Play all audios:
ABSTRACT Glycosaminoglycans (GAGs) regulate many important physiological processes. A pertinent issue to address is whether GAGs encode important functional information via introduction of
position specific sulfate groups in the GAG structure. However, procurement of pure, homogenous GAG motifs to probe the “sulfation code” is a challenging task due to isolation difficulty and
structural complexity. To this end, we devised a versatile synthetic strategy to obtain all the 16 theoretically possible sulfation patterns in the chondroitin sulfate (CS) repeating unit;
these include rare but potentially important sulfated motifs which have not been isolated earlier. Biological evaluation indicated that CS sulfation patterns had differing effects for
different breast cancer cell types and the greatest inhibitory effect was observed for the most aggressive, triple negative breast cancer cell line MDA-MB-231. SIMILAR CONTENT BEING VIEWED
BY OTHERS CHEMOENZYMATIC SYNTHESIS OF SULFUR-LINKED SUGAR POLYMERS AS HEPARANASE INHIBITORS Article Open access 02 December 2022 EFFICIENT PLATFORM FOR SYNTHESIZING COMPREHENSIVE HEPARAN
SULFATE OLIGOSACCHARIDE LIBRARIES FOR DECODING GLYCOSAMINOGLYCAN–PROTEIN INTERACTIONS Article 22 June 2023 TUMOR-AGNOSTIC CANCER THERAPY USING ANTIBODIES TARGETING ONCOFETAL CHONDROITIN
SULFATE Article Open access 30 August 2024 INTRODUCTION Glycosaminoglycans (GAGs) are heterogeneous polysaccharides comprising of repeating uronic acid and amino sugar disaccharide units.
These macromolecules can be covalently attached to core proteins to form proteoglycan side chains, or located in the extracellular matrix and intracellular secretory granules1,2,3. GAGs have
gained interest as potential therapeutic agents in cancer treatment, with studies showing their involvement in various pathobiological cancer stages4,5 and interactions with various
effective molecules such as growth factors and cytokines6,7. Overexpression of chondroitin sulfate (CS) has been identified in various cancer phenotypes such as prostate, testicular,
gastric, pancreatic and breast cancer8,9,10,11,12. For instance, compositional analysis of GAG side chains isolated from malignant breast tissues indicate an elevation in CS
expression13,14,15, with an increase in CS-A and CS-E sulfation sequences and a decrease in CS-C and CS-D16,17,18. These indicate that the sulfate groups present on CS might play an
important role in the cellular processes involved in the progression of breast cancer7,8,19,20. To investigate the molecular interactions of CS, chemical synthesis provides a viable
alternative to prepare pure, homogenous CS sequences via careful control on the site(s) of sulfation to probe structural activity relationship. Notable work has been achieved by various
groups in the synthesis of different CS analogues, such as CS-A, CS-C, CS-D, CS-E, CS-R, CS-K, CS-L, CS-M21,22,23,24,25,26,27,28,29,30,31,32,33,34 and some of these analogues have been
studied for their biological effects24,35,36. However, based on the current synthetic strategies reported, not all the sulfation patterns possible in the CS repeating unit can be obtained.
We opined that CS sulfation motifs which are not commonly expressed could encode important regulatory information. Thus, we envisioned a synthetic strategy which would allow for the
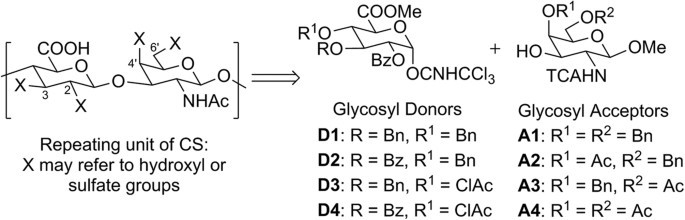
synthesis of all the sulfation patterns possible in CS. In CS, sulfation may occur on the C-2, C-3 positions of D-glucuronic acid and the C-4′, C-6′ positions of D-N-acetyl galactosamine
(Fig. 1) thus accounting for a total of 16 disaccharide possibilities. As with other saccharide synthesis, many key protection steps are required to control the site of sulfation in the
desired analogue37,38,39. Our synthetic strategy utilizes the benzyl ether and ester protecting groups as orthogonal handles to direct regioselective sulfation in the final product. Prior
research work has demonstrated that the C-2 ester directing group is pivotal to direct β-stereoselective glycosylation25,34. Since C-2 may contain a sulfate group, ester protected hydroxyl
groups are thus required as both sulfation and non-sulfation sites depending on the target compound. With this in mind, we modified some of the currently available CS
precursors24,31,35,38,40,41 to obtain glycosyl donors D1–D4 and acceptors A1–A4; these building blocks enable the synthesis of all 16 sulfation patterns theoretically possible in the CS
repeating unit. RESULTS AND DISCUSSION SYNTHESIS OF MONOMERIC BUILDING BLOCKS To obtain the glycosyl donors, intermediate 3 (Fig. 2) was subjected to different protection steps in a
divergent mode. C-2 ester protection was required for all 4 donors to direct β-stereoselective glycosylation and the C-3 hydroxyl group was protected either as an ester or a benzyl ether via
the dibutyl tin oxide mediated approach42. For D1 and D2, ester protected hydroxyl groups were denoted as sulfation sites; hence an orthogonal benzyl ether protecting group was required on
C-4, achieved by the use of CoCl2 and BH3.THF to direct complete regio-reductive ring opening of the benzylidene acetal protecting group43,44 (intermediates 7 and 8). Conversely, D3 and D4
mark benzyl ether protected hydroxyl groups as sulfation sites and hence require the orthogonal ester protection at C-4; chloroacetyl ester was chosen as this group could be selectively
cleaved to allow for the synthesis of longer CS fragments when required45,46. Intermediates 7, 8, 9, 10 were subjected to C-6 oxidation and carboxylate methylation, followed by C-4
chloroacetylation (for intermediates 15 and 16). This was followed by anomeric thiophenol deprotection and attachment of the trichloroacetimidate glycosyl auxiliary to furnish donors D1–D4.
To obtain the glycosyl acceptors, known intermediate 2347,48,49 was modified to introduce key protecting groups in common intermediate 26 (Fig. 3). Regio-reductive ring opening of the
benzylidene acetal in 26 enabled the formation of the benzyl ether on either the C-4′ position or C-6′ position depending on the choice of Lewis acid used. Ring opening using
triethylsilane/TfOH system enabled the formation of the benzyl ether on the C-6′ position in complete regioselectivity50 (intermediate 27), ascertained by 2D NMR. Alternatively, the benzyl
ether could be obtained on the C-4′ position with complete regioselectivity via triethylsilane/PhBCl2 reductive system50 (intermediate 29). Protection of the corresponding hydroxyl groups as
esters formed intermediates 28 and 30. The benzylidene acetal could also be cleaved via acidic hydrolysis, with both hydroxyl groups protected as benzyl ethers or esters (32 and 33). The
C-2′ azide in intermediates 28, 30, 32 and 33 were next converted to the _N_-trichloroacetyl group (TCAHN), this C-2 participating group directed β-stereoselective glycosylation of the
methyl ether at the anomeric position in the subsequent step. Finally, cleavage of the C-3′ naphthyl ether via DDQ oxidation furnished glycosyl acceptors A1–A4. GLYCOSYLATION OF MONOMERIC
BUILDING BLOCKS With glycosyl donors D1–D4 and glycosyl acceptors A1–A4 on hand, any sulfation pattern required in the final CS disaccharide can be obtained by the judicious choice of donor
and acceptor building blocks. D1–D4 were first glycosylated with A1–A4 using TMSOTf catalyst to form the protected disaccharides (Fig. 4). The C-2 participating ester group present in D1–D4
enabled exclusive formation of the β-product25. Upon glycosylation, the trichloroacetyl group was reduced to the acetyl group by radical mediated tributylstannane reduction. Any C-4
chloroacetyl groups present were also reduced to the form acetyl esters (intermediates 46A–46H). For intermediates 42A–42H, the ester protecting groups were next liberated via basic
hydrolysis and the free hydroxyl groups reacted with the sulfating agent. SO3.TEA was utilised to enable complete sulfation of the C-2 hydroxyl group in the glucuronic acid moiety; 5 equiv.
sulfating agent was required per –OH to ensure complete sulfation of the desired sites. Fortunately, the C-6 carboxylate group generated from the ester deprotection step did not affect the
sulfation step. With the sulfate groups attached at the required positions, global deprotection by hydrogenation of the remaining benzyl ether groups proceeded in the final step to furnish 8
distinct CS disaccharides 45A–45H. Due to the high negative charge in tetrasulfated disaccharide 45H, an additional step was introduced to protect the C-6 carboxylate group as a benzyl
ester, which facilitated product isolation during sulfation. For intermediates 46A–46H, the benzyl ethers were first cleaved via hydrogenation and the free hydroxyl groups were reacted with
SO3.TEA. Subsequently, global deprotection of the remaining ester protecting groups via basic hydrolysis51 furnished another 8 CS disaccharides 49A–49H. Through this strategy, all 16 CS
disaccharides were synthesized, which include those already reported21,30,52,53,54,55, in addition to novel sulfation motifs. By the incorporation of orthogonal protecting groups in the
monomeric building blocks, we were able to direct site specific sulfation of the CS disaccharide to obtain all the possible isomers, which were characterised by NMR and high resolution mass
spectrometry (ESI) techniques. The complete CS disaccharide library thus enables us to probe the “sulfation code” of CS in biological systems via structural activity relationship studies.
EVALUATING THE CS DISACCHARIDE LIBRARY ON BREAST CANCER CELL VIABILITY To achieve this, we proceeded to test the effect of CS sulfation patterns on breast cancer cell viability. The
synthesized CS disaccharides were tested on 4 different human breast cell lines. This included the non-tumorigenic breast epithelial cell line MCF-12A, to evaluate compound cytotoxicity and
3 breast cancer cell lines: MCF-7, T47D and MDA-MB-231. MCF-7 and T47D are low grade breast cancer cells which express the estrogen receptor and hence can be targeted using hormonal
therapy56,57,58,59. MDA-MB-231 cells are high grade triple negative breast cancer cells (TNBC) which do not express the estrogen receptor, progesterone receptor nor the human epidermal
growth factor receptor 260,61. TNBC tumor subtypes show low response to chemotherapy and are more challenging to treat due to the lack of known therapeutic targets, thus resulting in higher
patient mortality62,63,64. The biological effect of each CS disaccharide was investigated by incubating the cells with the CS disaccharide for 72 hours, prior to addition of the MTS reagent
to determine number of viable cells after treatment period. 4 different CS disaccharide concentrations were tested (0.1 μg/mL, 1 μg/mL, 10 μg/mL and 100 μg/mL). We first screened the 16 CS
disaccharides on MCF-12A cells and the results indicated that there was no significant change in cell viability (Supplementary Fig. 1). Hence these 16 CS disaccharides were not cytotoxic to
normal breast cells. Interestingly, when the 16 CS disaccharides were tested on the more aggressive MDA-MB-231 cell line, a statistically significant decrease in cell viability was observed
(via one way ANOVA analysis) for CS disaccharides 49F, 45B and 45D at 100 μg/mL concentration (Fig. 5; Supplementary Fig. 2 and 3). These inhibitory effects suggest that the sulfate groups
present on CS could encode important regulatory information for cellular processes involved in breast cancer survival. The results from the preliminary CS disaccharide screening also
indicate that both the number and position of the sulfate groups present in the CS disaccharide have an effect on MDA-MB-231 cell viability. The non-sulfated and fully sulfated CS
disaccharides, 49H and 45H, have no effect on cell viability suggesting that the presence of some sulfate groups are required for CS to elicit an inhibitory effect on MDA-MB-231 cells but
saturating all the possible sulfation sites would lead to a loss of activity. We next proceeded to screen the CS disaccharides on low grade breast cancer cells MCF-7 and T47D. The MTS
results showed no change in the number of viable cells after treatment with the CS disaccharides, indicating that all 16 CS disaccharides had no significant effect on MCF-7 cells
(Supplementary Fig. 4). The same observation was noted in T47D cells (Supplementary Fig. 5). To further evaluate the active CS disaccharides (49F, 45B and 45D), apoptosis assays were
subsequently conducted with the Caspase-Glo 3/7 kit which monitored the amount of caspase-3 and -7 activities present in the MDA-MB-231 cells after treatment with the respective CS
disaccharides. Results from the caspase assay showed an increase in luminescence when MDA-MB-231 cells were treated with CS disaccharides 49F, 45B and 45D (Fig. 6), indicating an increase in
caspase-3 and -7 activities. This suggests that the CS disaccharides could induce death of breast cancer cells via apoptosis. Interestingly, the largest decrease in cancer cell viability
and highest amount of caspase activity were seen in the CS disaccharide 45B–treated group. CONCLUSION In summary, a versatile synthetic strategy has been devised for the chemical synthesis
of all the sulfation patterns possible in the CS repeating unit. A total of 16 different CS disaccharides have been synthesized; these include analogues currently available as well as novel
sulfation motifs. Biological evaluation indicated that CS sulfation patterns had differential effects on different types of breast cancer cells. High grade breast tumor cells (MDA-MB-231)
showed significant reduction in cell viability upon treatment with CS disaccharides 49F, 45B and 45D while low grade breast tumor cells (MCF-7, T47D) and normal breast cells (MCF-12A) were
unaffected. Apoptosis assay suggests that these CS disaccharides could induce apoptosis. Since longer CS sequences could provide stronger activities than the disaccharides24,65, further
studies are presently ongoing to synthesize and evaluate CS oligosaccharides with the active sulfation profiles for their effect on MDA-MD-231 cells. METHODS CHEMICAL SYNTHESIS OF CS
DISACCHARIDES Detailed experimental procedures and compound characterization data can be found in the supplementary information, available in the online version of the paper. MTS ASSAY The
breast cells were plated onto a 96-well plate and cultured for 24 h. After 24 h, the cells were treated with the desired CS disaccharide at 4 different concentrations: 0.1 μg/mL, 1 μg/mL, 10
μg/mL and 100 μg/mL. A control group was included where only the drug vehicle was used; 6 replicates were made for each data set (n = 6). Cells were treated with each compound for 72 hours
and then washed with phosphate-buffered saline (PBS). CellTiter 96® AQueous One Solution (MTS reagent) was added to each well. Absorbance readings (λ = 490 nm) were taken after 3 h and the
data analyzed using one-way Analysis of Variance (ANOVA) with post-hoc Dunnett’s test. Statistical significance was defined as p < 0.05. APOPTOSIS ASSAY MDA-MB-231 cells were plated on a
6-well plate and treated with the selected CS disaccharide at 100 μg/mL concentration for 48 h. A control set was included where only the drug vehicle was used. After 48 h, the cells were
collected by trypsinization and reseeded into a white opaque 96-well plate to facilitate luminescence measurement (n = 6). After 24 h, 100 μL of Caspase-Glo® 3/7 reagent was added to each
well and then allowed to incubate for 1 h at room temperature in the dark. Luminescence readings were then measured. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Poh, Z.W. _et al._
Divergent Synthesis of Chondroitin Sulfate Disaccharides and Identification of Sulfate Motifs that Inhibit Triple Negative Breast Cancer. _Sci. Rep._ 5, 14355; doi: 10.1038/srep14355 (2015).
REFERENCES * Kjellen, L. & Lindahl, U. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 60, 443–475 (1991). Article CAS Google Scholar * Iozzo, R. V. Matrix
proteoglycans: From molecular design to cellular function. Annu. Rev. Biochem. 67, 609–652 (1998). Article CAS Google Scholar * Kolset, S. O., Prydz, K. & Pejler, G. Intracellular
proteoglycans. Biochem. J. 379, 217–227 (2004). Article CAS Google Scholar * Iozzo, R. V. Basement membrane proteoglycans: from cellar to ceiling. Nat. Rev. Mol. Cell Biol. 6, 646–656
(2005). Article CAS Google Scholar * Yang, J. et al. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J. Cell Biol. 165, 881–891 (2004).
Article CAS Google Scholar * Asimakopoulou, A. P., Theocharis, A. D., Tzanakakis, G. N. & Karamanos, N. K. The biological role of chondroitin sulfate in cancer and chondroitin-based
anticancer agents. In Vivo 22, 385–389 (2008). CAS PubMed Google Scholar * Afratis, N. et al. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 279, 1177–1197
(2012). Article CAS Google Scholar * Svensson, K. J. et al. Chondroitin sulfate expression predicts poor outcome in breast cancer. Int. J. Oncol. 39, 1421–1428 (2011). CAS PubMed Google
Scholar * Ricciardelli, C. et al. Elevated stromal chondroitin sulfate glycosaminoglycan predicts progression in early-stage prostate cancer. Clin. Cancer Res. 3, 983–992 (1997). CAS
PubMed Google Scholar * Labropoulou, V. T. et al. Versican but not decorin accumulation is related to metastatic potential and neovascularization in testicular germ cell tumours.
Histopathology 49, 582–593 (2006). Article CAS Google Scholar * Theocharis, A. D., Tsara, M. E., Papageorgacopoulou, N., Karavias, D. D. & Theocharis, D. A. Pancreatic carcinoma is
characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim. Biophys. Acta 1502, 201–206 (2000). Article CAS Google Scholar *
Theocharis, A. D., Vynios, D. H., Papageorgakopoulou, N., Skandalis, S. S. & Theocharis, D. A. Altered content composition and structure of glycosaminoglycans and proteoglycans in
gastric carcinoma. Int. J. Biochem. Cell Biol. 35, 376–390 (2003). Article CAS Google Scholar * Alini, M. & Losa, G. A. Partial characterization of proteoglycans isolated from
neoplastic and nonneoplastic human breast tissues. Cancer Res. 51, 1443–1447 (1991). CAS PubMed Google Scholar * Olsen, E. B., Trier, K., Eldov, K. & Ammitzboll, T. Glycosaminoglycans
in human breast cancer. Acta Obstet. Gynecol. Scand. 67, 539–542 (1988). Article CAS Google Scholar * Cooney, C. A. et al. Chondroitin sulfates play a major role in breast cancer
metastasis: a role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast Cancer Res. 13, R58 (2011). Article CAS ADS Google
Scholar * Potapenko, I. O. et al. Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression. Mol. Oncol. 4, 98–118 (2010). Article
CAS Google Scholar * Iida, J. et al. Role for chondroitin sulfate glycosaminoglycan in NEDD9-mediated breast cancer cell growth. Exp. Cell Res. 330, 358–370 (2015). Article CAS Google
Scholar * Willis, C. M. & Klüppel, M. Chondroitin sulfate-E is a negative regulator of a pro-tumorigenic Wnt/Beta-catenin-collagen 1 axis in breast cancer cells. PLoS ONE 9, e103966
(2014). Article ADS Google Scholar * Prinz, R. D., Willis, C. M., Viloria-Petit, A. & Kluppel, M. Elimination of breast tumor-associated chondroitin sulfate promotes metastasis.
Genet. Mol. Res. 10, 3901–3913 (2011). Article CAS Google Scholar * Yip, G. W., Smollich, M. & Gotte, M. Therapeutic value of glycosaminoglycans in cancer. Mol. Cancer Ther. 5,
2139–2148 (2006). Article CAS Google Scholar * Jacquinet, J.-C., Lopin-Bon, C. & Vibert, A. From polymer to size-defined oligomers: a highly divergent and stereocontrolled
construction of chondroitin sulfate A, C, D, E, K, L and M oligomers from a single precursor: part 2. Chem. - Eur. J. 15, 9579–9595 (2009). Article CAS Google Scholar * Vibert, A.,
Lopin-Bon, C. & Jacquinet, J.-C. From polymer to size-defined oligomers: a step economy process for the efficient and stereocontrolled construction of chondroitin oligosaccharides and
biotinylated conjugates thereof: part 1. Chem. - Eur. J. 15, 9561–9578 (2009). Article CAS Google Scholar * Jacquinet, J.-C., Rochepeau-Jobron, L. & Combal, J.-P. Multigram syntheses
of the disaccharide repeating units of chondroitin 4- and 6-sulfates. Carbohydr. Res. 314, 283–288 (1998). Article CAS Google Scholar * Tully, S. E. et al. A chondroitin sulfate small
molecule that stimulates neuronal growth. J. Am. Chem. Soc. 126, 7736–7737 (2004). Article CAS Google Scholar * Gama, C. I. et al. Sulfation patterns of glycosaminoglycans encode
molecular recognition and activity. Nat. Chem. Biol. 2, 467–473 (2006). Article CAS Google Scholar * Tamura, J.-I., Neumann, K. W. & Ogawa, T. A regio- and stereoselective synthesis
of 4-O-sulfated chondroitin di- and tetrasaccharides based on the strategy designed for the elongation of the repeating unit. Bioorg. Med. Chem. Lett. 5, 1351–1354 (1995). Article CAS
Google Scholar * Tamura, J.-I., Neumann, K. W., Kurono, S. & Ogawa, T. Synthetic approach towards sulfated chondroitin di-, tri- and tetrasaccharides corresponding to the repeating
unit. Carbohydr. Res. 305, 43–63 (1997). Article CAS Google Scholar * Tamura, J., Nakada, Y., Taniguchi, K. & Yamane, M. Synthesis of chondroitin sulfate E octasaccharide in a
repeating region involving an acetamide auxiliary. Carbohydr. Res. 343, 39–47 (2008). Article CAS Google Scholar * Lubineau, A. & Bonnaffé, D. Access to molecular diversity in
glycosaminoglycans: combinatorial synthesis of eight chondroitin sulfate disaccharides. Eur. J. Org. Chem. 1999, 2523–2532 (1999). Article Google Scholar * Karst, N. & Jacquinet, J.-C.
Chemical synthesis of the disaccharide repeating unit of shark cartilage chondroitin sulfate D and of its methyl D-glycoside derivative. J. Chem. Soc., Perkin Trans. 1, 2709–2717 (2000).
Article Google Scholar * Vibert, A., Jacquinet, J.-C. & Lopin-Bon, C. Recent advances in the chemical and enzymatic chondroitin sulfate synthesis. J. Carbohydr. Chem. 30, 393–414
(2011). Article CAS Google Scholar * Bedini, E. et al. Semi-synthesis of unusual chondroitin sulfate polysaccharides containing GlcA(3-O-sulfate) or GlcA(2,3-di-O-sulfate) units.
Chemistry 18, 2123–2130 (2012). Article CAS Google Scholar * Bedini, E. & Parrilli, M. Synthetic and semi-synthetic chondroitin sulfate oligosaccharides, polysaccharides and
glycomimetics. Carbohydr. Res. 356, 75–85 (2012). Article CAS Google Scholar * Karst, N. & Jacquinet, J.-C. Stereocontrolled total syntheses of shark cartilage chondroitin sulfate
D-related tetra- and hexasaccharide methyl glycosides. Eur. J. Org. Chem. 2002, 815–825 (2002). Article Google Scholar * Tamura, J. et al. Synthesis and interaction with midkine of
biotinylated chondroitin sulfate tetrasaccharides. Bioorg. Med. Chem. Lett. 22, 1371–1374 (2012). Article CAS Google Scholar * Despras, G. et al. Toward libraries of biotinylated
chondroitin sulfate analogues: from synthesis to _in vivo_ studies. Chem. - Eur. J. 19, 531–540 (2013). Article CAS Google Scholar * Orgueira, H. A. et al. Modular synthesis of heparin
oligosaccharides. Chemistry 9, 140–169 (2003). Article CAS Google Scholar * Karst, N. A. & Linhardt, R. J. Recent chemical and enzymatic approaches to the synthesis of
glycosaminoglycan oligosaccharides. Curr. Med. Chem. 10, 1993–2031 (2003). Article CAS Google Scholar * Litjens, R. E., den Heeten, R., Timmer, M. S., Overkleeft, H. S. & van der
Marel, G. A. An expedient synthesis of the repeating unit of the acidic polysaccharide of the bacteriolytic complex of lysoamidase. Chemistry 11, 1010–1016 (2005). Article CAS Google
Scholar * Yeung, B. K. S., Chong, P. Y. C. & Petillo, P. A. Synthesis of glycosaminoglycans. J. Carbohydr. Chem. 21, 799–865 (2002). Article CAS Google Scholar * Coutant, C. &
Jacquinet, J.-C. 2-Deoxy-2-trichloroacetamido-D-glucopyranose derivatives in oligosaccharide synthesis: from hyaluronic acid to chondroitin 4-sulfate trisaccharides. J. Chem. Soc., Perkin
Trans. 1, 1573–1581 (1995). Article Google Scholar * Hada, N., Sonoda, Y. & Takeda, T. Synthesis of a novel glycosphingolipid from the millipede, Parafontaria laminata armigera and the
assembly of its carbohydrate moiety into multivalent structures. Carbohydr. Res. 341, 1341–1352 (2006). Article CAS Google Scholar * Ohlin, M., Johnsson, R. & Ellervik, U.
Regioselective reductive openings of 4,6-benzylidene acetals: synthetic and mechanistic aspects. Carbohydr. Res. 346, 1358–1370 (2011). Article CAS Google Scholar * Tani, S., Sawadi, S.,
Kojima, M., Akai, S. & Sato, K.-I. A novel method for regioselective ring-opening reduction of 4,6-O-benzylidene hexopyranoside derivatives using CoCl2 and BH3·THF. Tetrahedron Lett. 48,
3103–3104 (2007). Article CAS Google Scholar * Blatter, G. & Jacquinet, J. C. The use of 2-deoxy-2-trichloroacetamido-D-glucopyranose derivatives in syntheses of hyaluronic
acid-related tetra-, hexa- and octa-saccharides having a methyl beta-D-glucopyranosiduronic acid at the reducing end. Carbohydr. Res. 288, 109–125 (1996). CAS PubMed Google Scholar *
Lopin, C. & Jacquinet, J. C. From polymer to size-defined oligomers: an expeditious route for the preparation of chondroitin oligosaccharides. Angew. Chem. Int. Ed. Engl. 45, 2574–2578
(2006). Article CAS Google Scholar * Pilgrim, W. & Murphy, P. V. α-glycosphingolipids via chelation-induced anomerization of O- and S-glucuronic and galacturonic acid derivatives.
Org. Lett. 11, 939–942 (2009). Article CAS Google Scholar * Banaag, A. R. & Tius, M. A. Design of chiral auxiliaries for the allene ether nazarov cyclization. J. Am. Chem. Soc. 129,
5328–5329 (2007). Article CAS Google Scholar * Lemieux, R. U. & Ratcliffe, R. M. The azidonitration of tri-O-acetyl-D-galactal. Can. J. Chem. 57, 1244–1251 (1979). Article CAS
Google Scholar * Sakagami, M. & Hamana, H. A selective ring opening reaction of 4,6-O-benzylidene acetals in carbohydrates using trialkylsilane derivatives. Tetrahedron Lett. 41,
5547–5551 (2000). Article CAS Google Scholar * Lucas, H. et al. Syntheses of heparin - like pentamers containing “opened” uronic acid moieties. Tetrahedron 46, 8207–8228 (1990). Article
CAS Google Scholar * Sugahara, K. et al. Structural studies on the chondroitinase ABC-resistant sulfated tetrasaccharides isolated from various chondroitin sulfate isomers. Carbohydr. Res.
255, 145–163 (1994). Article CAS Google Scholar * Sugahara, K. et al. Chondroitinase ABC-resistant sulfated trisaccharides isolated from digests of chondroitin/dermatan sulfate chains.
Carbohydr. Res. 255, 165–182 (1994). Article CAS Google Scholar * Gargiulo, V., Lanzetta, R., Parrilli, M. & De Castro, C. Structural analysis of chondroitin sulfate from Scyliorhinus
canicula: a useful source of this polysaccharide. Glycobiology 19, 1485–1491 (2009). Article CAS Google Scholar * Akatsu, C. et al. Dermatan sulfate epimerase 2 is the predominant
isozyme in the formation of the chondroitin sulfate/dermatan sulfate hybrid structure in postnatal developing mouse brain. Glycobiology 21, 565–574 (2011). Article CAS Google Scholar *
Levenson, A. S. & Jordan, V. C. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 57, 3071–3078 (1997). CAS PubMed Google Scholar * Weisz, A. et al. Molecular
identification of ERα-positive breast cancer cells by the expression profile of an intrinsic set of estrogen regulated genes. J. Cell. Physiol. 200, 440–450 (2004). Article CAS Google
Scholar * Koboldt, D. et al. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012). Article CAS ADS Google Scholar * Osborne, C. K., Hobbs, K. & Clark,
G. M. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 45, 584–590 (1985). CAS PubMed Google Scholar * Neve, R. M. et al. A
collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10, 515–527 (2006). Article CAS Google Scholar * Holliday, D. L. & Speirs,
V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 13, 215 (2011). Article Google Scholar * Fulford, L. G. et al. Basal-like grade III invasive ductal carcinoma
of the breast: patterns of metastasis and long-term survival. Breast Cancer Res. 9, R4 (2007). Article Google Scholar * Carey, L. A. et al. The triple negative paradox: primary tumor
chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 13, 2329–2334 (2007). Article CAS ADS Google Scholar * O’Toole, S. A. et al. Therapeutic targets in triple negative breast
cancer. J. Clin. Pathol. 66, 530–542 (2013). Article Google Scholar * Hsu, C.-H., Hung, S.-C., Wu, C.-Y. & Wong, C.-H. Toward automated oligosaccharide synthesis. Angew. Chem. Int. Ed.
50, 11872–11923 (2011). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors thank the National University of Singapore (NUS) (ARF: R-143-000-554-112) for the
financial support and NUS Graduate School for Integrative Sciences & Engineering for a Ph.D. scholarship (to Z.W.P.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Chemistry, National University of Singapore (NUS), Singapore Zhong Wei Poh, Chin Heng Gan, Eric J. Lee & Yulin Lam * NUS Graduate School for Integrative Sciences and Engineering (NGS),
Singapore Zhong Wei Poh & Yulin Lam * Department of Anatomy, National University of Singapore (NUS), Singapore Suxian Guo & George W. Yip Authors * Zhong Wei Poh View author
publications You can also search for this author inPubMed Google Scholar * Chin Heng Gan View author publications You can also search for this author inPubMed Google Scholar * Eric J. Lee
View author publications You can also search for this author inPubMed Google Scholar * Suxian Guo View author publications You can also search for this author inPubMed Google Scholar *
George W. Yip View author publications You can also search for this author inPubMed Google Scholar * Yulin Lam View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS Z.W.P. synthesized and characterized the CS disaccharides with the assistance of C.H.G., E.J.L. and Z.W.P. conducted the MTS and apoptosis assays with the assistance of
S.G., Z.W.P., G.W.Y. and Y.L. analyzed the results and wrote the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC
SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party
material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons
license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wei Poh, Z., Heng Gan, C., Lee, E. _et al._ Divergent Synthesis of Chondroitin Sulfate Disaccharides and Identification of Sulfate Motifs
that Inhibit Triple Negative Breast Cancer. _Sci Rep_ 5, 14355 (2015). https://doi.org/10.1038/srep14355 Download citation * Received: 17 June 2015 * Accepted: 24 August 2015 * Published:
24 September 2015 * DOI: https://doi.org/10.1038/srep14355 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative