Play all audios:
ABSTRACT The transcription factor FOXP3 is essential for the differentiation and function of regulatory T cells (Treg). It is established that the transcription factor GATA-3 is induced in
Treg cells under inflammatory conditions. GATA-3 stabilizes FOXP3 levels to avoid the differentiation of Treg cells into inflammatory-like T cells. The IL-6 signal pathway influences the
sensitivity of Treg cells towards instability. The mechanism of GATA-3 in regulating FOXP3 and its relation to the IL-6 pathway remains unclear. Here we report how miR-125a-5p plays an
important role in regulating the conversion of Treg cells by IL-6. miR-125a-5p expression is low in Treg cells under steady state conditions and can be induced by GATA-3 to inhibit the
expression of IL-6R and STAT3. This finding reveals a GATA3/miR-125a-5p/IL-6R and STAT3/FOXP3 regulatory pathway, which determines how Treg cells respond to inflammatory IL-6-rich
conditions. SIMILAR CONTENT BEING VIEWED BY OTHERS THE GSK3Β-Β-CATENIN-TCF1 PATHWAY IMPROVES NAIVE T CELL ACTIVATION IN OLD ADULTS BY UPREGULATING MIR-181A Article Open access 08 February
2021 MIR-146A REGULATES REGULATORY T CELLS TO SUPPRESS HEART TRANSPLANT REJECTION IN MICE Article Open access 17 June 2021 THE MIR-641-STIM1 AND SATB1 AXES PLAY IMPORTANT ROLES IN THE
REGULATION OF THE TH17/TREG BALANCE IN ITP Article Open access 16 May 2024 INTRODUCTION Treg cells maintain the balance of immune self-tolerance and homeostasis via limiting aberrant or
excessive inflammation1,2. Treg cells are not terminally differentiated cells, as they can lose the expression of FOXP3 and become pro-inflammatory cells when induced by certain
cytokines3,4,5. IL-6 is one of the most likely candidates to induce FOXP3 downregulation as it plays a critical role in determining the balance between Th17 and Treg differentiation6. In
year 2003, Pasare and Medzhitov reported that Toll-like receptor-induced IL-6 expression led to the loss of Treg cell function7. More recently, activated Treg cells were found to
differentiate into Th17 cells in the presence of IL-6 and in the absence of exogenous TGF-β8. IL-6, together with IL-1, may induce downregulation of Foxp3 in a signal transducer and
activator of transcription 3 (STAT3)-dependent pathway9. _In vivo_ experiments have also shown that in autoimmune arthritis FOXP3+ Treg cells lose FOXP3 expression and undergo conversion
into Th17 cells. This process was found dependent on IL-610. It has been reported that IL-6 downregulates FOXP3 mRNA expression via epigenetic changes11. Furthermore, our laboratory has
shown that IL-6 and TGF-β treatment can downregulate FOXP3 by promoting FOXP3 protein degradation _in vitro_12. However, how the IL-6 signal pathway is regulated in Treg cells remains
unclear. MicroRNAs (miRNAs) have emerged as important regulators in many physiological and pathological processes including development, differentiation, metabolism, immunity, cell
proliferation and apoptosis13,14. miRNAs are small non-coding RNAs, which repress translation or cleave messenger RNAs (mRNAs) via binding to the coding sequence (CDS) regions or the 3′
untranslated regions (3′UTR) of target genes. For instance, miR-146a has been reported as indispensable for Treg-mediated suppression through regulating the IFNγ response by targeting
STAT115. FOXP3 directly induces miR-155 and is responsible for the survival of Treg cells through repression of suppressor of cytokine signaling 1 (SOCS1)16,17. All these findings suggest
that miRNAs play important roles in the function of Treg cells. In this study, we performed a miRNA microarray and Real-Time-PCR analysis on a human Treg-like cell line to correlate miRNA
expression with FOXP3 expression. We identified that miR-125a-5p, a GATA3-inducible miRNA, targets interleukin 6 receptor (IL-6R) and STAT3 transcripts. We propose a regulatory mechanism by
which miR-125a-5p reduces the sensitivity of Treg cells toward IL-6 conversion. RESULTS MIR-125A-5P EXPRESSION IS REGULATED IN HUMAN T CELL LINES AND T SUBSETS FOXP3 is the master
transcription factor in Treg cells, which regulates the function of Treg cells via modulating gene transcription. We were interested in knowing whether any miRNAs were related to FOXP3
expression. The SZ-4 cell line was originally identified in a Sezary disease patient and expresses FOXP318. This cell line has been used to investigate Sezary Syndrome and the regulation of
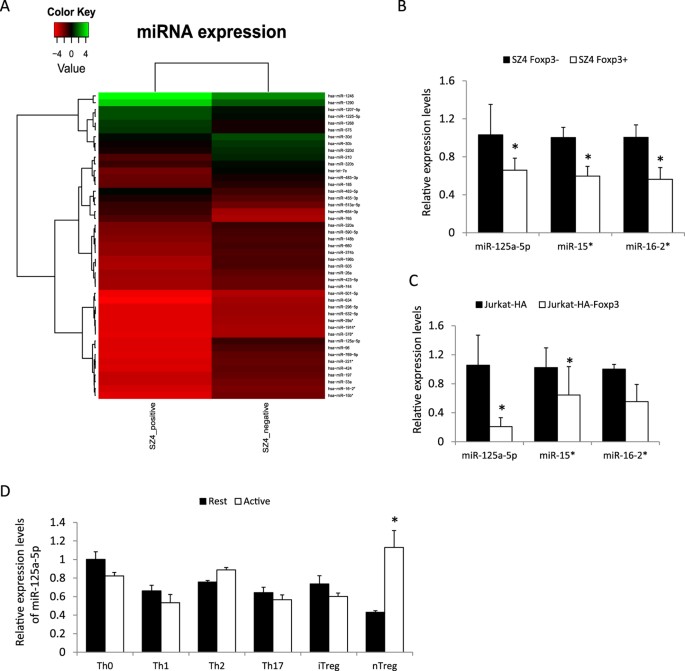
genes in T cells19. We purified RNA from the FOXP3-positive SZ-4 cell line and FOXP3-negative SZ-4 cell line (derived from subcloning) and carried out miRNA microarray to compare expression
levels of miRNA. The clusters of miRNAs that had more than two-fold change in expression were rendered into a heat-map diagram (Fig. 1A). miR-125-5p was one of the most downregulated miRNAs
revealed by the microarray data. We then used Real-Time-PCR to confirm the expression levels of several miRNAs in the SZ-4 (Fig. 1B) and Jurkat (Fig. 1C) cell lines. All of these results
indicated that miR-125a-5p is downregulated in FOXP3-positive T cells. We then checked the expression levels of miR-125a-5p in primary Th0, Th1, Th2, Th17, iTreg and nTreg cells. The
expression of miR-125a-5p was found lower in resting nTreg cells compared with effector T cells, but could be upregulated after TCR activation (Fig. 1D). GATA3 INDUCES THE EXPRESSION OF
MIR-125A-5P IN TREG CELLS In order to know whether the expression of miR-125a-5p is regulated by FOXP3, we analyzed the promoter region of miR-125a-5p for transcription factor binding DNA
epitopes. There were no predicted binding sites for FOXP3, but rather those for GATA3 binding were found (Supplemental Sequence 1). We then cloned the promoter region of the gene
transcribing miR-125a-5p into the pGL3-basic vector and carried out a luciferase assay in 293T cells (Fig. 2A). The data indicated that the activity of the miR-125a-5p promoter could be
induced by GATA3 in a dose dependent manner. Site-directed mutations suggested that site four was the most important binding site of GATA3 as luciferase activity after mutation of the fourth
site showed no change of luciferase activity with different levels of PIP-Myc-GATA3 (Fig. 2B). We then examined whether GATA3 could upregulate miR-125a-5p levels. GATA3 was overexpressed in
Jurkat and Jurkat-HA-FOXP3 cells by electroporation and we found the expression level of miR-125a-5p was upregulated (Fig. 2C). Endogenous GATA3 was then induced by anti-CD3/CD28 antibodies
in Jurkat-HA-FOXP3 cells and primary human nTreg cells. Protein levels of GATA3 was tested via Western blotting (Supplemental Fig. 1A) or flow cytometry (Supplemental Fig. 1B) and the
expression level of miR-125a-5p though Real-Time PCR (Fig. 2D). These results show that miR-125a-5p could be upregulated and this correlated with the endogenous induction of GATA3
expression. We then depleted GATA3 via lentivirus transduction in nTreg cells followed by anti-CD3/CD28 activation and detected GATA3 protein and miR-125a-5p levels. The results show that
the upregulation of miR-125a-5p in activated nTreg cells was dependent on endogenous GATA3 expression (Fig. 2E & Supplemental Fig. 1C). All the above data suggested that under TCR
stimulation the expression of miR-125a-5p could be upregulated via GATA3 in human nTreg cells. MIR-125A-5P DIRECTLY TARGETS IL-6R AND STAT3 In order to investigate the function of
miR-125a-5p we first identified its target genes. We searched for different target genes in TargetScan and then selected 42 target genes related to immune function. The 3′UTR of these genes
were cloned into the pGL3 vector for luciferase assays. We overexpressed or knocked down miR-125a-5p in 293T cells and analyzed for changes in luciferase activity. The results show that
miR-125a-5p significantly reduced luciferase activity using the 3′UTR of IL-6R and STAT3 but not after mutation of the miRNA interaction sites (MutIL6R-2 and MutSTAT3, respectively) (Fig.
3A,B). We then checked the protein levels of the target genes after overexpression or knockdown of miR-125a-5p in nTreg cells. Western blotting analysis showed that the protein level of
STAT3 was reduced by miR-125a-5p (Fig. 3C & Supplemental Fig. 2) and by flow cytometry IL-6R was found decreased by miR-125a-5p (Fig. 3D). The miRNA binding sites and mutations are shown
in Supplemental tables 1 and 2. MIR-125A-5P DECREASES THE SENSITIVITY OF TREG CELLS TOWARD IL-6 CONVERSION It is well known that IL-6 stimulation can reduce FOXP3 levels and decrease the
suppressive function of Treg cells, which is dependent on STAT3. As IL-6R and STAT3 are both targets of miR-125a-5p, we hypothesized that miR-125a-5p can influence the sensitivity of Treg
cells towards IL-6 signals. After overexpressing or knockdown of miR-125a-5p in primary nTreg cells, the cells were activated with anti-CD3/CD28 beads with or without IL-6 for 48 hours. The
expression level of FOXP3 in nTreg cells was then detected by flow cytometry. In the control groups (ago-NC and anti-NC), FOXP3 was downregulated when treated with IL-6. However, the
expression of FOXP3 had almost no change upon IL-6 treatment in the miR-125a-5p overexpression group and decreased significantly in the miR-125a-5p knockdown group (Fig. 4A). We also carried
out a suppression assay with the same group of nTreg cells. The suppressive functions of the four different groups of Treg cells were similar when treated only with anti-CD3/CD28 beads
(Fig. 4B). After IL-6 stimulation, the suppressive function of Treg cells decreased in all of the groups. Knockdown of miR-125a-5p highly sensitized the Treg cells to IL-6-mediated
downregulation of Treg suppression, but the overexpression of miR-125a-5p increased the suppressive function of these Treg cells under IL-6 stimulation (Fig. 4C). All these data suggest that
miR-125a-5p decreases the sensitivity of Treg cells toward IL-6-induced conversion. GATA3, MIR-125A-5P, IL-6R, STAT3 AND FOXP3 FORMS A REGULATORY LOOP As the decrease and dysfunction of
Treg cells has been found in some human diseases we tested whether miR-125a-5p expression correlated with disease occurrence. Our published work has already shown how GATA3 expression is
high in Treg cells of asthma patients20. Here, we investigated the expression level of miR-125a-5p and its target genes in asthma patients. The expression levels of GATA3 and miR-125a-5p
were significantly upregulated in the Treg cells of asthma patients compared with healthy donors (Fig. 5A,B). In addition, the expression level of IL-6R was significantly downregulated in
asthma patients (Fig. 5C). There was no difference in the expression of STAT3 between the asthma patients and healthy donors, albeit a cluster of patients did show relatively lower
expression of STAT3 in the asthma patients (Fig. 5D). The expression of FOXP3 was also upregulated (Fig. 5E). These results reveal how miR-125a-5p and IL-6R may play important roles in
asthma and show a regulatory pathway that regulates the sensitivity of Treg cells toward IL-6 conversion. DISCUSSION We investigated the role of miR-125a-5p in human Treg cells. mir-125a-5p
has been reported to act as a tumor suppression modulator in several kinds of cancers, such as glioblastoma, lung cancer and hepatocellular carcinoma etc.21,22,23,24,25 and has also been
reported to play a role in the immune system. In Drosophila, miR-125 was identified as a regulator of innate immunity26. In oxidized low-density lipoprotein-stimulated monocyte-derived
macrophages, miR-125a-5p was found to decrease lipid uptake and secretion of inflammatory cytokines27. mir-125a-5p was also reported to downregulate the expression of a hepatitis B virus
surface antigen28. During macrophage activation and polarization, miR-125a-5p promotes M2 macrophages and reduces M1 phenotype polarization29. However, the expression and function of
miR-125a-5p in Treg cells remained unclear. The expression of miR-125a-5p is lower in CD4+ CD25+ T cells compared to CD4+ CD25− cells purified from human cord blood30. Valproate treatment of
human cord blood CD4+ effector T cells could repress miR-125a-5p expression and upregulate FOXP331. In purified T cell subsets from PBMC of human, Real-Time-PCR results indicated that the
expression of miR-125a-5p is downregulated in Treg cells (resting) compared to effector T cells32. Our data also suggested that miR-125a-5p is expressed lower in FOXP3+ human T cell lines.
Many reports have indicated that miRNA expression can be regulated by transcription factors. The transcription factor Twist can induce miR-10b expression33 and HIF-1α can upregulate
miR-21034,35. Our data showed that TCR and IL-2 activation could upregulate miR-125a-5p and this process was dependent on GATA3. GATA3 is the canonical transcription factor of Th2
cells36,37. GATA3 is required for T cell lineage commitment as GATA3 is expressed in Linloc-KithiCD25− early T cell progenitors and lack of GATA3 results in failed development of T cells38.
GATA3 is indispensable for the early development of CD4+ T cells during the ß selection process39. It is reported that GATA3 binds to and promotes ThPOk to induce CD4+ and CD8+ T cell
differentiation40. In Treg cells, the expression of GATA3 is critical for Treg cell physiology during inflammation41. GATA3 is also reported as essential for the function of Treg cells via
binding to and promoting cis-acting elements of Foxp342. We found that GATA3 upregulates miR-125a-5p in Treg cells during TCR stimulation. But in Th2 cells whether GATA3 can up regulate
miR-125a-5p remains unknown. The expression of miR-125a-5p in resting Th1 and Th17 cells is higher than resting Treg cells, which may reflect other mechanisms that regulate miR-125a-5p in
other T cell subsets. In animals, miRNA sequences and their targets are not perfectly matched, which makes the search for targets complicated. Using a computer-based method, we identified
IL-6R and STAT3 as two novel targets of miR-125a-5p. IL-6 transduces two signal pathways via binding to IL-6R: one dependent on STAT3 activation and another on Src homology region 2
domain-containing phosphatase 2 (SHP2). It is reported that the expression level of IL-6R is responsible for the sensitivity of Treg cells toward IL-6 conversion43. IL-6-induced inhibition
of FOXP3 is dependent on STAT344. Also, STAT3 has been reported to promote the instability of Treg cells45. Thus we predicted that miR-125a-5p could regulate the sensitivity of Treg cells
under IL-6 stimulation and our _in vitro_ data had confirmed our prediction. Our data also showed that without IL-6 stimulation, overexpression or knock down of miR-125a-5p did not change
the expression level of FOXP3 or the suppressive function of Treg cells. Similar results have been reported that show how the overexpression of miR-125a have no effect on FOXP3 expression or
cell phenotype30. Fayyad-Kazan H _et al._ indicated that valproate treatment induces FOXP3 expression in CD4+ effector T cells by increasing the binding of Ets-1 and Ets-2 to the FOXP3
promoter, instead of a miR-125a-5p dependent mechanism31. It is probable that different cell types and experimental conditions led to these different observations. The IL-6 signal pathway is
also important for the differentiation of iTreg and Th17 cells. However, changes in the expression level of miR-125a-5p in naïve T cells had no effect on the polarization of both cell types
(data not shown). Also, the IL-6 signal pathway has been reported to be regulated by several miRNAs. In the malignant transformation of MCF-10A, Lin28 and let-7a regulate the activity of
the IL-6/STAT3 axis46. It is reported that there is one feedback loop comprised of IL-6-STAT3-miR-24/miR-629-HNF4-miR-124, which regulates hepatocellular oncogenesis47. miR-93 influences
proliferation and differentiation states of breast cancer stem cells (BCSCs) by targeting several genes including STAT348. All these studies were performed in cancer cells and whether these
miRNAs have similar roles to miR-125a-5p in Treg cells requires more investigation. Asthma is a chronic inflammatory disease characterized by T helper cell 2 (Th2) inflammation leading to
airway hyper-responsiveness (AHR). Evidence has indicated that Treg cells are involved in this disease49. Our data showed that GATA3, miR-125a-5p, FOXP3 and IL-6R are perturbed in the Treg
cells of asthma patients. Further studies on functional and tissue-specific Treg cell subsets of asthma patients would be meaningful to reveal the functional consequences of altering this
signal pathway. In conclusion, we have identified a novel signal pathway in which miR-125a-5p decreases the sensitivity of Treg cells toward IL-6-mediated conversion. This signal pathway
links two critical transcription factors in Treg cells with the function of one miRNA, which supports the notion that miRNAs are important regulators in Treg cells. Furthermore, miR-125a-5p
and IL-6R are perturbed in asthma patients, which provides basis for development of new therapeutic strategies against asthma. METHODS CELL CULTURE AND TRANSFECTION HEK293T cells were
cultured in DMEM containing 10% fetal bovine serum (FBS) and transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Jurkat and SZ4 cell lines were
cultured in RPMI-1640 containing 10% FBS. Electroporation of Jurkat cells was performed with the NEPA21 apparatus (NEPAGENE, Japan). MIRNA MICROARRAY The microarray experiment was performed
using the Agilent-021827 Human miRNA Microarray. The microarray data analyzed for this publication has been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series
accession number; GSE64074 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=gjwremicrnkdjcl&acc=GSE64074) REAL-TIME POLYMERASE CHAIN REACTION ASSAYS Total RNA was extracted using
TRIzol reagent (Invitrogen). cDNA was synthesized using a reverse transcriptase kit (TaKaRa, Japan), followed by Real-Time-PCR analysis (SYBR Green; TaKaRa). The primers that were used are
as follows: ß-actin forward, 5′-GGACTTCGAGCAAGAGATGG-3′ and reverse, 5′-AGCACTGTGTTGGCGTACAG-3′; IL-6R forward, 5′-CCTGACGACAAAGGCTGTGCTCT-3′ and reverse, 5′-GCTGAACTTGCTCCCGACACTACTG-3′;
STAT3 forward, 5′-GGGGCTTTTGTCAGCGATGGAGTA-3′and reverse, 5′-ATTTGTTGACGGGTCTGAAGTTGAG-3′; GATA3 forward, 5′- CTCATTAAGCCCAAGCGAAG-3′ and reverse, 5′- TTTTTCGGTTTCTGGTCTGG-3′; FOXP3 forward,
5′-TCCCAGAGTTCCTCCACAAC-3′ and reverse, 5′-ATTGAGTGTCCGCTGCTTCT-3′. The expression level of miR-125a-5p was assayed with Hairpin-itTM MicroRNAs Quantitation PCR Kit (GenePharma, Shanghai,
China). Each sample was analyzed in triplicate and U6 RNA was used to normalize miRNA levels. WESTERN BLOT ANALYSIS Cells were washed with pre-chilled phosphate-buffered saline (PBS) and
lysed in radioimmune precipitation assay buffer. These cell lysates were separated on SDS-PAGE gels and transferred to PVDF membrane. All the primary antibodies were incubated overnight at 4
degrees Celsius, followed by incubation with HRP-conjugated goat anti-rabbit or goat anti-mouse secondary antibody and detected with ECL solution (Millipore). Antibodies against ß-actin
were purchased from Sigma; anti-STAT3 (79D7) was purchased from Cell Signaling. Anti-GATA3 (HG3-31) antibody was purchased from Santa Cruz Biotechnology. LUCIFERASE ACTIVITY ASSAY The human
3′ UTR regions of IL-6R or STAT3 were amplified by PCR and cloned into the EcoRI and XhoI sites or KpnI and XbaI sites of the pGL3-control vector (Promega). The promoter region of
miR-125a-5p was amplified by PCR and cloned into the KpnI and XhoI sites of the pGL3-basic vector (Promega). Nucleotide-substitution mutations were carried out using PCR-based methods. All
primers used in vector construction are listed in the supplemental tables. All constructs were verified by sequencing. For the luciferase assay, 293T cells were cultured in 12-well plates
and transfected with 100 ng luciferase reporter plasmid, 5 ng pRL-TK vector expressing the Renilla luciferase (Promega) and 25nM, 50nM or 100 nM of ago miR-125a-5p or anta miR-125a-5p or
miRNA negative control. For the promoter activity detection, 100 ng of luciferase reporter vector, 5 ng of pRL-TK vector and PIP-Flag-GATA3 or PIP-Flag-Blank vectors were co-transfected into
293T cells. After transfection for 48 hours, firefly and renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay (Promega). LENTIVIRAL CONSTRUCTS AND INFECTION
The shRNA lentiviral vectors pLKO.1 shGATA3-2 or pLKO.1 shCK were transfected into HEK 293T cells with the lentivirus packing vector Delta 8.9 and VSVG envelope glycoprotein. Viral
supernatants were harvested after 48 h. Primary human Treg cells were transduced with virus along with a secondary anti-CD3/CD28 stimulus (four cells to one bead). The following shRNA
sequence was used in this experiment: 5′-AGCCTAAACGCGATGGATATA-3′ (shGATA3-2). HUMAN T CELL CULTURE Naïve human CD4+ T cells (CD4+ CD25lowCD127highCD45RAhigh) and primary human CD4+
CD25highCD127low Treg cells from healthy donors were isolated by FACS on a BD FACS ARIA II sorter (BD Biosciences). Primary Treg cells were expanded using anti-CD3/CD28 dynabeads
(Invitrogen) in X-VIVO-15 medium (Lonza, Switzerland) supplemented with 10% human AB serum, 1% GlutaMax (GIBCO), 1% sodium pyruvate (GIBCO), 1% Pen/Strep (GIBCO) and 100 U/ml IL-2. Naïve T
cells were activated with anti-CD3/CD28 dynabeads in X-VIVO-15 medium and polarized into other T subsets under the following conditions: Th1: rhIL-12 (1 ng/ml) and anti-IL-4 (10 μg/ml)
antibody; Th2: rhIL-4 (20 ng/ml) and anti-IFNγ (10 μg/ml) antibody; Th17: rhTGF-β1 (2.5 ng/ml), rhIL-6 (50 ng/ml), rhIL-1β (10 ng/ml) and rhIL-23 (100 ng/ml) and iTreg: 10 ng/ml TGF-ß, 100
ng/ml all-trans retinoic acid (atRA) and 100 units/ml rIL-2. SUPPRESSION ASSAY After culture for seven days, the Treg cells are restimulated with anti-CD3/CD28 dynabeads, 12.5 U/ml IL-2 and
with or without 20 ng/ml IL-6 for two days. To measure suppression function, CFSE-labeled PBMC cells were stimulated with anti-CD3/CD28 dynabeads. To the responder cells, Tregs stimulated
with or without IL-6 were added at different ratios and suppression of CFSE-labeled T cells was assessed as described50. ASTHMA PATIENTS AND TREG ISOLATION The human patients and healthy
control samples were from Ruijin Hospital (Shanghai, China). The study was approved by the Ruijin Hospital Ethics Committee, Shanghai Jiao Tong University School of Medicine (Permit number
2013-51) and was carried out in accordance with the approved guidelines. All participants provided written inform consent. The isolation of PBMC and Treg cells was performed as previously
described20. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Li, D. _et al._ MiR-125a-5p Decreases the Sensitivity of Treg cells Toward IL-6-Mediated Conversion by Inhibiting IL-6R and
STAT3 Expression. _Sci. Rep._ 5, 14615; doi: 10.1038/srep14615 (2015). REFERENCES * Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133,
775–787, 10.1016/j.cell.2008.05.009 (2008). Article CAS PubMed Google Scholar * Wing, K. & Sakaguchi, S. Regulatory T cells exert checks and balances on self tolerance and
autoimmunity. Nat Immunol 11, 7–13, 10.1038/ni.1818 (2010). Article CAS PubMed Google Scholar * Koenen, H. J. et al. Human CD25highFoxp3pos regulatory T cells differentiate into
IL-17-producing cells. Blood 112, 2340-2352, 10.1182/blood-2008-01-133967 (2008). Article CAS Google Scholar * Zhou, X., Bailey-Bucktrout, S., Jeker, L. T. & Bluestone, J. A.
Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol 21, 281–285, 10.1016/j.coi.2009.05.007 (2009). Article CAS PubMed PubMed Central Google Scholar * Sakaguchi, S., Vignali, D. A.,
Rudensky, A. Y., Niec, R. E. & Waldmann, H. The plasticity and stability of regulatory T cells. Nat Rev Immunol 13, 461–467, 10.1038/nri3464 (2013). Article CAS PubMed Google Scholar
* Kimura, A. & Kishimoto, T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 40, 1830–1835, 10.1002/eji.201040391 (2010). Article CAS PubMed Google Scholar * Pasare, C. &
Medzhitov, R. Toll pathway-dependent blockade of CD4+CD25+T cell-mediated suppression by dendritic cells. Science 299, 1033–1036, 10.1126/science.1078231 (2003). Article CAS PubMed ADS
Google Scholar * Xu, L., Kitani, A., Fuss, I. & Strober, W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of
exogenous TGF-beta. J Immunol 178, 6725–6729 (2007). Article CAS PubMed Google Scholar * Yang, X. O. et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell
programs. Immunity 29, 44–56, 10.1016/j.immuni.2008.05.007 (2008). Article CAS PubMed PubMed Central Google Scholar * Komatsu, N. et al. Pathogenic conversion of Foxp3(+) T cells into
TH17 cells in autoimmune arthritis. Nat Med 20, 62–68, 10.1038/nm.3432 (2014). Article CAS PubMed Google Scholar * Lal, G. et al. Epigenetic regulation of Foxp3 expression in regulatory
T cells by DNA methylation. J Immunol 182, 259–273 (2009). Article CAS PubMed Google Scholar * Gao, Z. et al. Synergy between IL-6 and TGF-beta signaling promotes FOXP3 degradation. Int
J Clin Exp Pathol 5, 626–633 (2012). CAS PubMed PubMed Central Google Scholar * He, L. & Hannon, G. J. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5,
522–531, 10.1038/nrg1379 (2004). Article CAS PubMed Google Scholar * Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism and function. Cell 116, 281–297 (2004). Article CAS PubMed
Google Scholar * Lu, L. F. et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 142, 914–929, 10.1016/j.cell.2010.08.012 (2010). Article CAS
PubMed PubMed Central Google Scholar * Lu, L. F. et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 30, 80–91,
10.1016/j.immuni.2008.11.010 (2009). Article CAS PubMed PubMed Central Google Scholar * Kohlhaas, S. et al. Cutting edge: the Foxp3 target miR-155 contributes to the development of
regulatory T cells. J Immunol 182, 2578–2582, 10.4049/jimmunol.0803162 (2009). Article CAS PubMed Google Scholar * Abrams, J. T. et al. A clonal CD4-positive T-cell line established from
the blood of a patient with Sezary syndrome. J Invest Dermatol 96, 31–37 (1991). Article CAS PubMed Google Scholar * Samanta, A. et al. TGF-beta and IL-6 signals modulate chromatin
binding and promoter occupancy by acetylated FOXP3. Proc Natl Acad Sci USA 105, 14023–14027, 10.1073/pnas.0806726105 (2008). Article PubMed ADS PubMed Central Google Scholar * Zhang, J.
et al. Identification of the E3 deubiquitinase ubiquitin-specific peptidase 21 (USP21) as a positive regulator of the transcription factor GATA3. J Biol Chem 288, 9373–9382,
10.1074/jbc.M112.374744 (2013). Article CAS PubMed PubMed Central Google Scholar * Cortez, M. A. et al. miR-29b and miR-125a regulate podoplanin and suppress invasion in glioblastoma.
Genes Chromosomes Cancer 49, 981–990, 10.1002/gcc.20808 (2010). Article CAS PubMed PubMed Central Google Scholar * Jiang, L., Huang, Q., Chang, J., Wang, E. & Qiu, X. MicroRNA
HSA-miR-125a-5p induces apoptosis by activating p53 in lung cancer cells. Exp Lung Res 37, 387–398, 10.3109/01902148.2010.492068 (2011). Article CAS PubMed Google Scholar * Kim, J. K. et
al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology 57, 1055–1067, 10.1002/hep.26101 (2013).
Article CAS PubMed Google Scholar * Scott, G. K. et al. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J Biol Chem 282, 1479–1486,
10.1074/jbc.M609383200 (2007). Article CAS PubMed Google Scholar * Zhu, W. Y. et al. Differential expression of miR-125a-5p and let-7e predicts the progression and prognosis of non-small
cell lung cancer. Cancer Invest 32, 394–401, 10.3109/07357907.2014.922569 (2014). Article CAS PubMed Google Scholar * Garbuzov, A. & Tatar, M. Hormonal regulation of Drosophila
microRNA let-7 and miR-125 that target innate immunity. Fly (Austin) 4, 306–311 (2010). Article CAS Google Scholar * Chen, T. et al. MicroRNA-125a-5p partly regulates the inflammatory
response, lipid uptake and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res 83, 131–139, 10.1093/cvr/cvp121 (2009). Article CAS PubMed ADS Google Scholar *
Potenza, N. et al. Human microRNA hsa-miR-125a-5p interferes with expression of hepatitis B virus surface antigen. Nucleic Acids Res 39, 5157–5163, 10.1093/nar/gkr067 (2011). Article CAS
PubMed PubMed Central Google Scholar * Banerjee, S. et al. miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem 288, 35428–35436,
10.1074/jbc.M112.426866 (2013). Article CAS PubMed PubMed Central Google Scholar * Rouas, R. et al. Human natural Treg microRNA signature: role of microRNA-31 and microRNA-21 in FOXP3
expression. Eur J Immunol 39, 1608–1618, 10.1002/eji.200838509 (2009). Article CAS PubMed Google Scholar * Fayyad-Kazan, H. et al. Valproate treatment of human cord blood CD4-positive
effector T cells confers on them the molecular profile (microRNA signature and FOXP3 expression) of natural regulatory CD4-positive cells through inhibition of histone deacetylase. J Biol
Chem 285, 20481–20491, 10.1074/jbc.M110.119628 (2010). Article CAS PubMed PubMed Central Google Scholar * Rossi, R. L. et al. Distinct microRNA signatures in human lymphocyte subsets
and enforcement of the naive state in CD4+T cells by the microRNA miR-125b. Nat Immunol 12, 796–803, 10.1038/ni.2057 (2011). Article CAS PubMed Google Scholar * Ma, L., Teruya-Feldstein,
J. & Weinberg, R. A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449, 682–688, 10.1038/nature06174 (2007). Article CAS PubMed ADS Google
Scholar * Camps, C. et al. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res 14, 1340–1348, 10.1158/1078-0432.CCR-07-1755 (2008).
Article CAS PubMed Google Scholar * Pulkkinen, K., Malm, T., Turunen, M., Koistinaho, J. & Yla-Herttuala, S. Hypoxia induces microRNA miR-210 _in vitro_ and _in vivo_ ephrin-A3 and
neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett 582, 2397–2401, 10.1016/j.febslet.2008.05.048 (2008). Article CAS PubMed Google Scholar * Zheng, W. & Flavell, R.
A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997). Article CAS PubMed Google Scholar * Zhu, J. et
al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol 5, 1157–1165, 10.1038/ni1128 (2004). Article CAS PubMed Google Scholar * Hosoya, T.
et al. GATA-3 is required for early T lineage progenitor development. The Journal of Experimental Medicine 206, 2987–3000, 10.1084/jem.20090934 (2009). Article CAS PubMed PubMed Central
Google Scholar * Pai, S. Y. et al. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity 19, 863–875 (2003). Article CAS PubMed Google Scholar * Wang, L. et
al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat Immunol 9, 1122–1130, 10.1038/ni.1647 (2008). Article CAS
PubMed PubMed Central Google Scholar * Wohlfert, E. A. et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest 121, 4503–4515, 10.1172/JCI57456
(2011). Article CAS PubMed PubMed Central Google Scholar * Wang, Y., Su, M. A. & Wan, Y. Y. An essential role of the transcription factor GATA-3 for the function of regulatory T
cells. Immunity 35, 337–348, 10.1016/j.immuni.2011.08.012 (2011). Article CAS PubMed PubMed Central Google Scholar * Zheng, S. G., Wang, J. & Horwitz, D. A. Cutting edge:
Foxp3+CD4+CD25+regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol 180, 7112–7116 (2008). Article CAS PubMed Google Scholar * Yao, Z. et
al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109, 4368–4375, 10.1182/blood-2006-11-055756 (2007). Article CAS PubMed PubMed Central Google Scholar * Laurence,
A. et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity 37, 209–222,
10.1016/j.immuni.2012.05.027 (2012). Article CAS PubMed PubMed Central Google Scholar * Iliopoulos, D., Hirsch, H. A. & Struhl, K. An epigenetic switch involving NF-kappaB, Lin28,
Let-7 MicroRNA and IL6 links inflammation to cell transformation. Cell 139, 693–706, 10.1016/j.cell.2009.10.014 (2009). Article CAS PubMed PubMed Central Google Scholar *
Hatziapostolou, M. et al. An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell 147, 1233–1247, 10.1016/j.cell.2011.10.043 (2011). Article CAS PubMed
PubMed Central Google Scholar * Liu, S. et al. MicroRNA93 regulates proliferation and differentiation of normal and malignant breast stem cells. PLoS Genet 8, e1002751,
10.1371/journal.pgen.1002751 (2012). Article CAS PubMed PubMed Central Google Scholar * Lloyd, C. M. & Hawrylowicz, C. M. Regulatory T cells in asthma. Immunity 31, 438–449,
10.1016/j.immuni.2009.08.007 (2009). Article CAS PubMed PubMed Central Google Scholar * Zheng, S. G. et al. CD4+ and CD8+ regulatory T cells generated _ex vivo_ with IL-2 and TGF-beta
suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol 172, 1531–1539 (2004). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS Our
research is supported by the grants from National Basic Research Program of China (973 Program) 2014CB541803 and 2014CB541903, National Science Foundation of China 31200647, 81330072,
31370863, 31170825, 81270083, 31200646, 81271835, 81302532 and 31300711, Shanghai Postdoctoral Sustentation Fund 12R21417100, China Postdoctoral Science Foundation 2012M520946 and National
Science and Technology Major Project Grants 2012ZX10002007-003, 2013ZX10003009–002, SMCST 11ZR1404900 and 14JC1406100. We gratefully acknowledge the Knowledge Innovation Program of Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences 2012KIP204. AUTHOR INFORMATION Author notes * Li Dan and Kong Chao contributed equally to this work. AUTHORS AND AFFILIATIONS
* Key Laboratory of Molecular Virology & Immunology, Unit of Molecular Immunology, Institut Pasteur of Shanghai, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences,
Shanghai, 200031, China Dan Li, Chao Kong, Andy Tsun, Chen Chen & Bin Li * Shanghai Key Laboratory of Bio-energy Crops, College of Life Science, Shanghai University, Shanghai, 200444,
China Chao Kong * Department of Pulmonary Medicine, Rui Jin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200025, China Huihui Song & Guochao Shi * State Key
Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China Wen Pan, Dai Dai & Nan Shen * Joint
Molecular Rheumatology Laboratory of the Institute of Health Sciences and Shanghai Renji Hospital, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences and Shanghai
Jiaotong University School of Medicine, Shanghai, 200025, China Wen Pan, Dai Dai & Nan Shen * Division of Rheumatology and the Center for Autoimmune Genomics and Etiology (CAGE),
Cincinnati Children’s Hospital Medical Center, Cincinnati, 45229, OH, USA Nan Shen * Department of Genetics, Yale University School of Medicine, New Haven, 06520, CT, USA Wen Pan Authors *
Dan Li View author publications You can also search for this author inPubMed Google Scholar * Chao Kong View author publications You can also search for this author inPubMed Google Scholar *
Andy Tsun View author publications You can also search for this author inPubMed Google Scholar * Chen Chen View author publications You can also search for this author inPubMed Google
Scholar * Huihui Song View author publications You can also search for this author inPubMed Google Scholar * Guochao Shi View author publications You can also search for this author inPubMed
Google Scholar * Wen Pan View author publications You can also search for this author inPubMed Google Scholar * Dai Dai View author publications You can also search for this author inPubMed
Google Scholar * Nan Shen View author publications You can also search for this author inPubMed Google Scholar * Bin Li View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS D.L. designed the research; B.L. supervised the research; D.L., C.K. and A.T. conducted the experiments; D.L. wrote the manuscript; A.T. revised the manuscript;
H.S. and G.S. provided clinical samples; C.C., W.P., D.D. and N.S. helped the experiments. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other
third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative
Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Li, D., Kong, C., Tsun, A. _et al._ MiR-125a-5p Decreases the Sensitivity of Treg cells Toward IL-6-Mediated Conversion by
Inhibiting IL-6R and STAT3 Expression. _Sci Rep_ 5, 14615 (2015). https://doi.org/10.1038/srep14615 Download citation * Received: 20 March 2015 * Accepted: 02 September 2015 * Published: 01
October 2015 * DOI: https://doi.org/10.1038/srep14615 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative