Play all audios:
ABSTRACT Arbuscular mycorrhiza fungi (AMF) can colonize the roots of _Amorpha fruticosa_, a perennial leguminous woody shrub and form arbuscular mycorrhiza (AM). AMF have significant
promoting effects on _A. fruticosa_ growth as the intensity of fungal colonization increases. Taking AMF-_A. fruticosa_ symbionts as the experimental material, gel-free isobaric tags for
relative and absolute quantification (iTRAQ) coupled with two-dimensional liquid chromatography-tandem mass spectrometry (LC-MS/MS) were used to investigate the expression of _A. fruticosa_
mycorrhizal proteins at the maturation stage. A total of 3,473 proteins were identified, of which 77 showed dramatic changes in their root expression levels; 33 increased and 44 decreased.
We also found nine AMF proteins that were expressed with AMF treatment. The 77 proteins were classified according to function. Plant proteins were assigned into 11 categories:
metabolism-related (32%), protein folding and degradation-related (22%), energy-related (10%), protein synthesis-related (8%), stress and defense-related (24%), transcription-related (6%),
membrane and transport-related (4%), cellular structure-related (2.5%), signaling transduction-related (11%) and unknown proteins (5%). The results of the study provide a foundation for
further investigation of the metabolic characteristics and molecular mechanisms of AM. SIMILAR CONTENT BEING VIEWED BY OTHERS METABOLOMICS AND TRANSCRIPTOMICS TO DECIPHER MOLECULAR
MECHANISMS UNDERLYING ECTOMYCORRHIZAL ROOT COLONIZATION OF AN OAK TREE Article Open access 21 April 2021 TISSUE-SPECIFIC SIGNATURES OF METABOLITES AND PROTEINS IN ASPARAGUS ROOTS AND
EXUDATES Article Open access 01 April 2021 COMPARATIVE SINGLE-NUCLEUS RNA-SEQ ANALYSIS REVEALED LOCALIZED AND CELL TYPE-SPECIFIC PATHWAYS GOVERNING ROOT-MICROBIOME INTERACTIONS Article Open
access 03 April 2025 INTRODUCTION Arbuscular mycorrhiza (AM), representing the most widely distributed mutualistic root symbiosis in nature, are the result of long-term evolution between
plant and soil fungi. AM fungi (AMF) are obligate symbionts and are recalcitrant to pure culture on synthetic media; they grow only in living plants. Their hereditary variability and
heterogeneous characteristics are essential for colonizing a large number of potential host plants. However, different host plants may simultaneously induce the expression of different
symbiosis-related genes. AMF are some of the most widespread microorganisms and they can form symbionts with more than two-thirds of the vascular plants in natural or artificial ecosystems.
These plants include important agricultural species, such as wheat, rice and the model plant _Populus trichocarpa_1,2. The foundation of mycorrhizal symbiosis is the ability of AMF, using
their multicore hyphae, to provide nutrients (especially phosphorous) to host plants that have long-distance illiquidity3. New physiological and molecular evidence has shown that, for
phosphorus, the mycorrhizal pathway (MP) is operational regardless of plant growth responses. Meanwhile, the contribution of the direct pathway (DP) is decreased, which results in a greater
dependence of host plants on the nutrients that AMF provide4. AMF can utilize only simple carbon and nitrogen sources from their hosts to complete their life cycles. This may be due to the
loss of some enzyme-encoding genes and to macromolecular synthesis defects that have arisen during the long-term evolution of symbiosis with plants5,6. AM play a significant role in
promoting the growth of host plants and researchers have increased their efforts to study the interactions between AMF and host plants. Recorbet and colleagues have compared the root
proteome responses of _Medicago truncatula_ upon colonization with two AM fungi, i.e., _Glomus mosseae_ (GM) and _G. intraradices_, using two-dimensional electrophoresis (2-DE)7. They found
42 symbiosis proteins; of these, 32 could be confidently identified and retrieved following MS/MS and matching with a database encompassing 21 fungal proteins. To test the mechanisms by
which shoots of Cd-treated mycorrhizal plants avoid metal toxicity, Aloui has performed a 2-DE/MALDI-TOF-based comparative proteomic analysis of the _M. truncatula_ shoot responses upon
mycorrhization and Cd exposure8. finding that Cd triggers an opposite response than mycorrhization, which is coupled with an increase in molecular chaperones in the shoots of mycorrhizal
plants relative to those that are metal-free. Wang has studied the dynamic changes in maize leaf protein expression profiles under AMF colonization9. In that study, the differentially
expressed proteins in maize leaves were separated by 2-DE and the results reveal 21 differentially expressed gel spots in maize leaves. Among them, 8 proteins were successfully identified.
With the development of molecular biology techniques, quantitative analysis of the differences in protein expression profiles during the colonization process of pathogenic or symbiotic
microorganisms has become possible; these techniques have played a critical role in analyzing pathogenic mechanisms. Isobaric tags for relative and absolute quantification (iTRAQ) represents
one of the new and powerful techniques for simultaneous analysis of multiple samples and provide relative quantification of hundreds of proteins. iTRAQ reagents produce high-quality,
reproducible results from complex samples and iTRAQ has thus become widely used. However, iTRAQ-based studies on the symbiotic mechanisms of AMF and host plants have rarely been reported.
Our group has studied the symbiotic relationship between plants and fungi at the mRNA level, Zhang xingxing identified 30 symbiosis-related genes expressed in _Amorpha fruticosa_ roots
colonized by GM at different stages by using mRNA differential-display PCR (DDRT-PCR). The expressed genes were confirmed by reverse Northern blotting. Eleven fragments were sequenced and
putatively identified by homologous alignment and these genes were found to relate to defense and signal transduction10. Kong xiangshi also has found 47 symbiosis-related unigenes during AMF
treatment by using suppression subtractive hybridization (SSH) and subsequent Gene Ontology (GO) database, BLAST annotation and literature searches to categorize each of the identified
genes. Among the expressed genes, those related to plant metabolism and stress and defense show important roles during the symbiotic process of AMF-_A. fruticosa_11. Based on our previous
work, we have continued to use _A. fruticosa_, a perennial leguminous woody shrub plant, as a host. Using AMF-_A. fruticosa_ symbionts as the experimental materials, we used iTRAQ combined
with 2-D LC-MS/MS to investigate the expression of _A. fruticosa_ mycorrhizal proteins at the maturation stage. The results of the study provide a theoretical basis for the further analysis
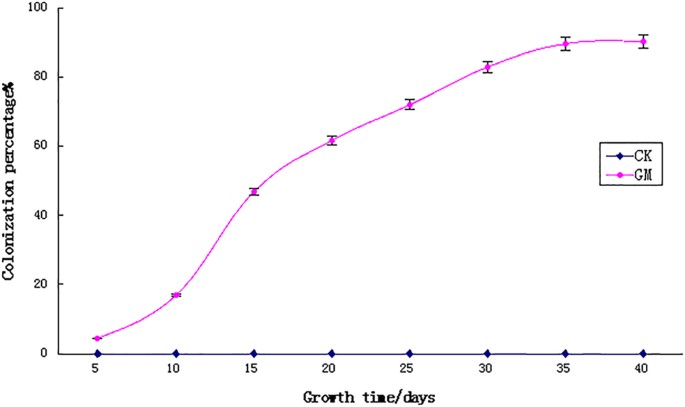
of the metabolic characteristics and molecular mechanisms of symbiosis between AMF and _A. fruticosa_. RESULTS AMF COLONIZATION The colonization percentage of _A. fruticosa_ roots is shown
in Fig. 1. At day 5, the roots were relatively small and only a few hyphae were detected. Most of the spores were not in contact with the host roots and were mainly in their vegetative
growth stage12,13. The infection rate began to increase at day 10 and the growth status of GM-inoculated seedlings was significantly better than that of non-inoculated plants. The
colonization percentage increased rapidly at day 15. At that time, there was a large quantity of mycelium-infected roots that increased over time. The colonization percentage reached its
peak at the vigorous phase (i.e., 30 days) and a large number of vesicles and arbuscules were observed within the roots. At 40 days, we observed that the hyphae had extended from the plant
root surface and had infected neighboring roots. No colonization by AM was observed in the non-inoculated plants, because the mixed soil used for culture had been autoclaved thoroughly.
IDENTIFICATION OF SYMBIOSIS-RELATED PROTEINS USING ITRAQ LC-MS/MS The roots of _A. fruticosa_ changed their protein levels when colonized by GM and mutualistic symbionts formed. Using an
iTRAQ approach, 86 differentially expressed symbiosis-related proteins were successfully identified. Among them, 77 were plant proteins, with 33 proteins showing increases and 44 showing
decreases (Table 1) and 9 were fungal proteins (Table 2). More detailed information in supplementary information. AMF proteins play an import role in symbiotic systems, but they show high
expression in only the AMF themselves. The low overall concentration of AMF proteins and the limitations of the technology, resulted in few AMF proteins being detected14. CLASSIFICATION OF
SYMBIOSIS-RELATED PROTEINS The GO database, BLAST annotations and information reported in the literature were used to categorize each of the identified proteins11. The 77 differentially
expressed proteins in _A. fruticosa_ were categorized into different functional classes and assigned to 11 categories. The functional categories are shown in Fig. 2; they include
metabolism-related (32%), protein folding and degradation-related (22%), energy-related (10%), protein synthesis-related (8%), stress and defense-related (24%), transcription-related (6%),
membrane and transport-related (4%), cellular structure-related (2.5%), signaling transduction-related (11%) and unknown (5%). Among these classes, proteins related to plant metabolism,
protein folding and degradation and energy (totaling 64% of the identified proteins) play important roles during the symbiotic process of AMF-_A. fruticosa._ DISCUSSION Previous studies have
demonstrated that colonization is a multi-step, genetically regulated process under the control of specific loci15,16. AMF interact with host plants as cell walls, cell membranes and
cellular components undergo dramatic changes17. During the colonization process, functional proteins are induced to express and regulate this process, ultimately forming stable mutualistic
symbionts. SIGNALING-RELATED PROTEINS Mutualistic symbionts are the result of a mutual recognition and interaction process between AMF and plant signaling molecules. During the colonization
process, signal transduction occurs so that the symbiotic partners recognize each other and the host plants decrease their defense responses. At the same time, AMF are prepared to colonize
and to form appressoria and, subsequently, to form arbuscules, vesicles and spores. Because of their mutual nutritional relationship, a real-time dynamic signal dialogue between fungi and
host plants is continually present. In this study, we found that the protein levels of Rho GDP-dissociation inhibitor 1 and somatic embryogenesis receptor-like kinase were significantly
increased in the symbiotic roots. Rho GDP-dissociation inhibitor 1, a regulator of Rho GTPase, regulates the balance of Rho GTPase bound to GTP or GDP. There are 2 conformational states of
Rho GTPase: the GTP-bound ‘active’ state and the GDP-bound ‘inactive’ state, in which GTP has been hydrolyzed to GDP18. As a member of the subfamily of small G proteins, Rho GTPase regulates
a number of important signal-transduction pathways in eukaryotic cells. Rho GTPase, called Rop (Rho-related GTPase) in plants, has different isomers in animals and fungi19. Rho GTPases are
widely distributed in plants and the corresponding genes in _Arabidopsis_, maize, barley, rice, peas and alfalfa have been cloned20,21,22. Rho GTPases participate in the regulation of a
variety of cellular processes, e.g., gene expression, cell wall synthesis, H2O2 production, actin rearrangement processes, signal transduction pathways of MAP kinase23,24 and cytoskeletal
assembly and reassembly, to produce a variety of cellular responses. As a regulatory factor, RhoGDI1 was significantly increased in _A. fruticosa_ AM. Clearly, this protein is closely
related to signal transduction between _A. fruticosa_ and GM. Multiple somatic embryogenesis receptor-like kinases (SERKs) have been defined, including the leucine-rich repeat receptor-like
kinase (LRR-RLK) subfamily members and a family of transmembrane signal-transduction proteins25. They are characterized by a predicted signal sequence, a single transmembrane region and a
cytoplasmic kinase domain. These features suggest that some SERK family protein kinases may play pivotal roles in communication between cells and the environment or in cell-cell
interactions. Currently, SERK genes have been cloned from various plant species. The AtSERK3 gene participates in the brassinolide (brassinosteroid, BR) signal-transduction pathway. BR is an
important hormone that regulates plant growth and development. Functional analysis has shown that the _Arabidopsis thaliana_ mutant became a dwarf when the AtSERK3 gene is knocked out26.
The overexpression of the OsSERK1 gene in rice cultivars leads to an increase in host resistance to blast fungus27; in contrast, transcripts of the lettuce LsSERK gene not only are decreased
in _in vitro_ somatic embryonic structures but also easily infect _Sclerotinia_28. Studies have also shown that the SERK gene is closely related to antibiotic stress. Plant root
colonization by AMF results in increased levels of somatic embryogenesis receptor-like kinase, which plays a major role in promoting plant growth and enhancing plant disease resistance.
STRESS AND DEFENSE-RELATED PROTEINS Inoculation with AMF has strong growth-promoting effects on _A. fruticosa_, especially at the mature stage of symbiont formation. These effects are
mediated by increased action of SERK in BR signal-transduction pathways, which have a key role in the regulation of autoimmune responses and of plant root cell elongation and division.
However, such regulation is not determined by a single factor. At an early stage of symbiosis, a weak defense response emerges when roots are stimulated by AMF colonization. Lectin plays a
crucial role in this defense response by recognizing and binding to the sugar molecules of intruders and interfering with their function on plants. Many plant lectins can bind to glucose,
mannitol, galactose or other monosaccharides and they exhibit high affinity to the oligosaccharides of alien plants. Studies have shown that lectins on leguminous tree surfaces can gather
rhizobia around the roots29. As AMF infect the roots of _A. fruticosa_, plant defense responses are initiated, resulting in agglutinin-2 accumulation. Agglutinin-2 is an important factor for
the identification of AMF, similarly to rhizobia. When _A. fruticosa_ is colonized by AMF, the abscisic acid (ABA) content increases rapidly, leading to the closing of plant stomata and
decreased transpiration; this response also activates the genes encoding soluble osmolytes, thus decreasing stress injuries and the impact of stress-induced reactive oxygen and ethylene30.
Therefore, ABA accumulation may stimulate metabolic enzymes to produce a feedback effect31. The major ABA catabolic route is decomposition via ABA 8′-hydroxylase to form phaseic acid.
Therefore, ABA 8′-hydroxylase accumulation in _A. fruticosa_ may represent a mechanism for regulating ABA levels. In multiple rice mapping populations, germin-like protein (GLP) markers have
been associated with quantitative trait loci (QTL) for resistance to rice blast pathogens. At the early stage of rice blast fungus infection or mechanical damage, some OsGLPs are
transiently induced and expressed. Varying 5′ regulatory regions and the differential expression of some protein family members between resistant and susceptible cultivars correspond with
differential hydrogen peroxide (H2O2) accumulation levels after fungal infection32. Wang discovered a new wheat germin-like protein33 that is up-regulated in both resistant and susceptible
plants. It has been speculated to be involved in wheat defense responses. GLP is significantly increased at the early stage of AMF infection in roots of _A. fruticosa_ and it may participate
in biotic stress responses. PROTEIN FOLDING AND DEGRADATION-RELATED PROTEINS During the symbiosis process, the modification and degradation of peptides and proteins are critical for
maintaining cell function. Protein disulfide isomerase, bi-ubiquitin, serine carboxypeptidase, proteasome subunit beta type-6 and subtilisin-like protease SDD1 accumulate in plant roots to
ensure proper cell function. Plants use the proteasome pathway for selective protein degradation and the proteasome plays pivotal roles in removing abnormally modified proteins and
non-targeted proteins. Interactions between bi-ubiquitin and proteasome subunit beta type-6 provide an effective way to degrade proteins. Bi-ubiquitin is highly conserved in eukaryotes and
it is covalently bound to target proteins through post-translational modification to mediate degradation. Serine carboxypeptidase (SCP), an enzyme that catalyzes the hydrolysis of proteins
in eukaryotes, has been found in rice, _Arabidopsis_ and peas. It has been shown that SCP has broad functions in plants, including protein turnover and secondary metabolism synthesis and it
plays an important role in improving plant stress resistance. Liu showed that the expression of _OsBISCPL1_ was induced by rice blast fungi and antiviral signaling molecules (salicylic acid
and jasmonic acid)34 and that overexpression of _OsBISCPL1_ could enhance disease resistance, oxidative stress tolerance and ABA sensitivity in transgenic _Arabidopsis_ plants. _OsBISCPL1_
is expressed ubiquitously and differentially in rice and it is induced by antiviral signaling molecules (BTH, JA, SA and ACC) and is up-regulated by incompatible interactions between rice
and the blast fungus. Liu has shown that the expression of the _ZmSCP_ gene in corn is up-regulated under induction by _Rhizoctonia solani_ and that the ZmSCP protein are associated with
various abiotic stresses35. The subtilisin-like protease SDD1 is a member of the processing-type proteases in eukaryotes. As a preproprotein, it can direct peptides for transport to the
cytoplasm. SDD1 is a crucial gene that regulates stomatal development and encodes a subtilisin-like serine protease. As a processive enzyme, it may activate a protein molecule or a signal
that directs receptors into contact with epidermal cells during stomatal development processes. Liang has shown that the serine protease-encoding gene _SDD1_ is widely expressed acts on the
development of stomata and is also necessary for normal root development36. Protein disulfide isomerase (PDI), a multifunctional protein, is distributed widely in eukaryotic organisms and is
involved in modifying/folding newly synthesized proteins. The catalytic thiol-disulfide exchange reaction to form disulfide is involved in many physiological processes, such as auxiliary
protein folding in the endoplasmic reticulum, reconstruction of misfolded proteins and the repair and refolding of damaged proteins under stress37. Additionally, as a chaperone, PDI can
assemble heterogeneous protein peptides and regulate disulfide bonds in an ATP-dependent manner and it may also be closely related to sugar transport, protein synthesis and other metabolic
processes in eukaryotic organisms. ENERGY-RELATED PROTEINS During the symbiosis process, dihydrolipoyl dehydrogenase, aldehyde dehydrogenase and isocitrate dehydrogenase [NAD] regulatory
subunit 1 accumulated in plant roots. AMF colonization significantly enhances the energy metabolism of plants. The Krebs cycle provides more energy than glycolysis and it is an important
pathway not only an important for sugar metabolism but also for the metabolism of lipids, proteins and nucleic acids, which are eventually oxidized to carbon dioxide and water. Isocitrate
dehydrogenase (IDH) is considered to be the rate-limiting enzyme of the Krebs cycle; it catalyzes decarboxylation to ketoglutarate while reducing NAD+ to NADH38. Therefore, the activity of
NAD-IDH has a significant impact on cellular metabolism. Isocitrate dehydrogenase [NAD] regulatory subunit 1, a regulatory factor, controls the activity of NAD-IDH and thus affects metabolic
activity. Kuhlemeier has explored the energy metabolism of tobacco pollen and has found that, in vegetative tissues39, pyruvate enters the Krebs cycle by pyruvate dehydrogenase (PDH);
however, in reproductive organs, it is converted to acetaldehyde by pyruvate decarboxylase (PDC) and then enters the Krebs cycle via aldehyde dehydrogenase (ALDH) and acetyl coenzyme A
synthetase (ACS). Thus, ALDH plays an important role in the pyruvate metabolism pathway of PDC/ALDH/ACS. Under stress conditions, plant cells quickly accumulate excessive reactive oxygen
species (ROS), which cause oxidative stress and result in the accumulation of large amounts of toxic substance and eventually in plant death40. Aldehydes are an important component of
peroxidation reaction products and they play a crucial role in the oxidation of carboxylic aldehydes, the removal of toxic aldehydes and the reduction of lipid peroxidation, thereby
improving plant tolerance41. As an important member of the pyruvate dehydrogenase family, dihydrolipoyl dehydrogenases ensure the production of oxidatively decarboxylated pyruvate CoA and
CoA then enters the Krebs cycle to produce large amounts of energy for plant growth. CELLULAR STRUCTURE-RELATED PROTEINS Dramatic changes in plant morphology and in the penetrating mycelium,
dynamic reorganization of cytoskeletal elements and organelle transformation occur when arbuscular vesicles develop42. Tubulin is an important component of the cytoskeleton and it plays an
important role in maintaining intracellular structural order and cell morphology. Meanwhile, tubulin is closely related to cellular transport, cell differentiation, cell motility, signal
recognition, cell division and other developmental activities. Mills has revealed dramatic changes in both microtubules and actin arrangement in the host cell and further studies have found
that microtubules and actin rearrangement in the host cell are necessary for expression in non-host plants43. Studies on plant tubulin have primarily been focused on annual plants, such as
_Arabidopsis_, tobacco and rice, but study of the tubulin gene in perennial trees has been rare44. MEMBRANE AND TRANSPORT-RELATED PROTEINS A K+ efflux antiporter and coatomer, which are
membrane and transport-related proteins, respectively, were found in the _A. fruticosa_ mycorrhizea. The K+ efflux antiporter is mainly responsible for maintaining the intracellular ion
balance and regulating the cells’ osmotic pressure. During AMF colonization, AMF invasion affects the ion balance of plant root cells and plants maintain the intracellular ion balance to
stimulate K+ increases. Coatomer, a coat protein, transports vesicles and vesicle-mediated non-selective transport ensures the accurate transport of proteins and lipids. METABOLISM-RELATED
PROTEINS During mycorrhizal symbiosis, increased levels of 3-oxoacyl-[acyl-carrier-protein] synthase, neutral ceramidase and caffeic acid 3-O-methyltransferase (COMT), which are
metabolism-related proteins, were observed. Lipid metabolism is one of the basic metabolic pathways in plants. The β-ketoacyl-acyl carrier protein synthase (KASI)-mediated acyl chain
extension is important in the _de novo_ synthesis of fatty acids. Ceramides, which are central molecules in the sphingolipid signaling pathway, play important roles as second messengers in
plants and participate in many significant plant signaling pathways, such as cell growth, proliferation, differentiation, senescence and apoptosis45. Neuraminidase is a key enzyme that
regulates ceramide. Neutral neuraminidase hydrolyzes ceramide to form sphingosine (ref). Liu has found that AtCER is involved in H2O2-induced oxidative stress46. During cell morphogenesis,
lignin plays an important role in the growth and development of vascular tissues and is involved in cell wall lignification, which increases the hardness or compressive strength of the cell
wall. It also promotes the formation of mechanical tissues while also having a major impact on plant lodging, disease and stress resistance47. There are 3 types of monomeric lignin
biosynthesis pathways,: the shikimate pathway, the phenylketonuria pathway and the lignin biosynthesis-specific pathway48. COMT is a key enzyme in the specific lignin pathway and is involved
in the synthesis of S-lignin49. AMF colonization enhanced the synthesis of woody _amorpha_ lignin, thus affecting the growth and development of plants. TRANSCRIPTION AND PROTEIN
SYNTHESIS-RELATED PROTEINS Ribosomal proteins are important components of the ribosome and they have important roles in translation efficiency and ribosome stability. They also participate
in important cellular processes, such as DNA repair, apoptosis and regulation of gene expression; e.g., 40S ribosomal proteins showed significant accumulation in plant roots after AMF
invasion. Nascent polypeptide-associated complex subunit alpha (NAC), which is located at the top of the newly synthesized polypeptide, can reversibly bind to eukaryotic ribosomes and guide
the correct distribution and translocation of newly synthesized polypeptides in the cell. The observed increases in 40S ribosomal protein and NAC levels, combined with folding- and
degradation-associated proteins, ensure the fast and accurate synthesis and distribution of AM symbiosis-related proteins. Transcriptional regulation is an important aspect of the regulation
of gene expression. The results show significant accumulation of histone H3 and histone H4 in the host plant roots. Nucleosomes constitute the basic unit of chromatin in eukaryotes.
Histones, which are structural proteins of chromosomes, play important roles in DNA folding and packing, protecting DNA from digestive enzymes and gene regulation, tumor formation and
apoptosis. The N-terminal amino acids of histones participate in acetylation, methylation, phosphorylation, ubiquitination and other covalent modifications. Studies have shown that histones
may change the structure of chromatin via post-translational modifications, thus modulating gene expression50. UNKNOWN PROTEINS During AMF symbiosis, the expression levels of proteins within
_A. fruticosa_ roots were changed; some proteins disappeared and new symbiosis proteins arose. The functional analysis of symbiotic proteins in _A. fruticosa_, a non-model plant, is not
difficult. Because these proteins were differentially expressed in the symbiotic system, they are targets for future studies. AM-A. FRUTICOSA MOLECULAR REGULATION MODEL By using
bioinformatics analysis, we found that mycorrhizal proteins were involved in several biological processes and cellular activities (Fig. 3) and we verified that the symbiosis formed between
AMF and _A. fruticosa_ is a uniform and harmonious result of symbiotic interactions. METHODS _G. mosseae_ (GM) was harvested from sorghum, which was supplied by the Ecology Laboratory of
Heilongjiang University, by co-culturing for longer than 40 days. Inocula contained a mixture of the rhizosphere that consisted of AM fungal spores, hyphae and mycorrhizal fragments. The
inocula contained approximately 500 spores per 20 g. SEEDLING CULTURE Seeds of _A. fruticosa_ were purchased from the Academy of Agricultural Sciences of Heilongjiang Province. _A.
fruticosa_ seeds were sterilized with 0.4% K2MnO4 for 20 min, rinsed and then covered with a layer of white gauze to keep them moist. Germination was conducted in an incubator at 30 °C for
60 h after soaking for 24 h. The growing medium was 50% peat soil, 30% vermiculite and 20% sand. It was sterilized in an autoclave at 121°C for 2 h and then air dried for 1 week before the
start of the experiments10,11. MYCORRHIZAL COLONIZATION PERCENTAGE DETERMINATION The germinated seeds were then planted in a pre-sterilized mixed matrix and grown under a 16-h photoperiod at
temperatures of 25/18 °C (day/night) with 60% relative humidity. One group was inoculated with GM inoculum and the other was inoculated with sterilized inoculum as a control (CK). Each
treatment was repeated 10 times. A total of 20 pots were arranged randomly and watered every 2 days. The mycorrhizal colonization percentage of the seedlings was determined using the Phillip
and Hayman staining method (KOH bleaching-acid fuchsin stain) with some modifications (Phillips J M, 1970)51. PROTEIN EXTRACTION, PROTEIN QUANTIFICATION AND SDS-PAGE At the maturation
stage, _A. fruticosa_ roots were harvested and total root protein was precipitated with 10% (w/v) trichloroacetic acid (TCA) in acetone at −20 °C overnight. After centrifugation at 40,000 ×
g at 4 °C for 1 h, the pellets were washed 3 times with cold 80% acetone. A 2-D Quant kit (GE Healthcare, USA) was used to determine the protein concentrations. SDS-polyacrylamide gel (12%)
electrophoresis was performed with 30-μg samples at 120-V constant voltage for 2 h. The gel was stained with Coomassie blue and visualized52,53. ITRAQ LABELING The CK group and the GM group
each included 3 biological replicates. After digesting with trypsin, the proteins from the non-infected and infected samples were labeled with iTRAQ reagents 115 (CK1), 116 (CK2), 117 (CK3),
118 (GM1), 119 (GM2) and 121 (GM3) and were then combined following the manufacturer’s protocol at a ratio of 1:1:1:1:1:1 for LC-MS/MS analysis54,55. LC-MS/MS MEASUREMENTS The labeled
samples were pooled and purified using a strong cation-exchange chromatography (SCX) column and were then separated on an analytical column (1.7 μm, 100 μm × 100 mm) at a flow rate of 300
nL/min using a linear gradient of 5–35% acetonitrile (ACN) over 40 min. The ion spray voltage was 4.5 kV and nitrogen was used as a nebulizing gas (30 psi) and a curtain gas (15 psi). From
each MS scan, the 30 most intense precursor ions were selected for MS/MS fragmentation and were detected at a mass resolution of 30,000 at m/z 40056. Data analysis was performed with a
Triple TOF 5600 System and then the iTRAQ data were compared with the protein sequences of homologous species after genome annotation. PROTEIN IDENTIFICATION Protein Pilot 4.0 (AB Sciex
Inc., USA) was used to simultaneously identify and quantify proteins57,58. Differentially expressed proteins were required to satisfy 3 conditions for identification: (1) each confident
protein identification involved at least 1 unique peptide; (2) the _P_-value was less than 0.05; and (3) changes of greater than 1.2-fold or less than 0.8 fold were considered significant.
All of the identified proteins were classified according to the annotations acquired by using the UniProt knowledge base and the GO database. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE:
Song, F. _et al._ Proteomic analysis of symbiotic proteins of _Glomus mosseae_ and _Amorpha fruticosa_. _Sci. Rep._ 5, 18031; doi: 10.1038/srep18031 (2015). REFERENCES * Fitter, A.,
Heinemeyer, A. & Staddon, P. The impact of elevated CO2 and global climate change on arbuscular mycorrhizas: a mycocentric approach. New Phytologist. 147, 179–187 (2000). Article CAS
Google Scholar * Rosendahl, S. Populations and individuals of arbuscular mycorrhizal fungi. New Phytologist. 178, 253–266 (2008). Article Google Scholar * Smith, S. & Read, D.
Mycorrhizal symbiosis from cellular to ecosystem scales. Annual review of plant biology. 62, 227–250 (2008). Article Google Scholar * Smith, S. E. & Smith, F. A. Roles of arbuscular
mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annual review of plant biology. 62, 227–250 (2011). Article CAS Google Scholar * Kiers, E. T.
et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science. 333, 880–882 (2011). Article CAS ADS Google Scholar * Trépanier, M. et al. Dependence of
arbuscular-mycorrhizal fungi on their plant host for palmitic acid synthesis. Applied and environmental microbiology. 71, 5341–5347 (2005). Article Google Scholar * Recorbet, G. et al.
Identification of in plant-expressed arbuscular mycorrhizal fungal proteins upon comparison of the root proteomes of _Medicago truncatula_ colonised with two Glomus species. Fungal Genetics
and Biology. 47, 608–618 (2010). Article CAS Google Scholar * Aloui, A. et al. Arbuscular mycorrhizal symbiosis elicits shoot proteome changes that are modified during cadmium stress
alleviation in _Medicago truncatula_. BMC plant biology. 11, 75 (2011). Article CAS Google Scholar * Wang, Z. H., Yuan, K. & Yang, L. F. Identification and Functional Analysis of
Maize Leaf Proteins Responding to the Abuscular Mycorrhizal Fungi (AMF). Chinese Journal of Tropical Agriculture. 33, 40–44 (2013). Google Scholar * Song, F. Q., Li, J. Z. & Zhang, X.
X. Characterization of expressed genes in the establishment of arbuscular mycorrhiza between _Amorpha fruticosa_ and _Glomus mosseae_. Journal of Forestry Research. 25, 541–548 (2014).
Article CAS Google Scholar * Song, F. Q., Kong, X. S., Li, J. Z. & Chang, W. Screening the related genes in the AM Fungi and _Amorpha fruticosa_ symbiosis with the subtractive
hybridization technique. Scienta Slivae Sinicae. 50, 64–74 (2014). Google Scholar * Heikham, E., Rupam, K. & Bhoopander, G. Arbuscular mycorrhizal fungi in alleviation of salt stress: a
review. Annals of Botany. 104, 1263–1280 (2009). Article Google Scholar * Song, F. Q., Kong, X. S., Dong, A. R. & Liu, X. F. Impact of arbuscular mycorrhizal fungi on the growth and
related physiological indexes of _Amorpha fruticosa_. Journal of Medicinal Plants Research. 6, 3648–3655 (2012). CAS Google Scholar * Claudia, H. & Helge, K. A roadmap of cell-type
specific gene expression during sequential stages of the arbuscular mycorrhiza symbiosis. BMC Genomics. 14, 306–324 (2013). Article Google Scholar * Bonfante, P. et al. The _Lotus
japonicus LjSym4_ gene is required for the successful symbiotic infection of root epidermal cells. Molecular plant-microbe interactions: MPMI. 13, 1109–1120 (2000). Article CAS Google
Scholar * Novero, M. et al. Dual requirement of the _LjSym4_ gene for mycorrhizal development in epidermal and cortical cells of _Lotus japonicus_ roots. New phytologist. 154, 741–749
(2002). Article CAS Google Scholar * Bonfante, P. et al. At the interface between mycorrhizal fungi and plants: the structural organization of cell wall, plasma membrane and cytoskeleton.
Fungal Associations. 9, 45–61 (2001). Article CAS Google Scholar * Etienne Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature. 420, 629–635 (2002). Article CAS ADS
Google Scholar * Li, H. et al. Arabidopsis Rho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiology. 118, 407–417 (1998).
Article CAS Google Scholar * Winge, P., Brembu, T., Kristensen, R. & Bones, A. M. Genetic structure and evolution of RAC-GTPases in _Arabidopsis thaliana_. Genetics. 156, 1959–1971
(2000). CAS PubMed PubMed Central Google Scholar * Christensen, T. M. et al. Conserved subgroups and developmental regulation in the monocot rop gene family. Plant physiology. 133,
1791–1808 (2003). Article CAS Google Scholar * Zheng, Z. L. & Yang, Z. The Rop GTPase: an emerging signaling switch in plants. Plant molecular biology. 44, 1–9 (2000). Article CAS
Google Scholar * Takai, Y., Sasaki, T. & Matozaki, T. Small GTP-binding proteins. Physiological reviews. 81, 153–208 (2001). Article CAS Google Scholar * Yang, Z. et al. Small
GTPases versatile signaling switches in plants. The Plant Cell. 14, 375–388 (2002). Article ADS Google Scholar * Shiu, S. H. & Bleecker, A. B. Receptor-like kinases from _Arabidopsis_
form a monophyletic gene family related to animal receptor kinases. Proceedings of the National Academy of Sciences. 98, 10763–10768 (2001). Article CAS ADS Google Scholar * Noguchi, T.
et al. Brassinosteroid-insensitive dwarf mutants of _Arabidopsis_ accumulate brassinosteroids. Plant Physiology. 121, 743–752 (1999). Article CAS Google Scholar * Hu, H., Xiong, L. &
Yang, Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta. 222, 107–117 (2005). Article CAS Google
Scholar * Santos, M. et al. Suppression of SERK gene expression affects fungus tolerance and somatic embryogenesis in transgenic lettuce. Plant Biology. 11, 83–89 (2009). Article CAS ADS
Google Scholar * Yin, A. H. & Han, S. F. Relationship of Nodulation with Reactions of Letins of Leguminous Trees with EPS of _Rhizobium_. Journal of Nanjing Forestry University. 29,
88–90 (2005). Google Scholar * Verslues, P. & Zhu, J. Plant signaling from genes to biochemistry. Biochemical Society Transactions. 33 (2005). * Cutler, A. J. & Krochko, J. E.
Formation and breakdown of ABA. Trends in plant science. 4, 472–478 (1999). Article CAS Google Scholar * Davidson, R. M. et al. Rice germin-like proteins: Allelic diversity and
relationships to early stress responses. Rice. 3, 43–55 (2010). Article Google Scholar * Wang, J. M. et al. Cloning and Chromosome Mapping of a Germin-Like Protein Gene in Wheat and Its
Expression in Response to Infection with Wheat Powdery Mildew. Scientia Agricultura Sinica. 42, 3104–3111 (2009). CAS Google Scholar * Liu, H. et al. A rice serine carboxypeptidase-like
gene _OsBISCPL1_ is involved in regulation of defense responses against biotic and oxidative stress. Gene. 420, 57–65 (2008). Article CAS Google Scholar * Liu, L. et al. Cloning and
Expression Analysis of Serine Carboxypeptidases in Maize (_Zea mays L_.). Acta Agronomica Sinica. 39, 164–171 (2013). Article CAS Google Scholar * Liang, K. et al. Identification and
Genetic Analysis of Two Putative SDD1 Alleles in _Arabidopsis_. Chin Bull Bot. 3, 318–326 (2010). Google Scholar * Kim, Y. J. et al. Protein disulfide isomerase-like protein 1-1 controls
endosperm development through regulation of the amount and composition of seed proteins in rice. PlOS one. 7, 44493 (2012). Article ADS Google Scholar * Chen, R. D. & Gadal, P.
Structure, functions and regulation of NAD and NADP dependent isocitrate dehydrogenases in higher plants and in other organisms. Plant Physiology and Biochemistry. 28, 411–427 (1990). CAS
Google Scholar * Kuhlemeier, C. Aldehyde dehydrnase in tobacco pollen. Plant Mol Bio. 1, 355–365 (1997). Google Scholar * Mittler, R. Oxidative stress, antioxidants and stress tolerance.
Trends in plant science. 7, 405–410 (2002). Article CAS Google Scholar * Li, X. L., Yang, C. P. & Xu, X. L. Molecular Cloning and Expression Analysis of Aldehyde Dehydrogenase (ALDH)
Gene Segment in Leymus Chinensis. Chinese Agricultural Science Bulletin. 23, 115–120 (2007). Google Scholar * Genre, A. & Bonfante, P. Actin versus tubulin configuration in
arbuscule-containing cells from mycorrhizal tobacco roots. New phytologist. 140, 745–752 (1998). Article CAS Google Scholar * Mills, D., Kunoh, H., Keen, N. & Mayama, S. Molecular
aspects of pathogenicity and resistance: requirement for signal transduction. American Phytopathological Society, 257–267 (1996). * Rao, G. D. & Zhang, J. G. Advance of Studies on Plant
Tubulin Gene. World Forestry Research. 26, 11–16 (2013). Google Scholar * Liang, H. et al. Ceramides modulate programmed cell death in plants. Genes & Development. 17, 2636–2641 (2003).
Article CAS Google Scholar * Liu, R. H., Jiang, W. B. & Yu, D. Q. The Response of _AtCER_ to Oxidative Stress in _Arabidopsis thaliana_. Acta Botanica Yunnaica. 31, 326–334 (2009).
Article CAS Google Scholar * Li, B. et al. Advance on Key Enzyme Gene (COMT) Involved in Lignin Biosynthesis. Molecular Plant Breeding. 1, 117–124 (2010). ADS Google Scholar * Hamada,
K. et al. 4-Coumarate: coenzyme A ligase in black locust (_Robinia pseudoacacia_) catalyses the conversion of sinapate to sinapoyl-CoA. Journal of plant research. 117, 303–310 (2004).
Article CAS Google Scholar * Kang, C. et al. Carbon Emission Flow in Networks. Sci. Rep. 2, 479, 10.1038/srep00479 (2012). Article CAS PubMed PubMed Central Google Scholar *
O'Malley, D. M. et al. The role of laccase in lignification. The Plant Journa. 4, 751–757 (1993). Article CAS Google Scholar * Wei, Q. D. et al. Advancement of Histone and Its
Relationship with Regulation of Gene Expression. Journal of Tropical Medicine. 6, 596–598 (2006). CAS Google Scholar * Phillips, J. & Hayman, D. Improved procedures for clearing roots
and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British mycological Society. 55, 158–161 (1970). Article Google
Scholar * Sophie, A., Leslie, M. H. & Sona, P. ABA-Dependent and Independent G-Protein Signaling in _Arabidopsis_ Roots Revealed through an iTRAQ Proteomics Approach. Journal of
proteome research. 10, 3107–3122 (2011). Article Google Scholar * Zhang, K. R., Carolyn, M., Charles, H. H. & Michael, A. D. The _Medicago truncatula_ Small Protein Proteome and
Peptidome. Journal of Proteome Research. 5, 3355–3367 (2006). Article CAS Google Scholar * Liu, J. Y. et al. Comparative analysis of proteomic profile at different development stages of
_Volvariella volvacea_ by iTRAQ-coupled 2D LC-MSMS. Microbiology China. 39, 853–864 (2012). ADS Google Scholar * Pan, X. Q. et al. iTRAQ Protein Profile Analysis of Tomato _Green-ripe_
Mutant Reveals New Aspects Critical for Fruit Ripening. Journal of Proteome Research. 13, 1979–1993 (2014). Article CAS Google Scholar * Alexey, C., Consuelo, M. V., Neus, V. & Roman,
A. Z. Functional Identification of Target by Expression Proteomics (FITExP) reveals protein targets and highlights mechanisms of action of small molecule drugs. Scientific Reports. 5,
11176, 10.1038/srep11176 (2015). Article Google Scholar * Jose, A. M. et al. Quantitative proteomic analysis of host—pathogen interactions: a study of _Acinetobacter baumannii_ responses
to host airways. BMC Genomics. 16, 422–443 (2015). Article Google Scholar Download references ACKNOWLEDGEMENTS We sincerely thank Prof. sixue Chen for greatly improved the manuscript. This
study was supported by National Natural Science Foundation of China (31070576 and 31270535), Natural Science Foundation of Heilongjiang Province of China (No. ZD201206), Excellent Youth
Foundation of Heilongjiang Province of China (No. JC201306) and High-level Talents Support Program of Heilongjiang University (Ecological Restoration Team). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Heilongjiang University, Harbin, Heilongjiang, China Fuqiang Song, Dandan Qi, Xuan Liu, Xiangshi Kong, Yang Gao, Zixin Zhou & Qi Wu Authors * Fuqiang Song View author
publications You can also search for this author inPubMed Google Scholar * Dandan Qi View author publications You can also search for this author inPubMed Google Scholar * Xuan Liu View
author publications You can also search for this author inPubMed Google Scholar * Xiangshi Kong View author publications You can also search for this author inPubMed Google Scholar * Yang
Gao View author publications You can also search for this author inPubMed Google Scholar * Zixin Zhou View author publications You can also search for this author inPubMed Google Scholar *
Qi Wu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS F.S. designed the research, D.Q. wrote the main manuscript text of chinese and
interpreted the proteomics data, X.K. and X.L. wrote the main manuscript text of english and edited language, Y.G., Z.Z. and Q.W. extract proteins. All authors reviewed the manuscript.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is
licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license,
unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce
the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Song, F., Qi, D., Liu, X. _et
al._ Proteomic analysis of symbiotic proteins of _Glomus mosseae_ and _Amorpha fruticosa_. _Sci Rep_ 5, 18031 (2016). https://doi.org/10.1038/srep18031 Download citation * Received: 30 June
2015 * Accepted: 10 November 2015 * Published: 10 December 2015 * DOI: https://doi.org/10.1038/srep18031 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative