Play all audios:
ABSTRACT Triple negative breast cancer (TNBC) features among the most aggressive manifestations of cancer due to its enhanced metastatic potential and immunity to therapeutics which target
hormone receptors. Under such scenarios, anti-cancer compounds with an ability to influence multiple targets, or an entire process, will have an advantage over specific signal transduction
inhibitors. To counter the metastatic threat it is essential to target cellular components central to the processes of cancer cell migration and adaptation. Our previous work on a novel
triterpenoid, AECHL-1, explored its anti-cancer potential, and linked it to elevated ER stress in cancer cells, while its anti-angiogenic potential was credited for its ability to manipulate
the cytoskeleton. Here, we broaden its range of action by showing that it curbs the metastatic ability of TNBC cells, both _in vitro_ in MDA-MB-231 cell line and _in vivo_, in mouse models
of metastasis. AECHL-1 does so by disrupting the cytoskeletal network, and also suppressing NF-κB and β-Catenin mediated key molecular pathways. These activities also contributed to AECHL-1
mediated suppression of TGF-β/TNF-α induced Epithelial to Mesenchymal Transition (EMT) and cancer stem cell characteristic. Thus, we present AECHL-1 as a promising therapeutic inhibitor of
metastatic disease. SIMILAR CONTENT BEING VIEWED BY OTHERS TARGETING THE ILK/YAP AXIS BY LFG-500 BLOCKS EPITHELIAL–MESENCHYMAL TRANSITION AND METASTASIS Article 20 April 2021 IDENTIFICATION
OF TRIPTONIDE AS A THERAPEUTIC AGENT FOR TRIPLE NEGATIVE BREAST CANCER TREATMENT Article Open access 28 January 2021 ASAH1 FACILITATES TNBC BY DUSP5 SUPPRESSION-DRIVEN ACTIVATION OF MAP
KINASE PATHWAY AND REPRESENTS A THERAPEUTIC VULNERABILITY Article Open access 26 June 2024 INTRODUCTION The tumor and its microenvironment are a hub of dynamic cellular activities. Several
molecular processes are orchestrated in a cancer cell in response to peripheral stimuli, which lead to cancer establishment and progression. Irrespective of the advances in clinical and
preclinical trials of cancer therapy, breast cancer remains one of the leading causes of mortality in women, with most of the fatalities being attributed to its metastasis1,2. Difficulties
in the treatment of metastasis are attributed mainly to the heterogeneous nature of tumor cells and their interactions with the microenvironment. To metastasize, the cancer cell remodels the
cytoskeleton and forms membrane protrusions, at the leading edge, thus initiating invasion and migration3,4,5. The nexus of migration-invasion-metastasis is often associated with the
process of epithelial to mesenchymal transition (EMT)6, which is characterized by the loss of epithelial markers, like E-cadherin and gain of mesenchymal markers, such as N-Cadherin,
Vimentin, Snail and Twist7,8. Associated perpetrators of breast cancer relapse, the cancer stem cells (CSCs) harbor an enhanced ability to escape chemo/radio-therapy, and an up-regulation of
CSC markers has been reported to be intimately linked to the process of EMT9,10. These interlinked mechanisms of EMT and metastasis have a cumulative effect in augmenting the complexity of
the disease, and hence targeting them is of paramount importance. Increase in the cases of relapse and resistance have elicited the need for the development of chemotherapeutics with
strategic modes of action11. Natural compounds have been shown to possess enormous potential as anti-proliferative as well as anti-metastatic agents against multiple cancer types12,13,14.
Earlier, we had reported the anticancer activity of a novel triterpenoid, AECHL-1, isolated from the root bark of _Ailanthus excelsa_ RoxB15 and recently we gained further insights into its
mechanism of action, and demonstrated that AECHL-1 could trigger apoptosis in breast cancer cells via mitochondrial perturbations and elevated ER stress16. Another line of investigation
revealed that AECHL-1 inhibits tumor angiogenesis of breast cancer cells via cytoskeletal disruption17. In the present study, we sought to determine the anti-migratory and anti-invasive
potential of AECHL-1 on TNBC MDA-MB-231 cells and in mice models of tumorigenesis and metastasis. Our findings demonstrate that AECHL-1 could inhibit cancer cell migration and invasion by
targeting the processes of actin nucleation and branch formation, both _in vitro_ and _in vivo_. AECHL-1 could also suppress the phenomenon of EMT and reduce the expression of CSC
indicators. AECHL-1 could execute these activities by down-regulating the expression of proteins such as β-catenin and NF-κB, which are engaged in regulation of the above mentioned
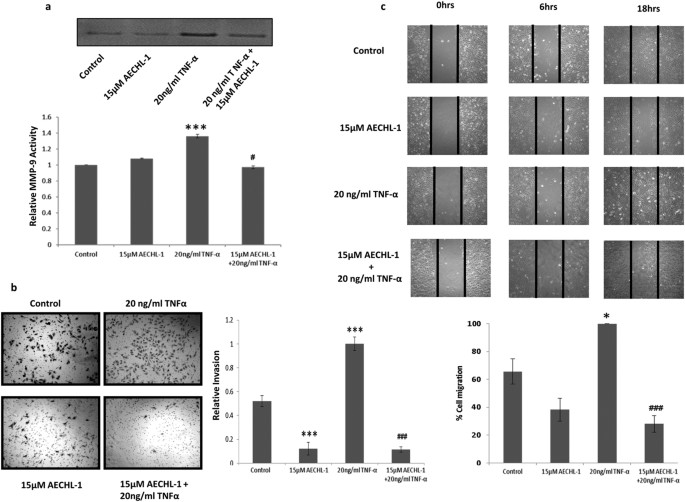
processes, thus making AECHL-1 an effective dispenser of anti-cancer activities. RESULTS AECHL-1 INHIBITS TNF-Α MEDIATED MDA-MB-231 MIGRATION AND INVASION THROUGH DOWN-REGULATION OF MMP-9
ACTIVITY In order to determine whether AECHL-1 affected invasiveness, MDA-MB-231 cell line was chosen, for its established invasive potential and mesenchymal phenotype. Matrix Metallo
Proteinase (MMP)-9 activity, essential for the cells to digest the ECM in order to migrate or invade, was studied following TNF-α induction. 15 μM AECHL-1 treatment reduced MMP 9 activity as
determined by zymography (Fig. 1a). This result also explained and strengthened our observation concerning AECHL-1 mediated inhibition of transwell invasion (Fig. 1b) and, migration across
a scratch wound (Fig. 1c). AECHL-1 HAMPERS BREAST CANCER CELL INVASION THROUGH SUPPRESSION OF NF-ΚB MEDIATED MAPK ACTIVITY AND DECREASES MESENCHYMAL MARKER EXPRESSION NF-κB is an important
regulator of many pro-survival, invasive and inflammatory pathways18,19. The status of NF-κB was determined in these cells after 15 μM AECHL-1 treatment, in the presence or absence of 20
ng/ml TNFα. A downregulation in phosphorylated p65 subunit of NF-κB was seen by western blotting (Fig. 2a). Immunofluorescence studies revealed decrease in p65 localization in the nucleus
(Fig. 2b). Whole cell protein lysates of treated MDA-MB-231 were also analyzed for the expression of pERK 1/2 and pMEK 1/2 via Western Blotting. This revealed a significant decrease in the
expression and activity of MAPK proteins as phosphorylated forms of both the proteins were reduced by AECHL-1 treatment (Fig. 2c). Since secretion of pro-angiogenic factors elicits a
pro-metastatic response from tumor cells and may induce them to switch phenotypes from epithelial to mesenchymal, thus initiating migration and invasion20, cells were exposed to AECHL-1
treatment 2 h prior to TNF-α induction. Following termination, cells were analyzed for expression of invasion and mesenchymal markers through either flow cytometry or confocal microscopy.
Pro-angiogenic/invasive growth factor secretion was studied by ELISA. AECHL-1 remarkably decreased bFGF/VEGF secretion into the conditioned media (CM) in the presence and absence of TNF-α
induction as determined by ELISA (Fig. 2d). Vimentin, CD-44 and αvβ3 are characteristic markers usually sported by the cancer cells having a mesenchymal phenotype21,22,23. Reduction in CD-44
and αvβ3 expression (Fig. 2e) was observed following TNF-α induction. AECHL-1 treatment also brought about a decrease in Vimentin expression, as observed by immunofluorescence analysis
(Fig. 2f). AECHL-1 AFFECTS CANCER CELL MIGRATION BY ALTERING CYTOSKELETAL DYNAMICS AECHL-1, in our previous study, had revealed an ability to de-regulate the actin cytoskeletal dynamics in
endothelial cells17. An observed decrease in migration and invasion by MDA-MB-231 cells on AECHL-1 treatment prompted us to explore this phenomenon further. _In vitro_ experiments involved a
typical scratch wound assay where cells were initially exposed to AECHL-1 for 2 h following a scratch infliction and TNF-α induction. Experiments were terminated at 9 h following wounding.
Cells were then lysed in RIPA and subjected to western blotting in order to study the expression of proteins involved in actin nucleation and branching during cancer cell migration. AECHL-1
could inhibit F-actin polymerization in migrating cells, and affected the localization of IQGAP-1 and WAVE-2 (Fig. 3a,b). AECHL-1 could also downregulate proteins belonging to the Rho family
of small GTPases-Rac/cdc42, and the actin branch generators ARP-2/3 (Fig. 3c). Interestingly, profilin another important protein known to be instrumental for the rapid polymerization of the
cytoskeleton24,25 was upregulated following AECHL-1 treatment. Our _in vivo_ results too displayed a similar trend. 5 μg/kg body weight AECHL-1, along with a significant regression in
MDA-MB-231 xenograft tumor volume, downregulated the expression of actin nucleation and branching proteins with respect to PBS treated control (Fig. 3d,e). Profilin, however was found to be
decreased in AECHL-1 treated mice, suggesting that profilin expression and translation may be situation dependent. β-catenin accumulation in the nucleus is often associated with loss of
E-cadherin and decrease in CD-44 expression. This correlates with susceptibility of the cell towards undergoing EMT, and acquisition of an invasive phenotype26. β-catenin dynamics at the
membrane is also affected by Rac/Cdc42 GTPase activity involving alteration of IQGAP1 affinity with this protein. This phenomenon alters cell-cell adhesion and contacts, thus modifying cell
polarity and shape. Since a change in morphology and cell-cell attachment was observed after AECHL-1 treatment, the status of β-catenin was also studied _in vitro_. β-catenin levels were
downregulated after AECHL-1 treatment and nuclear localization was decreased (Fig. 3f). It was observed that there was a slight membrane localization of β-catenin following AECHL-1
treatment, indicating an attempt by the cells towards maintenance of junctional integrity (white arrow, Fig. 3g). GSK-3β is a modulator of β-catenin stability and is known to mark β-catenin
for degradation by phosphorylating it. Phosphorylation of GSK-3β by Caesin kinase and AKT deactivates it26. As expected, AECHL-1 treatment could bring down phosphorylation of GSK-3β, thus
preserving the effective GSK-3β levels, which would in turn mark β-catenin for ubiquitination, in consequence restricting its nuclear localization (Fig. 3g) AECHL-1 INHIBITS METASTASIS OF
MDA-MB-231 CELLS IN AN _IN VIVO_ TAIL-VEIN MOUSE MODEL SCID female mice were inoculated with MDA-MB-231 cells via tail vein injection, and 5 μg/kg body weight of AECHL-1 was administered to
the mice intra-peritoneal (i.p.) for the duration of 10 days. Control mice were treated with PBS. Lungs were excised after the duration of 4 weeks and studied for morphological
characteristics typical of affected lungs. They were then processed for H&E staining to observe metastatic foci. Lungs from AECHL-1 treated mice showed normal alveolar appearance with
sparse metastatic foci, whereas lungs excised from the PBS treated control group sported larger numbers of dense metastatic foci (Fig. 4a). We further quantified the metastatic focal density
by grading them according to the number and continuity per sample. It was observed that AECHL-1 could decrease this parameter in the lungs of treated mice. Thus AECHL-1 could decrease
metastatic colonization by MDA-MB-231 cells in the lungs of treated mice, as depicted by the images (Fig. 4b). AECHL-1 DISCOURAGES EPITHELIAL TO MESENCHYMAL TRANSITION The remarkable ability
demonstrated by AECHL-1 in cumulatively modulating various factors, responsible for observable changes in morphological and invasive properties, as well as metastasis inhibition led us to
wonder whether AECHL-1 could prevent the onset of EMT, which follows similar tenets. Thus, EMT was induced in immortalized breast epithelial cell line MCF-10A by 2 ng/ml TGF-β and 10ng/ml
TNF-α, over a period of 3–7 days. The cells were treated with 15 μM AECHL-1, 18 h prior to induction, and analyzed for epithelial and mesenchymal markers via Western blotting and
Immunofluorescence assays. For a 7 day culture, media containing the appropriate quantities of the chemical EMT inducers was changed every 3 days. Acquisition of EMT phenotype was determined
by the observation that these cells attained spindle shaped morphology losing their typical hexagonal shape (Fig. 5a). Evidence of EMT was also confirmed by studying the status of
E-cadherin, an epithelial marker and vimentin, a mesenchymal marker. Western blotting and confocal analysis revealed a decrease in E-cadherin accompanied by a concomitant increase in
vimentin expression (Fig. 5a,b). Pre-treatment with AECHL-1 prior to EMT induction prevented the acquisition of a mesenchymal phenotype by the MCF-10A cells. NF-κB is also known to mediate
TNF-α induced EMT through upregulation of various transcriptional repressors such as ZEB1/2, TWIST and Snail (SNAI)27. AECHL-1 could also downregulate the expression of NF-κB regulatory
subunit p65, Snail and Twist (Fig. 5b). Our qRT-PCR revealed an increase in Vimentin along with a decrease in E-cadherin in stimulated MCF-10A cells. AECHL-1 treatment could decrease the
transcript levels of Vimentin and partially restore E-cadherin levels (Fig. 5c). AECHL-1 SUPPRESSES THE CANCER STEM CELL POPULATION _IN VITRO_ The process of EMT is closely associated with
promoting the incidence of cancer stem cells in a tumor niche28 and as AECHL-1 could discourage EMT in breast cancer cells, we investigated its effect on the subpopulation of breast cancers
cells exhibiting CSC like characteristics. Breast CSCs show a characteristic CD44+/CD24−/ESA+ phenotype, enhanced ALDH activity and possess a capability to form mammospheres in non-adherent
cultures29. Also, ABC transporters enable these cells to efflux the chemotherapeutics out, providing them a distinct signature in the Hoechst Side Population Assay30. Thus, to analyze these
parameters, cells were treated with AECHL-1 and 24 h post treatment, these trypsinized cells were subjected to different standardized assays. Immunophenotyping with CD44, CD24 and ESA
yielded a significant decrease in the CD44+/CD24− antigen positive cells in MCF7 (Fig. 6a) and ESA+ cells in MDA-MB-231 cells (Fig. 6b). A reduced ALDH activity was also noted in both these
cell lines (Fig. 6c). AECHL-1 decreased the Hoechst 33342 effluxing side-population (SP) in MCF7 and MDA-MB-231 cells (Fig. 6d). It was also observed that AECHL-1 treatment diminished the
mammosphere forming capability in MCF7 cells, 48 h post-treatment (Fig. 6e). The colony formation propensity, which is directly correlated to the regenerative characteristic, was seen to be
directly affected by AECHL-1 in MCF7 and MDA-MB-231 cells (Fig. 6f,g). AECHL-1 SUPPRESSES THE CANCER STEM CELL POPULATION _IN VIVO_ Xenograft tumors, obtained by injecting MCF7 cells in SCID
mice, were lysed fresh after harvesting and live cells were analyzed for cancer stem cell population. CD44+/CD24− Immunophenotyping revealed a significant decrease in their expression
levels in tumors harvested from AECHL-1 treated mice as opposed to the tumors belonging to control mice (Fig. 7a). The ALDH reducing population also decreased significantly in AECHL-1
treated mice as compared to the control, as seen by Flow Cytometry (Fig. 7b). Transcriptional regulators known for initiating and preserving cancer stem cell characteristics, viz. Oct3/4,
Sox2, Nanog, C-myc and Lin28A31, were also seen to be drastically downregulated in AECHL-1 treated mice as compared to control, as observed by flow cytometry (Fig. 7c). DISCUSSION Metastasis
requires clusters of tumor cells to make their way through the protein dense ECM and subsequently invade extra-tumoral tissues, including the vascular and lymphatic systems. These cancer
cells efficiently exploit their altered signaling profiles to initiate the process of collective cell migration and invasion, which requires extensive cytoskeleton remodeling. Thus,
therapeutic disruption of this process can derail the mechanisms integral to metastasis and provide an option for containing the devastating spread of cancer. In this study, our compound of
interest, AECHL-1 was tested for its ability to interfere with this transformative process that tumor cells undergo in order to form metastatic lesions. The pro-inflammatory cytokine TNF-α
plays a significant role in driving the progression of tumorigenesis, influencing metastasis and regulating angiogenesis through the activation of multiple inter connected networks,
including the upregulation of proteolytic enzymes such as MMP-9 and uPA via the NF-ĸB pathway18,20,32. However, despite TNF-α stimulation, we observed a marked reduction in the migratory and
invasive potential of AECHL-1 treated MDA-MB-231 cells. This observation could be explained by the AECHL-1 mediated inhibition of nuclear translocation and down regulation of the active
subunit of NF-ĸB, which is phosphorylated p6519,27, consequently effectuating a decrease in MMP-9 activity. TNF-α also has the ability to affect the cytoskeletal apparatus by associating
with the Rho family of GTPases, including Cdc42 and Rac133. Our studies indicated that the Rho family of cytoskeleton effector proteins could be a possible target of AECHL-1, since the TNF-α
induced pro-migratory phenotype was abrogated by AECHL-1 treatment. A decrease in actin polymerization, reduction in active leading edge protrusions and perturbed localization of IQGAP-1
and WAVE-2 was observed on AECHL-1 treatment, with or without TNF-α induction. These changes in the expression and patterning of proteins related to cytoskeletal organization and assembly
(ARP-2 and 3) could be an outcome of AECHL-1 interfering with the aberrantly over-stimulated signaling pathways, present in aggressive cancer cells. Thus, AECHL-1 could broaden its reach and
disturb myriad functions ranging from cell survival to motility. Other molecules that have been implicated in TNF-α stimulated modulation of the cytoskeleton through the GTPases, include
MAP kinases34,35, which were also found to be down regulated by AECHL-1 treatment, thus confirming our hypothesis that the principle proteins involved in cytoskeletal remodeling are actively
targeted by AECHL-1. However, contrary to these results, Profilin, an actin binding protein, which increases actin filament turnover rates, was up regulated by AECHL-1 treatment in actively
migrating cells. Further investigation into the reported literature revealed that in MDA-MB-231 cells, Profilin is usually present in insignificant amounts and acts as a tumor suppressor
through the PTEN pathway36. Interestingly, in triple negative breast cancer patients, lower Profilin levels are synonymous with poor prognosis37. The proteins involved in regulating the
cytoskeletal apparatus also work in tandem with junctional proteins such as β-catenin and E-cadherin. Their dynamics influence junctional integrity affecting cell migration, invasion and
polarity. β-catenin, the effector protein of the Wnt pathway, is responsible for maintaining cell-cell adhesive contact through α-actinin involvement, which connects it to the
cytoskeleton38. On the other hand, its translocation to the nucleus, elicits a migratory response in these cells, setting in motion the acquisition of a more invasive phenotype along with a
loss of a apico-basal polarity39. Also nuclear β-catenin is a co-activator to the Tcf/lef transcription factors which bind to the enhancer elements of genes falling under the Wnt pathway,
including pro-invasive markers such as CD-44 and other pro-survival genes26. On AECHL-1 treatment, we observed a decrease in the nuclear localization and expression of β-catenin along with a
down regulation of the phosphorylated form of its negative regulator GSK-3β, and maintenance of the activated GSK-3β at levels similar to that of the control cells. Further, an AECHL-1
mediated decrease in the secretion of TNF-α induced pro-angiogenic cytokines VEGF and bFGF contributed towards making the extracellular microenvironment less conducive for initiation of cell
migration and invasion, thus complementing the inhibitory modalities effectuated by AECHL-1 on these mechanisms at the intracellular level. Changes in cell morphology, polarity and
invasiveness are indisputably the most important factors linked with the events of EMT. Our findings with AECHL-1 in this direction revealed a suppression of mesenchymal marker Vimentin
along with a restoration of epithelial marker E-cadherin to functional levels, in MCF-10A breast epithelial cells, which had been induced to undergo EMT by TGF-β/TNF-α treatment. The
transcription factors responsible for the induction and maintenance of EMT, namely, NF-κB, Snail and Twist were also found to be downregulated by AECHL-1, only in cells subjected to EMT.
Also, evidence derived from these experiments indicate that AECHL-1 did not change the expression pattern of either group of markers or transcription factors responsible for EMT, in
non-induced MCF-10A cells, hence strengthening our hypothesis that AECHL-1 did not affect cells with normal functioning or non-erratic signaling. Since TNF-α, through NF-κB, is known to
regulate the expression and activity of β-catenin, Snail and GSK-3β40, our results indicate that NF-κB plays an indispensable role in implementing the anti-cancer program triggered by
AECHL-1. A previous study concerning the apoptosis inducing mechanism of AECHL-1 too highlighted the involvement of NF-κB16. A sub-population of cells within the tumor, known to be resistant
to chemo/radiation therapy, contributes heavily towards tumor relapse, by facilitating metastatic growth and establishing secondary lesions. Their presence always resonates with poor
prognosis in patients41. These cells have stem-cell like characteristics and are known to have distinct cellular and molecular signature which enables them to escape chemo/radiotherapy and
attain the property of self-renewal42. Recently, these CSCs are shown to be intimately linked with the process of EMT, and studies have shown a direct correlation between EMT induction and a
surge in the population of CSCs42,43. AECHL-1 treatment showed a significant decrease in the CSC sub-population _in vitro_ as well as _in vivo_, thus strengthening our hypothesis that
AECHL-1 could bring about an overall suppression of the disease. In mice orthotropically injected with MDA-MB-231, AECHL-1 could decrease the tumor volume and down regulate all molecular
players involved in invoking the invasive cytoskeletal phenotype. Surprisingly, Profilin in this case was found to be down regulated too, which could be attributed to a global protein down
regulation observed in these tumors after one month of treatment, especially when they were already undergoing recession16. These, AECHL-1 mediated, inhibitory effects were also responsible
for the decrease in presence of metastatic lesions in the lungs of animals, injected with MDA-MB-231 through the tail vein. Overall, AECHL-1 launched a multi-targeted attack on metastatic
breast cancer cells _in vitro_ and _in vivo_, which closely resembles its activities in stimulated endothelial cells (Fig. 8). This diversified approach executed by AECHL-1 should be
actively harnessed for development of anti-cancer leads, which in combination with appropriate regiments or alone would affect tumor development and disease progression in a comprehensive
manner. MATERIALS AND METHODS REAGENTS Liebovitz’s L15 Medium and 0.5% Trypsin Phosphate Versene Glucose (TPVG) was purchased from HiMedia (USA). M171 Medium and Mammary Epithelial Growth
Supplement (MEGS) were obtained from Life Technologies (USA). Fetal Bovine Serum (FBS), Penicillin, and Streptomycin (P&S) were purchased from Gibco (Grand island, NY, USA). All reagents
were procured from Sigma-Aldrich (St. Louis, MO, USA) except for if otherwise mentioned. Primary antibodies for Immunofluorescence and Western Blotting were purchased from Cell Signaling
Technologies (CST), USA and Santa Cruz Biotechnology (Santa Cruz, CA, USA). AlexaFluor secondary antibodies for Immunofluorescence and Flow Cytometry were obtained from Invitrogen (Life
Technologies, USA). HRP-Linked anti-rabbit or anti-mouse secondary antibodies for Western Blotting were obtained from BioRad. All ELISA kits were purchased from R&D Systems, USA. CELL
CULTURE MDA-MB-231 and MCF7 cells were obtained from American Type Culture Collection (ATCC) and maintained in Leibovitz’s L15 Medium and DMEM respectively, supplemented with 10% Fetal
Bovine Serum, FBS. MCF-10A cells, obtained from ATCC, were grown in M171 Medium supplemented with MEGS. Cultures were maintained at 37 °C with 5% CO2 in a humidified incubator. WOUND HEALING
ASSAY MDA-MB-231 cells were seeded in 24-well plates and grown up to nearly 100% confluency. The cells were scratched with a pipette tip to create wounds. Treatment with TNF-α (20 ng/ml)
and AECHL-1 (15 μM), alone and in combination with each other was given in serum-free medium after scratch was made. Randomly chosen fields were photographed at 10X magnification with an
inverted microscope, and the images were taken at identical locations at the indicated time points. Percent cell migration was calculated by comparing final gap width to initial gap width
using image pro-plus. MDA-MB-231 cell migration from TNF-α treated wells at the final time point was normalized to 100% migration. MATRIGEL INVASION ASSAY MDA-MB-231 cells were suspended in
serum-free culture medium and loaded onto Matrigel-coated inserts (BD Biosciences, USA), placed in a 24-well plate. The lower chamber, thus created, was filled with 500 μl 20% FCS
(chemo-attractant) containing culture medium. After 18 h, the upper surface of the insert was swabbed with a cotton bud and invasive cells on the lower surface were fixed in 3.7% PFA. The
inserts were then stained using 1% crystal violet and imaged (10X) using an inverted microscope (Nikon). Image J was used for counting the invasive cells. DETECTION OF PROTEIN LEVELS BY FLOW
CYTOMETRY Expression of αvβ3, CD24 and CD44 after AECHL-1 treatment were studied using flow cytometry. Cancer stem cell markers oct3/4, sox2, nanog, c-myc and lin28A were detected in
xenograft tumor cells via flow cytometry as well. Cells from monolayers were trypsinized whereas tissue was subjected to dissociation by collagenase-III (1 mg/ml) treatment to obtain a
single cell suspension, subsequently these cells were fixed with 3.7% PFA for 10 min on ice and incubated with the appropriate primary antibody (1:150) in 1% blocking buffer at 37 °C for 30
min followed by washing and binding with appropriate fluorescence tagged secondary antibody (AlexaFluor 488; 1:300) in 1% blocking buffer at 37 °C for 30 min. Cells analyzed for expression
of surface marker analysis were not subjected to PFA fixing and were stained live. Cells were then washed twice with PBS and immediately acquired using FACSCalibur. Appropriate isotype
controls were used to subtract background fluorescence intensities. Analysis was carried out using Cell Quest Pro software. NUCLEAR CYTOPLASMIC EXTRACTION Cells were trypsinized using 0.5%
TPVG (HiMedia) and incubated in Hypotonic buffer [10 mM HEPES pH 7.9, 10 mM KCl, 2 mM MgCl2, 0.1 mM EGTA, 0.2 mM PMSF, protease inhibitor cocktail (Roche), sodium pyrophosphate (0.2 mM),
fluoride (10 mM) and orthovanadate (50 mM)] for 15 min on ice. The cytosolic fraction was collected by centrifugation at 5000 g for 15 min at 4 °C. The pellet was washed once in hypotonic
buffer and resuspended in cell lysis buffer [50 mM Tris pH 7.4, 5 mM EDTA, 250 mM sodium chloride, 50 mM sodium fluoride, 0.5 mM sodium vandate and 0.5% w/v Triton X-100]. The resuspended
pellet was incubated for 30 min at 4 °C with rotation shaking. Nuclear extracts were centrifuged at 13000 g for 15 min at 4 °C. WESTERN BLOT ANALYSIS Whole cell protein lysates were prepared
using RIPA buffer containing 0.2% SDS, 1 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM PMSF and EDTA-free mini-complete protease
inhibitor cocktail tablet (Roche). Tissue samples or cells were homogenized in RIPA Lysis Buffer. The protein concentration was determined using Bradford’s Reagent (BioRad). 40 µg of protein
was subjected to polyacrylamide gel electrophoresis and transferred on to a nitrocellulose membrane (Merck Millipore). Membranes were incubated with respective primary antibody dilutions
prepared in Tris Buffered Saline (TBS)-Tween for 2 h at room temperature or overnight at 4 °C. Membranes were then washed in TBS-Tween and incubated with secondary antibodies anti-rabbit
IgG–HRP (BioRad) or anti mouse IgG-HRP (BioRad) diluted (1:10.000) in TBS-Tween for 1 h at room temperature. Protein-antibody complexes were detected by Substrate Detection Kit
(Thermofischer, USA). Quantification of signal was done via densitometry analysis, using the Image J software. Actin was used as loading control for whole cell lysates and Lamin A/C and
GAPDH were used as loading controls for nuclear and cytoplasmic fractions respectively. RNA EXTRACTION, QRT-PCR Total RNA was isolated from cells and tissue samples using TRIzol reagent
(Sigma-Aldrich, USA) and reverse transcribed using Thermoscript cDNA synthesis kit (Life Technologies) as per manufacturer’s protocol. cDNA was quantified by a spectrophotometer using
NanoDrop Analysis. Approximately 600ng of cDNA was subjected to qRT PCR using SYBR green (Life Technologies) according to manufacturer’s instructions on an Applied Biosystems real-time
thermocycler. The profile of thermal cycling consisted of initial denaturation at 95 °C for 2 min, and 40 cycles at 95 °C for 15 s and 60 °C for 45 s for primer annealing and extension.
Melting curve analysis was used to determine the specific PCR products. All primers used for Real-Time PCR analyses were synthesized by Eurofins Genomics India Pvt. Actin was used as a
positive control and appropriate negative control was used to validate the reaction. The list of primers is given below as Table 1. The changes in the threshold cycle (CT) values were
calculated by the equation ΔCT = CT (target) − CT (endogenous control) and fold difference was calculated as 2−Δ (ΔCT). IMMUNOFLUORESCENCE ASSAY AND CONFOCAL IMAGING Cells were fixed using
3.7% PFA followed by permeabilization (for intracellular proteins only) with 0.2% Triton X-100. 5% BSA was used for blocking followed by staining with protein specific primary antibody and
Alexa Fluor secondary antibody (Invitrogen). DAPI was used for nuclear staining. Cells were visualized on a Zeiss LSM510 META (Carl Zeiss, Germany) and Leica SP5 II system (Leica
microsystems, Germany) and images were analyzed using LSM 5 Image Browser software. GELATIN ZYMOGRAPHY MDA-MB-231 cells were cultured to 70% confluence followed by treatment with AECHL-1 for
18 h. Cell free supernatants of culture were used to analyze MMP-9 levels using Gelatin Zymography. Aliquots of media supernatant were diluted 1:1 in zymography sample buffer [62.5 mM
Tris–HCl (pH 6.8), 8.8% glycerol, 2% (w/v) SDS, 0.05% bromophenol blue] and electrophoresed on a 7.5% SDS-polyacrylamide gel containing 1% gelatin, at 4° C. After Electrophoresis, the gel
was washed twice for 30 min with 2.5% Triton X-100, to remove SDS followed by incubating in renaturation Buffer (1 M Tris HCl, pH 7.6, NaCl, CaCl2 and NaN3) for 18 h at 37 °C. Gels were
stained with Coomassie Brillant Blue (0.1%w/v) and destained in 30% methanol, 10% acetic acid. Gels were visualized in Gel Documentation System (BioRad) and images were analyzed using
GeneSnap (BioRad) and Image J Software. ELISA ELISA kits (R&D Systems) were used to detect human Basic fibroblast growth factor (bFGF) and Vascular Endothelial Growth Factor (VEGF).
MDA-MB-231 cells were treated with AECHL-1 for 18 h and cell free supernatants were used to detect the levels of bFGF and VEGF by following the manufacturer’s protocol. ANIMAL EXPERIMENTS
ETHICAL STATEMENT All experiments were performed in accordance with relevant guidelines and regulations as per the Institutional Animal Ethics Committee (IAEC) under reference number
EAF/2013/B-217 dated 29/03/2010 of National Centre for Cell Science (NCCS). All experimental protocols were approved by IAEC, a committee constituted at NCCS, as per regulations of Committee
for the Purpose of Control and Supervision of Experiments and Animals (CPCSEA), Government of India. TAIL VEIN METASTASIS Metastasis was assessed by injecting 1 × 106 MDA-MB-231 cells
suspended in 150 μL sterile Phosphate Buffer Saline (PBS), in the tail vein of 6–8 week old female SCID mice. Animals were treated intra-peritoneally (i.p.) with PBS or AECHL-1 5 μg/kg body
weight of the animal for 10 days following tail vein injection. Metastasis to the lung was detected by generating cryosections of lungs, after 4 weeks. Tissue samples were fixed in OCT
Compound at −20 °C overnight. The fixed tissue samples were then sliced into sections not larger than 8 μm using a Shandon microtome. Metastatic foci were identified by H&E staining of
the lung sections. Quantification was carried out by measuring the metastatic index in three random fields per three lung sections per animal (n = 5). Metastatic index was determined by
calculating the ratio of “area covered by metastatic foci per section” to “total section area”. MCF7 AND MDA-MB-231 XENOGRAFT STUDIES Six-week-old female SCID mice were injected
subcutaneously into the dorsolateral flank with 2 × 106 MCF7 or MDA-MB-231 cells. When tumor volume reached visible proportions, animals were treated intra-peritoneal (i.p.) with PBS or
AECHL-1,5 μg/kg body weight of the animal for 10 days. After harvesting tumors, part of the tissue was stored at −80 °C for flow cytometry or subjected to Western blotting. HOECHST 33342
SIDE POPULATION ANALYSIS Cells were plated in 60 mm petridish as 2.5 × 105 cells/petridish and were treated with increasing concentrations of AECHL-1 at 70% confluence for 24 h. Cells were
then trypsinized and stained with HOECHST 33342 for 90 min at 37 °C. The cells were then analyzed using FACS Aria UV laser within 30 min of staining. Verapamil was used as an inhibitor
control to back-gate the side population cells. Analysis was carried out using the FACS Diva software. ALDH ASSAY ALDH reducing population was analyzed using the ALDEFLUOR Kit by Stemcell
Technologies, USA as per the manufacturer’s protocol. Analysis was carried out using the Cell Quest Pro software. STATISTICAL ANALYSIS Significant differences were analyzed using the Student
t test and two-tailed distribution. Results were considered to be statistically significant if P < 0.05 and were expressed as mean ± SE between triplicate experiments performed thrice.
All statistical comparisons were made relative to untreated controls and significance of differences is indicated as *P < 0.05 and **P < 0.01. ADDITIONAL INFORMATION HOW TO CITE THIS
ARTICLE: Dasgupta, A. _et al_. AECHL-1 targets breast cancer progression via inhibition of metastasis, prevention of EMT and suppression of Cancer Stem Cell characteristics. _Sci. Rep._ 6,
38045; doi: 10.1038/srep38045 (2016). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES
* Siegel, R., Ma, J., Zou, Z. & Jemal, A. Cancer statistics, 2014. CA Cancer J Clin 64, 9–29 (2014). PubMed Google Scholar * Jemal, A. et al. Global cancer statistics. CA Cancer J
Clin 61, 69–90 (2011). PubMed Google Scholar * Nürnberg, A. et al. Nucleating actin for invasion. Nat Rev Cancer 11, 177–187 (2011). PubMed Google Scholar * Le Clainche, C. &
Carlier, M. F. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev 88, 489–513 (2008). CAS PubMed Google Scholar * dos Remedios, C. G. et
al. Actin binding proteins: regulation of cytoskeletal micro laments. Physiol Rev 83, 433–473 (2003). CAS PubMed Google Scholar * Li, L. & Li, W. Epithelial-mesenchymal transition in
human cancer: comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacology & Therapeutics. 150, 33–46 (2015). CAS Google Scholar * Papageorgis, P. TGF-β
signaling in Tumor Initiation, Epithelial-to-Mesenchymal Transition, and Metastasis. J Oncol 2015, 587193 (2015). PubMed PubMed Central Google Scholar * Gonzalez, D. M. & Medici, D.
Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 7, re8 (2014). PubMed PubMed Central Google Scholar * Mitra, A., Mishra, L. & Li, S. EMT, CTCs and CSCs in
tumor relapse and drug-resistance. Oncotarget 6, 10697–10711 (2015). PubMed PubMed Central Google Scholar * Sottoriva, A. et al. Cancer stem cell tumor model reveals invasive morphology
and increased phenotypical heterogeneity. Cancer Res 70, 46–56 (2010). CAS PubMed Google Scholar * Sledge, G. W., Mamounas, E. P., Health, F., Hortobagyi, G. N. & Burstein, H. J.
Past, Present, and Future Challenges in Breast Cancer Treatment. J Clin Oncol. 32, 15–9 (2014). Google Scholar * Ramadevi Subramani et al. Nimbolide inhibits pancreatic cancer growth and
metastasis through ROS-mediated apoptosis and inhibition of epithelial-to-mesenchymal transition. Sci Rep. 5, 17618 (2016). Google Scholar * Sangmin Kim et al. Zerumbone suppresses the
motility and tumorigenecity of triple negative breast cancer cells via the inhibition of TGF-β1 signaling pathway. Oncotarget. 7(2), 1545–1558 (2015). Google Scholar * Gao, X1. et al.
Synthetic oleanane triterpenoid, CDDO-Me, induces apoptosis in ovarian cancer cells by inhibiting prosurvival AKT/NF-κB/mTOR signaling. Anti cancer res. 31(11), 3673–3681 (2011). CAS Google
Scholar * Lavhale, M. S., Kumar, S., Mishra, S. H. & Sitasawad, S. L. A Novel Triterpenoid Isolated from the Root Bark of Ailanthus excelsa Roxb (Tree of Heaven), AECHL-1 as a
Potential Anti-Cancer Agent. PLoS One. 4(4), e5365 (2009). ADS PubMed PubMed Central Google Scholar * Sawant, M. A., Dasgupta, A., Lavhale, M. S. & Sitasawad, S. L. Novel
triterpenoid AECHL-1 induces apoptosis in breast cancer cells by perturbing the mitochondria – endoplasmic reticulum interactions and targeting diverse apoptotic pathways. BBA - Gen. Subj.
1860, 1056–1070 (2016). CAS Google Scholar * Dasgupta, A. et al. AECHL-1, a novel triterpenoid, targets tumor neo-vasculature and impairs the endothelial cell cytoskeleton Angiogenesis 18,
283–299, doi: 10.1007/s10456-015-9466-5 (2015). Article CAS PubMed PubMed Central Google Scholar * Ghosh, S. & Karin, M. Missing pieces in the NF-κB puzzle. Cell. 109, Suppl
S81–S96 (2002). CAS PubMed Google Scholar * Hayden, M. S. & Ghosh, S. Signaling to NF-κB. Genes Dev. 18, 2195–2224 (2004). CAS PubMed Google Scholar * Hagemann, T. et al. Enhanced
invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-α dependent up-regulation of matrix metalloproteases. Carcinogenesis. 25, 1543–1549 (2004). CAS
PubMed Google Scholar * Mendez, M. G., Kojima, S. & Goldman, R. D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB
J. 24, 1838–1851 (2010). CAS PubMed PubMed Central Google Scholar * Louderbough, J. M. & Schroeder, J. A. Understanding the dual nature of CD44 in breast cancer progression. Mol.
Cancer Res. 9, 1573–1586 (2011). CAS PubMed Google Scholar * Hodivala-Dilke, K. αvβ3 integrin and angiogenesis: a moody integrin in a changing environment. Curr. Opin. Cell Biol. 20,
514–519 (2008). CAS PubMed Google Scholar * Zou, L., Ding, Z. & Roy, P. Profilin-1 overexpression inhibits proliferation of MDA-MB-231 breast cancer cells partly through p27kip1
upregulation. J. Cell Physiol. 223, 623–629 (2010). CAS PubMed PubMed Central Google Scholar * Witke, W. The role of profilin complexes in cell motility and other cellular processes.
Trends Cell Biol. 14, 461–469 (2004). CAS PubMed Google Scholar * Kimelman D., Xu W. β-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 25,
7482–7491 (2006). PubMed Google Scholar * Oeckinghaus, A. & Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 1, a000034 (2009).
PubMed PubMed Central Google Scholar * Hinkal, G., Morel, A. & Lie, M. Generation of Breast Cancer Stem Cells through Epithelial-Mesenchymal Transition. 3, 1–7 (2008). Google Scholar
* Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7), 3983–8
(2003). ADS CAS PubMed PubMed Central Google Scholar * Patrawala, L. et al. Side population is enriched in tumorigenic; stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells
are similarly tumorigenic. Cancer Res. 65(14), 6207–19 (2005). CAS PubMed Google Scholar * Karamboulas, C. & Ailles, L. Developmental signaling pathways in cancer stem cells of solid
tumors. BBA - Gen. Subj. 1830, 2481–2495 (2013). CAS Google Scholar * Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 25, 409–416 (2006). CAS PubMed
Google Scholar * Bist, P., Phua, Q. H., Shu, S. et al. Annexin-A1 controls an ERK-RhoA-NFκB activation loop in breast cancer cells. Biochem. Biophys.Res. Commun. 461, 47–53 (2015). CAS
PubMed Google Scholar * Kutsuna, H. et al. Actin reorganization and morphological changes in human neutrophils stimulated by TNF, GM-CSF, and G-CSF: the role of MAP kinases. Am. J. Physiol
Cell Physiol. 286, C55–C64 (2004). CAS PubMed Google Scholar * Papakonstanti, E. A. & Stournaras, C. Tumor necrosis factor-α promotes survival of opossum kidney cells via
Cdc42-induced phospholipase C-γ1 activation and actin filament redistribution. Mol. Biol. Cell. 15, 1273–1286 (2004). CAS PubMed PubMed Central Google Scholar * Zou, L., Ding, Z. &
Roy, P. Profilin-1 overexpression inhibits proliferation of MDA-MB-231 breast cancer cells partly through p27kip1 upregulation. J. Cell Physiol. 223, 623–629 (2010). CAS PubMed PubMed
Central Google Scholar * Witke, W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 14, 461–469 (2004). CAS PubMed Google Scholar *
Noritake, J., Watanabe, T., Sato, K., Wang, S. & Kaibuchi, K. IQGAP1: a key regulator of adhesion and migration. J. Cell Sci. 118, 2085–2092 (2005). CAS PubMed Google Scholar *
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol. Cell Biol. 15, 178–196 (2014). CAS PubMed PubMed Central Google Scholar *
Wu, Y. & Zhou, B. P. TNF-α, NFκB, Snail Pathway in Cancer. BJC. 102, 639–644 (2010). CAS PubMed PubMed Central Google Scholar * Blick, T. et al. Epithelial Mesenchymal Transition
Traits in Human Breast Cancer Cell Lines Parallel the CD44 hi/CD24 lo/− Stem Cell Phenotype in Human Breast Cancer. Journal of Mammary Gland Biology and Neoplasia. 15(1), 235–252 (2010).
PubMed Google Scholar * Wang, X. et al. The role of cancer stem cells in cancer metastasis: New perspective and progress. Cancer Epidemiol. 37, 60–63 (2013). CAS PubMed Google Scholar *
Velasco-Velázquez, Marco A. et al. The Role of Breast Cancer Stem Cells in Metastasis and Therapeutic Implications. The American Journal of Pathology 179(1), 2–11 (2011). PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS We thank, Dr. S. C. Mande, Director, National Centre for Cell Science (Pune, India) for encouragement and support. We would like
to express gratitude to The Department of Science and Technology (DST), India for funding the project. AUTHOR INFORMATION Author notes * Dasgupta Aparajita, Sawant Mithila A. and Kavishwar
Gayatri contributed equally to this work. AUTHORS AND AFFILIATIONS * National Centre for Cell Science, NCCS Complex, S.P. Pune University, Ganeshkhind, Pune, 411007, Maharashtra, India
Aparajita Dasgupta, Mithila A. Sawant, Gayatri Kavishwar & Sandhya Sitasawad * Pharmazz India Private Limited, H-6, Site-C, Surajpur Industrial area, Greater Noida, 201307, UP, India
Manish Lavhale Authors * Aparajita Dasgupta View author publications You can also search for this author inPubMed Google Scholar * Mithila A. Sawant View author publications You can also
search for this author inPubMed Google Scholar * Gayatri Kavishwar View author publications You can also search for this author inPubMed Google Scholar * Manish Lavhale View author
publications You can also search for this author inPubMed Google Scholar * Sandhya Sitasawad View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS A.D., M.S., and G.K. designed research work, performed _in vitro_ and animal experiments and drafted the manuscript. S.S., the sole corresponding author, supervised the project
and helped to draft the manuscript. M.L. carried out the isolation, purification and characterization of AECHL-1. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not
included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Dasgupta, A., Sawant, M., Kavishwar, G. _et al._ AECHL-1 targets breast cancer
progression via inhibition of metastasis, prevention of EMT and suppression of Cancer Stem Cell characteristics. _Sci Rep_ 6, 38045 (2016). https://doi.org/10.1038/srep38045 Download
citation * Received: 24 June 2016 * Accepted: 02 November 2016 * Published: 15 December 2016 * DOI: https://doi.org/10.1038/srep38045 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative