Play all audios:
ABSTRACT Irreversible electroporation (IRE) is a promising non-thermal treatment for inoperable tumors which uses short (50–100 μs) high voltage monopolar pulses to disrupt the membranes of
cells within a well-defined volume. Challenges with IRE include complex treatment planning and the induction of intense muscle contractions. High frequency IRE (H-FIRE) uses bursts of
ultrashort (0.25–5 μs) alternating polarity pulses to produce more predictable ablations and alleviate muscle contractions associated with IRE. However, H-FIRE generally ablates smaller
volumes of tissue than IRE. This study shows that asymmetric H-FIRE waveforms can be used to create ablation volumes equivalent to standard IRE treatments. Lethal thresholds (LT) of 505 V/cm
and 1316 V/cm were found for brain cancer cells when 100 μs IRE and 2 μs symmetric H-FIRE waveforms were used. In contrast, LT as low as 536 V/cm were found for 2 μs asymmetric H-FIRE
waveforms. Reversible electroporation thresholds were 54% lower than LTs for symmetric waveforms and 33% lower for asymmetric waveforms indicating that waveform symmetry can be used to tune
the relative sizes of reversible and irreversible ablation zones. Numerical simulations predicted that asymmetric H-FIRE waveforms are capable of producing ablation volumes which were
5.8–6.3x larger than symmetric H-FIRE waveforms indicating that _in vivo_ investigation of asymmetric waveforms is warranted. SIMILAR CONTENT BEING VIEWED BY OTHERS ELECTROPORATION AND CELL
KILLING BY MILLI- TO NANOSECOND PULSES AND AVOIDING NEUROMUSCULAR STIMULATION IN CANCER ABLATION Article Open access 02 February 2022 EFFECTS OF BIPOLAR IRREVERSIBLE ELECTROPORATION WITH
DIFFERENT PULSE DURATIONS IN A PROSTATE CANCER MOUSE MODEL Article Open access 30 April 2024 EFFECTS OF DIFFERENT APPLIED VOLTAGES OF IRREVERSIBLE ELECTROPORATION ON PROSTATE CANCER IN A
MOUSE MODEL Article Open access 26 December 2022 INTRODUCTION Irreversible electroporation (IRE) is an emerging cancer therapy which uses high intensity electrical pulses to focally ablate
solid tumors1. Clinically, two or more needle electrodes are advanced around a target tumor. A series of approximately 100 electrical pulses, 1000 to 3000 V in amplitude and 50 to 100 μs in
duration, are then delivered. These electrical pulses locally increase cell transmembrane potentials above a critical lethal threshold to create permanent nanoscale defects, which result in
rapid cell death. IRE ablations exhibit a characteristic sub-millimeter transition between complete cell death to unaffected tissue due to the rapid change in electric field intensity near
the electrodes. Small cohort studies of IRE for the treatment of inoperable tumors smaller than 3 cm show complete response rates between 93 and 98%2,3. These favorable early clinical
results have encouraged significant interest in the use of IRE for the treatment of liver4, pancreatic5,6,7, kidney8, prostate9,10,11,12,13, and brain14,15,16,17 tumors. Though IRE is a
promising emerging procedure, there are some clinical challenges which may impede widespread adoption of the therapy. The long duration electrical pulses create local and systemic muscle
contractions and may inadvertently interact with cardiac rhythms18. To alleviate this, patients must receive significant doses of chemical paralytics and pulse delivery is synchronized with
the heart-beat to ensure pulses are delivered during the absolute refractory period19. This is challenging since it significantly changes the clinical workflow in comparison to other focal
therapies and can increase the overall treatment time. Additionally, the ablation zone produced by IRE is dependent on local dynamic electrical properties of the tissue and heterogeneities
can potentially distort the electric field and produce irregular shaped ablations20. High frequency irreversible electroporation (H-FIRE) is a new protocol designed to address these
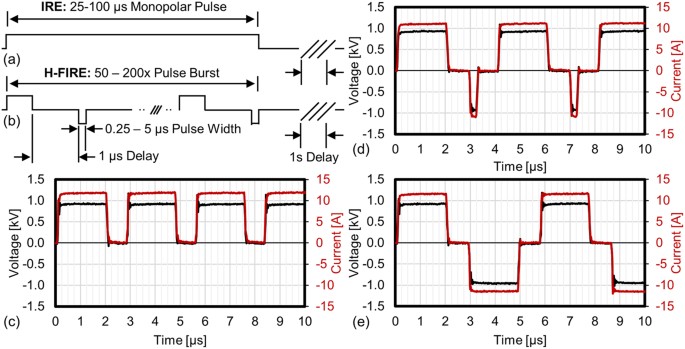
challenges. H-FIRE replaces the long duration monopolar IRE pulses (Fig. 1a) with a burst of alternating polarity pulses between 250 ns and 5 μs (Fig. 1b). The electrical conductivities of
skin, dura, tendons, blood vessels, and glandular tissues appear to converge at frequencies around 1 MHz21 resulting in H-FIRE ablations which more closely match analytical predictions in
simple tissue models22. Numerical analysis predict that these waveforms can short through skin and fat layers to produce more uniform ablations than IRE23. H-FIRE waveforms effectively
eliminate muscle contractions in small animal models24 and these treatments have been used to treat spontaneous tumors in large animals without the need for general anesthesia or chemical
paralysis25. H-FIRE treatments have been shown to significantly inhibit tumor growth in murine flank tumor models25 and it was recently demonstrated that H-FIRE treatments preferentially
target malignant cells in co-cultured 3D tumor models26. Limitations of H-FIRE include the ablation size, which is typically smaller than with conventional IRE and the need for specialized
electronics which can deliver the appropriate alternating polarity electrical pulses. _In vivo,_ IRE is typically observed in regions which are exposed to approximately 500–750 V/cm14,27,28.
Preliminary studies indicate that for equivalent energy treatments the lethal electric field is higher for H-FIRE than for IRE treatments25,26,29. Using a 3D _in vitro_ pancreatic tumor
model, Arena _et al_. found a lethal threshold of 500 V/cm when 80 × 100 μs IRE pulses were delivered30. The lethal electric field thresholds for equivalent energy H-FIRE protocols in the
same model were much higher, at 2022, 1687, 1070, and 755 V/cm for bursts containing 0.25, 0.5, 1, and 2 μs symmetric waveforms, respectively25. Given this increase in lethal threshold, to
successfully treat 1–3 cm tumors, these H-FIRE protocols would require a pulse generator which outputs significantly higher voltages than the 3000 V maximum currently implemented in the
clinic. Given these practical limitations, we present an alternate approach to decrease lethal thresholds and increase ablation volumes through the use of asymmetric pulse waveforms. Three
experiments were conducted to evaluate the implications of pulse asymmetry in electroporation therapies. First, to investigate the impacts of culture conditions on IRE and H-FIRE outcomes,
U87 cells were grown in either 2D monolayer or 3D cultures and exposed to an equivalent delivered energy in several different waveforms. These preliminary experiments mirror clinical
protocols which deliver 100 μs monopolar pulses 100 times. Second, to demonstrate clinical utility against multiple forms of cancer, the lethal thresholds for U87 and MDA-MB-231 BR3 cells
were examined in response to a broad array of pulse waveforms, comparing duration of energized bursts and pulse asymmetry. Third, to investigate if waveform asymmetry has any relevant
implications for electro-chemotherapy (ECT)31 or electro-gene therapy (EGT)32, reversible electroporation thresholds were evaluated by adding membrane impermeable propidium iodide (PI) to 2D
monolayers of each cell type prior to treatment. In this third experiment, cell cultures were exposed to lower energy treatments with 100x bursts where each burst was energized for 50 μs.
Finally, the reversible and lethal thresholds found _in vitro_ were incorporated into a three-dimensional finite element model to predict the size and shape of ablations which would be
created by H-FIRE waveforms if 3 kV pulses were delivered into live tissue through clinical electrodes. These models were then validated against ablations created in _ex vivo_ liver tissue.
It was found that both 2D and 3D culture conditions adequately replicate IRE lethal thresholds found _in vivo_ and that 2D cultures are acceptable for investigating H-FIRE protocols.
Waveforms which incorporate asymmetry had lower lethal thresholds and result in larger ablation zones than symmetric H-FIRE waveforms (Fig. 1c–e). This indicates that the elevated lethal
thresholds previously found in H-FIRE treatments are likely the result of waveform symmetry, and reduced lethal thresholds can be achieved with asymmetric H-FIRE waveforms. It was found that
the differences between reversible electroporation and lethal thresholds were substantially larger for symmetric waveforms than for asymmetric waveforms indicating that ECT and EGT
treatment protocols may benefit from the use of symmetric waveforms. Finally, numerical models using experimentally determined lethal thresholds indicate that asymmetric H-FIRE waveforms can
be used to produce 5.8–6.3x larger ablation volumes than symmetric waveforms. These results indicate that waveform asymmetry is a useful new parameter which can be used to modulate
treatment outcomes and _in vivo_ investigation of these waveforms is warranted. METHODS PULSE DELIVERY All treatments were delivered through two blunt 1.29 mm diameter (16 gauge) stainless
steel needles with a 3 mm edge-to-edge separation. A custom pulse generator was used to deliver IRE and H-FIRE protocols which were either monopolar: all pulses were positive polarity (Fig.
1c), asymmetric bipolar: positive and negative pulses had different durations (Fig. 1d), or symmetric bipolar: positive and negative pulses were the same duration (Fig. 1e). To simplify
discussion of these protocols we use the notation P - 1 - N where P indicates the positive pulse length and N indicates the negative pulse length in microseconds. All protocols used a 1 μs
delay between changes in pulse polarity. For example, 2–1–0.25 represents a pulse waveform with a 2 μs positive pulse, followed by a 1 μs delay, then a 0.25 μs negative pulse as shown in
Fig. 1d. To compare equivalent energy IRE and H-FIRE treatments, the H-FIRE waveforms were repeated in a burst such that the total energized time of each burst was equivalent to 50 or 100
μs. In cases where waveforms could not produce energized times of exactly 50 or 100 μs, the closest energized time without exceeding the target time was used. In all treatment groups bursts
were delivered with a repetition rate of 1 Hz and the output of the generator was set to deliver pulses which were 900 V in amplitude. CELL CULTURE _In vitro e_xperiments were conducted on a
glioblastoma cell line (U87, American Type Culture Collection Inc. Manassas, VA) and cells isolated from murine brain metastases after inoculation with human breast cancer cells (MDA-MB-231
BR3, a generous gift from the Laboratory of Martin Brown, Stanford, CA)33. These cells were chosen to model primary and metastatic brain tumors, respectively, and to evaluate potential
changes in treatment response by cells of different disease origins. The cells were cultured to 80% confluency in High Glucose Dulbecco’s Modified Eagle Medium with L-Glutamine (catalog
number 12800-082, Invitrogen Inc., Carlsbad, CA), which was supplemented with 10% heat inactivated Fetal Calf Serum (catalog number S11150, lot G14092, Atlanta Biologicals Inc.,
Lawrenceville, GA), 0.2 g/L Streptomycin Sulfate (catalog number 11860-038, Invitrogen Inc., Carlsbad, CA), and 0.126 g/L Penicillin G Potassium Salt (catalog number P7794-100MU,
Sigma-Aldrich Inc., St. Louis, MO). Cells were harvested via trypsinization and seeded either directly onto the bottom of 12 well plates (2D culture) or in a 50% mixture of Matrigel (catalog
number 356235, Corning Inc., Corning, NY) and culture media (3D culture) at a density of 100 k cells/mL. These cells were then allowed to reach confluent cultures over 4–7 days over which
time cell culture media was replaced as necessary. Prior to treatment, media was removed and replaced with 2 mL of fresh culture media. ASSESSMENT OF REVERSIBLE AND IRREVERSIBLE
ELECTROPORATION Stock solutions of 4 μM Calcein AM (Life Technologies, Carlsbad, CA) were prepared by adding 125 μL of sterile dimethyl sulfoxide to 50 μg of powdered dye and stored at −20
°C until use. Stock solutions of propidium iodide (PI) (MP Biomedicals Inc., Burlingame, CA) were prepared using a concentration of 1 mg/mL PI in sterile phosphate-buffered saline and stored
at 4 °C until use. Reversible electroporation thresholds were assessed by adding 100 μL/mL of PI stock solutions to each well 5 minutes prior to treatment. The samples were imaged
immediately after treatment using a fluorescence microscope with an automated stage (Leica DMI600 B, Buffalo Grove, IL) using a red fluorescent protein filter cube (540–552 nm/580–620 nm) to
visualize the electroporated population which had been counter-stained red by the PI (Fig. 2d). The height (y-axis) and width (x-axis) of each treatment zone was measured using the
microscope software’s built in measurement tool. To assess lethal thresholds, cells were treated with IRE or H-FIRE protocols and incubated at 37 °C in 5% CO2 for 24 hours prior to staining.
Calcein AM (2 μL/mL) and PI (100 μL/mL) stock solutions were then added to the cells and the ablation zone was imaged using green fluorescent protein (460–500 nm/512–542 nm) and red
fluorescent protein filter cubes (Fig. 3) and measured using the microscope software’s built in measurement tool. NUMERICAL CALCULATION OF REVERSIBLE AND LETHAL THRESHOLDS To calculate the
reversible electroporation or lethal threshold for each experimental treatment, the electric field distribution within a standard 12 well chamber was modeled numerically using COMSOL
Multiphysics (V5.2a, COMSOL Inc., Palo Alto, CA) via the 3D Electric Currents module. This module solves the equations: , , and where J is the local current density, Q is the electric
charge, E is the electric field, JEis the external current density, and V is the local voltage. A free tetrahedral mesh was generated in the media domain using the _Extremely Fine_ preset
while all other domains were meshed using the _Normal_ preset values. The media domain was then refined twice using a refinement method which splits each tetrahedron along the longest edge
(Fig. 2a,b). Experimental voltages were applied to the top most surface of one electrode. The top surface of the adjacent electrode was set to ground (0 V). All other external boundaries
were set as insulators (). The electrical conductivity (σ) was set to 1.2 S/m for the media, 2.22 × 106 S/m for the electrodes, and 1 × 10−16 S/m for the plastic well plate components. These
simulations required approximately 29 minutes to solve on an Intel i7 processor with 32 GB RAM. The electric field distributions (Fig. 2c) along the x- and y-axis were exported into a
spreadsheet where they were used to correlate treatment geometries (Fig. 2d) with electric field values. Each treatment zone was measured and an electric field value correlating the height
and width were determined. Each experimental parameter was repeated a minimum of three times (N = 3) yielding at least six electric field values which were averaged and reported as mean ±
standard deviation. Statistical significance between groups was determined using a two-sided Student’s T-test with unequal variances. An alpha value of 0.01 (α = 0.01), 99% confidence
interval, was used to determine if thresholds between treatment groups were statistically significantly different. Finite element models were used to predict clinical ablations created by
different H-FIRE waveforms (Supplemental Figure S1). These models used a dynamic tissue conductivity function8,14,34 with tissue conductivities derived from _in vivo_ experiments11 and
transitions between these conductivities centered at the reversible electroporation thresholds for each waveform found in this study. Methods for producing these models are presented in the
supplemental material. To validate these numerical models, predicted ablations were compared to ablations created in _ex vivo_ liver tissue using NanoKnife electrodes (AngioDynamics Inc.,
Latham, NY). To match the simulations, a single needle and grounding pad configuration35 was used. _Ex vivo_ porcine livers were obtained from the Stanford University Veterinary Service
Center Necropsy Laboratory in accordance with the University’s guidelines and regulations. Whole organs were submerged in a water tank containing a 3 g/L NaCl solution with a conductivity of
0.6 S/m. A single electrode was inserted into the tissue and a 0.254 mm aluminum sheet was placed 10 cm from the liver to serve as a current sink, simulating a distant grounding pad. 100 ×
2-1-2 H-FIRE bursts with each burst energized for 100 μs at approximately 3.5 kV were delivered to a minimum of three locations for each electrode configuration tested. The experiments were
repeated using two different clinical probes for the NanoKnife, the “monopolar probe” (MP probe), a 1 mm diameter 1 cm long electrode and the “bipolar probe” (BP probe) which contains two
0.7 cm electrodes on a single 1.27 mm diameter probe. For the BP probe, the distal electrode was energized and the proximal electrode was disconnected. All experiments were conducted within
four hours post-mortem. The tissue was sectioned and soaked in a 10 g/L tetrazolium chloride (TTC) solution for 20 minutes to dye metabolically active regions red and expose regions of
tissue which had been irreversibly electroporated (Supplemental Figure S2). The length and width of the ablation zones were then measured using calipers and values were reported as mean ±
standard deviation (Supplemental Figure S3). RESULTS 2D VERSUS 3D CULTURE CONDITIONS A total of 16 protocols were examined in both 2D monolayer cultures and 3D Matrigel cultures (Fig. 4).
All treatments delivered 100x bursts with each burst energized for a total of 100 μs. In 14 of 16 groups there was not a statistically significant difference (α = 0.01) between the lethal
thresholds found in 2D and 3D culture platforms. In the two treatments which were statistically different, the mean lethal thresholds differed by an average of 18%. In groups which were not
found to be statistically different, the mean lethal thresholds differed by an average of 9%. For U87 cells exposed to 100 μs monopolar IRE pulses, the lethal thresholds were 464 ± 38 V/cm
and 505 ± 23 V/cm for 3D and 2D culture, respectively. Ivey _et al_. found a lethal threshold of 492 ± 41 V/cm for U87 cells treated with an identical IRE protocol when cells were cultured
in 3D gels made with a 2% collagen mixture26 rather than the Matrigel material used in this study. These results indicated that culture conditions had a relatively small impact on lethal
thresholds in comparison to other experimental parameters (e.g. waveform symmetry). 2D culture protocols were substantially less complex and less expensive than those required for producing
and maintaining 3D cultures. Based on these factors, 2D culture conditions were used for all additional experiments to maximize the number of experimental parameters which could be
evaluated. EFFECT OF WAVEFORM ASYMMETRY ON LETHAL THRESHOLDS The lethal thresholds for all waveforms and cell types evaluated in 2D culture are presented in Table 1. Introducing asymmetry
into the pulse waveform produced measurable decreases in lethal thresholds. This effect was the largest for waveforms in which the longest pulse was 1 μs in duration (e.g. 1-1-1 vs. 1 μs
mono) and became less prominent as pulses became longer in duration. For U87 cells in 2D culture, the symmetric 50-1-50 and monopolar 100 μs waveforms had lethal thresholds of 603 ± 34 V/cm
and 505 ± 23 V/cm, respectively (Fig. 4a). A relative decrease of 98 V/cm. In contrast, the symmetric 5-1-5 and the monopolar 5 μs waveform H-FIRE waveforms had lethal thresholds of 967 ± 88
V/cm and 499 ± 26 V/cm, respectively. A relative decrease of 468 V/cm. Similar results were found for the 2 μs (Fig. 4c) and 1 μs (Fig. 4d) protocols. It is interesting to note that the
monopolar 5 μs and 2 μs waveforms had lethal thresholds which were not significantly different (α = 0.01) than the monopolar 100 μs waveform while the 5 μs and 2 μs symmetric waveforms had
substantially higher lethal thresholds than the monopolar 100 μs waveform. When the longest pulse in the waveform was either 1 μs or 2 μs, introducing asymmetry into the waveform by using a
0.5 μs pulse decreased the lethal threshold by an average of 25% and using a 0.25 μs pulse decreased the lethal threshold by an average of 45%, compared to the associated symmetric (1-1-1 or
2-1-2) waveforms. Use of a monopolar waveform decreased the lethal threshold by an average of 56% compared to the associated symmetric waveforms. All asymmetric H-FIRE treatment groups
which were monopolar or incorporated 0.25 μs pulses had statistically significantly lower (α = 0.01) lethal thresholds than the associated symmetric waveforms. Additionally, the lethal
thresholds for U87 cells exposed to 1-1-0.5 and 2-1-0.5 waveforms were significantly lower than those exposed to 1-1-1 and 2-1-2 waveforms, respectively. 50 ΜS VERSUS 100 ΜS ENERGIZED TIMES
IRE is often employed clinically when tumors are in close proximity to critical nerves or blood vessels where tissue heating can result in deleterious outcomes. A simple way to minimize
heating is to reduce the pulse-length (IRE) or energized time per burst (H-FIRE). Figure 5 shows the impact that reducing the energized time from 100 μs to 50 μs has on lethal thresholds for
the U87 cell lines. The results for both cell lines are summarized in Table 1. The IRE waveforms in Fig. 5a which were energized for 100 μs had lethal thresholds which were 20% lower on
average than those energized for 50 μs. For H-FIRE treatments (Table 1), most treatment groups which received bursts energized for 100 μs had statistically significantly lower lethal
thresholds than the 50 μs groups. However, this increase in energized time did not impact the lethal thresholds for U87 cells treated with 1-1-1 and 1-1-0.5 waveforms. Despite the relatively
small differences found for these two waveforms, the lethal thresholds found for 100 μs H-FIRE treatments were 20% lower than those energized for 50 μs when averaged across all H-FIRE
waveforms. REVERSIBLE ELECTROPORATION THRESHOLDS The reversible electroporation thresholds observed immediately after treatment are presented in Table 2 for both cell types and are shown in
Fig. 6 for U87 cells. For the 50 μs monopolar, 25-1-25, 13-1-37, and 10-1-40 waveforms (Fig. 6a), the average reversible electroporation threshold of 303 V/cm was 40% lower than the lethal
threshold when averaged across both cell types. There was not a statistically significant difference between the reversible electroporation thresholds between these waveforms. Interestingly,
there was some variation in reversible electroporation thresholds with H-FIRE waveforms (Fig. 6b–d). The largest differences between reversible and lethal thresholds occurred for the 1-1-1
and 2-1-2 symmetric bipolar waveforms. The 2-1-2 waveform resulted in the largest difference of 837 V/cm for U87 (49% lower). In contrast, the 2 μs monopolar waveform only had a difference
of 289 V/cm between the lethal and reversible electroporation thresholds and there was not a statistically significant difference for the 1-1-2 waveform for either cell type. For 1 μs H-FIRE
waveforms (Fig. 6c), the 1-1-1 waveform resulted in the largest difference of 992 V/cm (54% lower) for U87 cells between the reversible and lethal thresholds. The smallest difference
between these two thresholds of 315 V/cm was found for the 1-1-0.25 waveform. Sub-microsecond H-FIRE protocols were also evaluated (Fig. 6d). For U87 cells there was no statistically
significant difference between the reversible and lethal thresholds for the 0.25-1-0.25, 0.5-1-0.5, and 0.5-1-0.25 waveforms. However, the asymmetric 0.5-1-0.25 waveform had lower reversible
and irreversible thresholds than the 0.25-1-0.25 and 0.5-1-0.5 symmetric waveforms. U87 VS MDA-MB-231 BR3 CELL LINES The lethal thresholds for U87 cells were lower than for MDA-MB-231 BR3
cells for all waveforms in Table 1, the mean thresholds were 36% lower for U87 cells. The largest difference observed (137%) was for the 1-1-0.5 waveform when 100 × 50 μs bursts were
delivered. The smallest difference (13%) was observed for the 33-1-17 waveform. The 50 and 100 μs IRE groups in Fig. 5a had the smallest average difference in lethal threshold between cell
types at 26%. The average difference between cell types increased to 31% for 2 μs (Fig. 5b) and 49% for 1 μs (Fig. 5c) H-FIRE waveforms. The reversible electroporation thresholds were lower
for U87 cells than for MDA-MB-231 BR3 cells in 11 of 16 waveforms evaluated in Table 2. Only the 0.5-1-0.5 waveform had a reversible electroporation threshold for U87 cells which was
statistically significantly greater than for MDA-MB-231 BR3 cells. The 2-1-2, 1-1-2, 0.5-1-0.25, and 0.25-1-0.25 waveforms had also had mean reversible electroporation thresholds which were
higher for the U87 cells, however these were not statistically significant different between cell types. MODELING THE IMPACT OF PULSE ASYMMETRY ON CLINICAL ABLATIONS Finite element models
which incorporate the reversible and lethal electric field thresholds found here were used to predict how different H-FIRE waveforms may affect the size and geometry of ablations created
using clinical ablation electrodes and pulse voltages used in the treatment of solid tumors. The methods for creating these models and validation against ablations created in _ex vivo_ liver
tissue are presented in the supplemental document. Symmetric H-FIRE waveforms required significantly higher electric fields to induce cell death compared to asymmetric waveforms. This
resulted in the production of smaller predicted ablations for symmetric waveforms compared to the asymmetric waveforms when calculated using experimentally determined lethal thresholds
(Supplemental Figure S1c-g). A simulated treatment delivering 100 × 3.0 kV 50 μs monopolar pulses through a 1 cm electrode exposure (MP Probe) predict an ablation measuring 1.7 × 1.0 cm. For
the same 50 μs energized time, the 2 μs monopolar waveform produced the largest simulated H-FIRE ablation of 1.6 × 0.9 cm followed by 1.6 × 0.8 cm, 1.5 × 0.7 cm, and 1.3 × 0.4 cm for the
2-1-0.25, 2-1-0.5, and 2-1-2 waveforms, respectively (Supplemental Table S1). Volumetrically, ablations created by the 2 μs monopolar waveforms were 6.3x larger than the symmetric 2-1-2
waveform. Increasing the energized time from 50 μs to 100 μs resulted in a decrease in lethal threshold of approximately 20% averaged across all waveforms evaluated in this study. 1.8 × 1.1
cm, 1.7 × 1.0 cm, 1.7 × 1.0 cm, 1.6 × 0.9 cm, 1.4 × 0.5 cm simulated ablation zones were created for the 100 μs mono (505 V/cm), 2 μs mono (536 V/cm), 2-1-0.25 (594 V/cm), 2-1-0.5 (700
V/cm), and 2-1-2 (1316 V/cm) waveforms, respectively. Under these conditions, the 2 μs monopolar waveform produced a simulated ablation volume which was 5.8x larger than the symmetric 2-1-2
waveform. _EX VIVO_ RESULTS VERSUS NUMERICAL PREDICTIONS Experimental ablations were created in _ex vivo_ porcine liver by delivering 100 × 3.5 kV bursts of the 2-1-2 waveform (Supplemental
Figure S2). Each burst was energized for 100 μs and bursts were delivered at a repetition frequency of 1 Hz. Experimental ablations created using the clinical MP probe with a 1 cm electrode
exposure had an average length of 1.3 ± 0.3 cm and an average width of 0.7 ± 0.2 cm (Supplemental Figure S3). The numerical model of the MP probe predicted an ablation measuring 1.4 × 0.6 cm
when these experimental conditions were simulated (Supplemental Table S2) and the results were within 0.1 cm of the average ablation created experimentally (1.3 × 0.7 cm). Experimental
ablations created using the clinical BP probe had an average length of 1.4 ± 0.1 cm and an average width of 0.9 ± 0.2 cm (Supplemental Figure S3). The numerical model of the BP probe
predicted an ablation measuring 1.2 × 0.7 cm using these experimental parameters (Supplemental Table S3) and the results were within 0.2 cm of the average ablation created experimentally
(1.4 × 0.9 cm). This difference of one to two millimeters in length and width between the average experimental and predicted ablations serve as a preliminary validation of the ability of
these models, which incorporate dynamic tissue conductivity with reversible and lethal thresholds found _in vitro_, to predict ablation dimensions in tissue. DISCUSSION 2D VERSUS 3D CULTURE
CONDITIONS H-FIRE is a relatively new electroporation protocol. The symmetric waveforms were first proposed by Arena _et al_. as a method for creating more uniform ablations in heterogeneous
tissues23. Early _in vitro_ experiments on cells in suspension showed that these waveforms required substantially higher electric fields (2–2.7x) to induce cell death versus the standard
100 μs monopolar IRE waveforms29. When cells were grown in 3D collagen tumor models the lethal electric field threshold decreased to 1.5x and 2.2x the lethal threshold for equivalent energy
2 μs and 1 μs symmetric H-FIRE protocols, respectively. It is hypothesized that the decrease in the ratios of lethal thresholds between IRE and H-FIRE protocols is likely due to cells
transforming from a spherical shape in suspension into a more complex natural geometry when cultured in 3D models29. Arena _et al_. found a lethal threshold of 500 V/cm for pancreatic cancer
cells when delivering 80 × 100 μs monopolar IRE pulses in this 3D model30. The lethal thresholds for U87 brain cancer cells found in this study were 463 V/cm and 505 V/cm in 3D and 2D
models, respectively, when 100 × 100 μs monopolar IRE pulses were delivered. Previous studies found lethal thresholds in liver tissue between 300 and 640 V/cm using similar IRE
protocols36,37,38, indicating that both the 2D and 3D culture models recapitulate effects seen in mammalian tissue. Fourteen of sixteen protocols conducted in 2D and 3D conditions showed no
statistically significant difference in lethal threshold indicating that the 3D model has a limited effect on the lethal threshold and cells cultured in a 2D monolayer are a relatively
effective model for studying H-FIRE. 2D monolayers are a convenient model as they are relatively simple to generate, are less expensive, and less time intensive to create compared to 3D
cultures. However, when the thresholds were statistically different between groups, the 3D cultures all had lower lethal thresholds. This indicates that further investigation in 3D
constructs may be warranted, especially if _in vivo_ results are found to differ significantly from those found in 2D monolayers. U87 VS MDA-MB-231 BR3 CELL LINES The MDA-MB-231 BR3 cells
appeared to stretch out into a flattened oval shape with few protrusions. These cells continued to divide as the cultures approached confluency and appeared to overlap with each other to
cover the entire well plate surface. In contrast, the U87 cells formed more star shaped geometries with multiple elongated protrusions. They formed a web of interconnected cells and cell
division slowed as they became confluent. The networks of U87 cells had numerous gaps of unoccupied well plate area. While not extensively examined here, the differences in lethal thresholds
observed between cell types may be due to differences in cell geometry affecting the charging time of the cell membrane. Ivey _et al_. found identical lethal thresholds for U87, NHA, and
D1TNC1 cell lines after treatment with 100 μs monopolar pulses, but significantly different lethal thresholds between U87 cells and the astrocyte (NHA and D1TNC1) cell lines when using a
1-5-1 H-FIRE waveform26. These differences in lethal threshold were hypothesized to be a function of the morphology of the cell including the nucleus-to-cytoplasm ratio26 and morphological
factors may have impacted the observed differences between U87 and MDA-MB-231 BR3 cells shown here. EFFECTS OF WAVEFORM ASYMMETRY AND ENERGIZED TIME For H-FIRE, waveform asymmetry appeared
to be the biggest factor affecting the lethal threshold. Doubling the dose delivered by energizing the bursts for 100 μs rather than 50 μs decreased the lethal threshold by an average of
20%. In contrast, the lethal thresholds for asymmetric waveforms were 42% lower than the symmetric waveforms, averaged across all H-FIRE groups. Waveforms incorporating a 0.5 μs or 0.25 μs
pulses were 25% and 45% lower than symmetric waveforms, respectively. When only monopolar 1 μs or 2 μs pulses were used in the burst, the lethal thresholds were 56% lower on average compared
to their respective symmetric (1-1-1 or 2-1-2) counterparts. We hypothesize that two potential mechanisms contribute to the decrease in lethal threshold when asymmetric waveforms are used:
a cumulative charging of the cell membrane and enhanced electrokinetic transport. Symmetric pulses may be inducing a cancellation effect39 in which the alternating polarity pulses
sequentially charge then discharge the potential across the cell membrane faster than this potential would dissipate in the absence of an alternating pulse. The symmetric H-FIRE waveforms
had the largest differences between reversible and lethal thresholds indicating that these waveforms are effective at facilitating pore formation, but the rapid charging and discharging of
the membrane may not have efficiently driven the pore expansion processes necessary to permanently disrupt the cell membrane. When asymmetric waveforms are used, the forced discharge caused
by the alternate polarity pulse occurs to a lesser extent than for symmetric waveforms resulting in a cumulative charging of the cell membrane by the longer duration pulses in the burst.
This cumulative charging likely increases the potential across the cell membrane above the threshold required for pore formation and then then drives pore expansion. Monopolar IRE pulses act
as a baseline to indicate the lowest electroporation thresholds possible under these experimental conditions. Monopolar H-FIRE waveforms which contained 5 μs or 2 μs pulses had nearly
identical lethal thresholds to equivalent 50 or 100 μs pulses. This indicates that the induced membrane potential does not decrease substantially during the 1 μs delay between subsequent
pulses in a monopolar H-FIRE burst. However, 1 μs monopolar H-FIRE waveforms were less efficient at inducing cell death than 2 μs or 5 μs monopolar waveforms indicating that this may be the
lower limit for inducing pore expansion. This 1 μs threshold is likely related to the charging time of the cell membrane which is dependent on a number of factors including cell geometry and
medium conductivity, but is typically calculated to be approximately 1 μs29,40. Previous studies on sub-microsecond pulses showed that increasing the number of monopolar pulses increased
the number, but not the size of nanopores formed in the membrane41. A similar phenomena may be occurring here in which continued delivery of pulses 1 μs or shorter leads to the formation of
numerous smaller pores rather than rapid cell membrane destruction caused by pore expansion and any lethal effects are due to mechanisms which occur post-treatment. Symmetric pulses may also
be inducing a cancelation effect which results in zero net movement of charged species. The introduction of asymmetry in the H-FIRE waveform may result in net electrokinetic transport of
charged molecules across the cell membrane resulting in conditions which make it challenging for the cell to regain homeostasis. It was found that monopolar 300 ns pulses resulted in a
higher degree of calcium mobilization and lower viabilities 24 hours post treatment compared to bipolar waveforms with inter-pulse delays of up to 10 μs39. Additionally, 100 μs monopolar
pulses resulted in significantly higher magnitudes of PI influx compared to equivalent 1-1-1 H-FIRE waveforms42 for cells in suspension. A combination of these phenomena may be occurring
with asymmetric H-FIRE waveforms resulting in the formation of larger pores due to pore expansion and increased molecular transport due to electrophoresis, however, the degree to which these
phenomena occur have yet to be investigated for asymmetric H-FIRE waveforms. REVERSIBLE ELECTROPORATION Typical ECT32 and EGT43,44 protocols use 2 to 2445 monopolar pulses between 100 μs46
and 20 ms47 to electroporate cells and electrophoretically drive molecules across the cell membrane. A challenge with these monopolar waveforms is that they induce some degree of
irreversible electroporation _in vivo_ due to the relatively small difference between their reversible and lethal thresholds. Previous studies by Miklavcic _et. al_ found a difference of 275
V/cm between the reversible and lethal thresholds in _in vivo_ rabbit liver tissue after the delivery of eight 10 μs pulses27 and the monopolar IRE pulses investigated in this study had
approximately 225–277 V/cm between the reversible and lethal thresholds. From a clinical prospective, symmetric H-FIRE waveforms may be more advantageous in ECT and EGT protocols, where the
intent is to transfer membrane impermeable material into the cell rather than inducing cell death, because the symmetric waveforms have a very large difference between their reversible and
irreversible thresholds (837–1573 V/cm). It is unclear why symmetric waveforms resulted in a larger difference between reversible and irreversible thresholds. However, we hypothesize that
the rapid charging and discharging of the cell membrane by the symmetric pulses inhibits pore expansion and instead results in an increase in the number, but not size of the pores formed41.
However, it is unclear if these symmetric waveforms will be capable of transporting molecules across biological membranes efficiently. Numerical simulations indicate that electrophoretic and
diffusive transport of molecules play roughly equivalent roles in the transport of small molecules following electroporation48 while electrophoresis49 and electroporation induced
endocytosis50 are implicated in the transport of larger molecules51. Symmetric bipolar wave forms were recently reported to be an effective means for transiently disrupting the blood brain
barrier52, however, reversible electroporation for chemotherapy and gene transfer have yet to be demonstrated _in vitro_ or _in vivo_ with these waveforms. We hypothesize that the
introduction of a small degree of asymmetry into the H-FIRE waveform (e.g. a 2.0-1-2.1 waveform) may be sufficient to induce electrophoretic movement of charged molecules without producing
large lethal ablation zones or inducing muscle contractions53. Andre _et al_. demonstrated that high voltage pulses to induce electroporation followed by low voltage pulses to enhance
electrophoretic transport were advantageous for _in vivo_ EGT49 and a similar strategy may be necessary with H-FIRE. However, further work will be necessary to determine optimal protocols
for using H-FIRE waveforms for these non-lethal protocols. MODELING THE IMPACT OF PULSE ASYMMETRY ON CLINICAL ABLATIONS The use of numerical models for predictive treatment planning is
relatively common for IRE procedures8,11,17,54,55, in part because real time ultrasound visualization of the ablation zones is challenging and follow up CT or MRI imaging may be required to
confirm total tumor coverage56. The model presented here builds upon previous dynamic conductivity models11,17,34 by incorporating reversible and lethal thresholds found _in vitro_ into the
dynamic conductivity function. Given recent data which indicates that H-FIRE may be able to preferentially target malignant cells26, this modeling approach may be necessary for predicting
ablations margins created in _in vivo_. Finite element models simulating clinical electrodes and treatment voltages (Supplemental Figure S1) indicate that asymmetric H-FIRE waveforms can
potentially be used to produce equivalent ablation volumes to standard IRE waveforms (Supplemental Table S1). A 3 kV treatment using a 2-1-2 waveform energized for 100 μs produced a 1.4 ×
0.5 cm simulated ablation (0.18 cm3 volume) as shown in Supplemental Figure S1g. In contrast, switching to a 2-1-0.25 waveform would increase the ablation volume 5.5-fold to 1.0 cm3 (1.7 ×
1.1 cm). Clinically, a single electrode and grounding pad configuration is not implemented because 100 μs pulses would stimulate muscles over such a wide of a volume of the body that
chemical paralytics may no longer be sufficient to eliminate muscle contractions. Symmetric H-FIRE waveforms have been shown to mitigate these contractions24 and eliminate the need for
chemical paralytics in large animal studies25 at the expense of creating smaller ablations25. Extensive _in vivo_ investigation will be necessary to evaluate if muscle contractions are
present when asymmetric H-FIRE waveforms are used or if chemical paralytics will be required to mitigate them. However, based on previous studies it is feasible that asymmetric H-FIRE
waveforms will be able to produce clinically relevant ablations using a single electrode. STUDY LIMITATIONS There are a number of important limitations to the current study. 2D and 3D _in
vitro_ cell cultures have been found to adequately recapitulate the lethal electric field thresholds required for IRE treatments to induce cell death _in vivo_. However, data on _in vivo_
lethal thresholds for H-FIRE treatments have yet to be published and it is unknown if values determined _in vitro_ will match those found _in vivo_. Here and in prior studies25,30, lethal
thresholds for IRE and H-FIRE waveforms were investigated approximately 24 hours post treatment. The mechanisms of cell death following H-FIRE are unclear, but appear to be a combination of
physical necrosis due to cell membrane destruction and apoptosis. However, mechanistic studies conducted for durations longer than 24 hours have yet to be reported. Preliminary studies on
cells in suspension showed that viability after H-FIRE treatment continued to decline between 1 and 24 hours post treatment29 and it is unclear if the ablation zones created in 2D or 3D
culture would continue to evolve over longer time periods. Theoretical clinical ablation zones were generated using numerical models which have previously been used for clinical treatment
planning17 combined with experimental electroporation threshold data generated in 2D culture. These models predict that asymmetric waveforms may be useful in increasing the size of H-FIRE
ablations _in vivo_, however, extensive experimental examination will be required to validate these results. CONCLUSION _In vitro_ models of primary and metastatic brain cancer were used to
show that the lethal threshold for H-FIRE treatments is affected by the symmetry of the waveform. Asymmetric waveforms, including monopolar waveforms, have significantly lower lethal
thresholds than equivalent energy symmetric waveforms. The use of asymmetric H-FIRE waveforms clinically should result in the creation of equivalent ablation volumes to those seen in IRE
procedures while mitigating muscle contractions caused by long duration pulses. These results indicate that _in vivo_ testing to determine anti-tumor efficacy of asymmetric H-FIRE waveforms
is warranted. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Sano, M. B. _et al_. Asymmetric Waveforms Decrease Lethal Thresholds in High Frequency Irreversible Electroporation Therapies.
_Sci. Rep._ 7, 40747; doi: 10.1038/srep40747 (2017). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. REFERENCES * Davalos, R. V., Mir, L. M. & Rubinsky, B. Tissue ablation with irreversible electroporation. _Annals of Biomedical Engineering_ 33, 223–231 (2005). CAS PubMed
Google Scholar * Scheffer, H. J. et al. Irreversible Electroporation for Nonthermal Tumor Ablation in the Clinical Setting: A Systematic Review of Safety and Efficacy. _J Vasc Interv
Radiol_ 25, 997–1011 (2014). PubMed Google Scholar * Szot, C. S., Buchanan, C. F., Freeman, J. W. & Rylander, M. N. 3D _in vitro_ bioengineered tumors based on collagen I hydrogels.
_Biomaterials_ 32, 7905–7912 (2011). CAS PubMed PubMed Central Google Scholar * Dunki‐Jacobs, E., Philips, P. & Martin, I. Evaluation of thermal injury to liver, pancreas and kidney
during irreversible electroporation in an _in vivo_ experimental model. _Br J Surg_ 101, 1113–1121 (2014). PubMed Google Scholar * Martin, R. C., McFarland, K., Ellis, S. & Velanovich,
V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. _J Am Coll Surgeons_ 215, 361–369 (2012). Google Scholar * Martin, R. C.,
McFarland, K., Ellis, S. & Velanovich, V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. _Ann Surg Oncol_ 20, 443–449 (2013).
Google Scholar * Martin, R. C. et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. _Ann Surg_
262, 486–494 (2015). PubMed Google Scholar * Neal, R. E. et al. _In Vivo_ Irreversible Electroporation Kidney Ablation: Experimentally Correlated Numerical Models. _Biomedical Engineering,
IEEE Transactions on_ 62, 561–569 (2015). Google Scholar * Onik, G., Mikus, P. & Rubinsky, B. Irreversible electroporation: implications for prostate ablation. _Technol Cancer Res
Treat_ 6, 295–300 (2007). PubMed Google Scholar * Rubinsky, J., Onik, G., Mikus, P. & Rubinsky, B. Optimal Parameters for the Destruction of Prostate Cancer Using Irreversible
Electroporation. _The Journal of Urology_ 180, 2668–2674 (2008). PubMed Google Scholar * Neal, R. E. et al. _In vivo_ characterization and numerical simulation of prostate properties for
non‐thermal irreversible electroporation ablation. _The Prostate_ 74, 458–468 (2014). PubMed Google Scholar * Valerio, M. et al. Initial assessment of safety and clinical feasibility of
irreversible electroporation in the focal treatment of prostate cancer. _Prostate Cancer Prostatic Dis_ 17, 343–347 (2014). CAS PubMed PubMed Central Google Scholar * Tomihama, R.,
Günther, E., Kim, D. & Stehling, M. Irreversible electroporation treatment for prostate adenocarcinomas: a safety outcome study. _Journal of Vascular and Interventional Radiology_ 26,
S121–S122 (2015). Google Scholar * Garcia, P. A. et al. Intracranial nonthermal irreversible electroporation: _in vivo_ analysis. _J Membr Biol_ 236, 127–136 (2010). CAS PubMed Google
Scholar * Ellis, T. L. et al. Nonthermal irreversible electroporation for intracranial surgical applications: laboratory investigation. _Journal of neurosurgery_ 114, 681–688 (2011). PubMed
Google Scholar * Garcia, P. A., Rossmeisl, J. H., Ellis, T. L. & Davalos, R. V. Nonthermal Irreversible Electroporation as a Focal Ablation Treatment for Brain Cancer. In _Tumors of
the Central Nervous System_. Volume 12 171–182 (Springer, 2014). * Rossmeisl, J. H. Jr et al. Safety and feasibility of the NanoKnife system for irreversible electroporation ablative
treatment of canine spontaneous intracranial gliomas. _Journal of neurosurgery_ 123, 1008–1025 (2015). CAS PubMed Google Scholar * Thomson, K. R. et al. Investigation of the safety of
irreversible electroporation in humans. _J Vasc Interv Radiol_ 22, 611–621 (2011). PubMed Google Scholar * Martin, R. C., Schwartz, E., Adams, J., Farah, I. & Derhake, B. M. Intra -
operative Anesthesia Management in Patients Undergoing Surgical Irreversible Electroporation of the Pancreas, Liver, Kidney, and Retroperitoneal Tumors. _Anesth Pain Med_ 5, e22786 (2015).
PubMed PubMed Central Google Scholar * Golberg, A., Bruinsma, B. G., Uygun, B. E. & Yarmush, M. L. Tissue heterogeneity in structure and conductivity contribute to cell survival
during irreversible electroporation ablation by electric field sinks. _Scientific reports_ 5 (2015). * Gabriel, C. _Compilation of the Dielectric Properties of Body Tissues at RF and
Microwave Frequencies_. (DTIC Document, 1996). * Bhonsle, S. P., Arena, C. B., Sweeney, D. C. & Davalos, R. V. Mitigation of Impedance Changes Due to Electroporation Therapy Using Bursts
of High-Frequency Bipolar Pulses. _Biomed Eng Online_ doi: 10.1186/1475-925X-14-S3-S3 (2015). * Arena, C. B., Sano, M. B., Rylander, M. N. & Davalos, R. V. Theoretical considerations of
tissue electroporation with high-frequency bipolar pulses. _IEEE Trans Biomed Eng_ 58, 1474–1482 (2011). PubMed Google Scholar * Arena, C. B. et al. High-frequency irreversible
electroporation (H-FIRE) for non-thermal ablation without muscle contraction. _Biomed Eng Online_ 10, 102 (2011). PubMed PubMed Central Google Scholar * Sano, M. B. et al. Bursts of
Bipolar Microsecond Pulses Inhibit Tumor Growth. _Scientific reports_ 5 (2015). * Ivey, J. W. et al. Targeted cellular ablation based on the morphology of malignant cells. _Scientific
reports_ 5 (2015). * Miklavcic, D., Semrov, D., Mekid, H. & Mir, L. M. A validated model of _in vivo_ electric field distribution in tissues for electrochemotherapy and for DNA
electrotransfer for gene therapy. _Biochim Biophys Acta_ 1523, 73–83 (2000). CAS PubMed Google Scholar * Edd, J. F., Horowitz, L., Davalos, R. V., Mir, L. M. & Rubinsky, B. _In vivo_
results of a new focal tissue ablation technique: irreversible electroporation. _IEEE Trans Biomed Eng_ 53, 1409–1415 (2006). PubMed Google Scholar * Sano, M. B., Arena, C. B., DeWitt, M.
R., Saur, D. & Davalos, R. V. _In-vitro_ bipolar nano- and microsecond electro-pulse bursts for irreversible electroporation therapies. _Bioelectrochemistry_ 100, 69–79 (2014). CAS
PubMed Google Scholar * Arena, C. B., Szot, C. S., Garcia, P. A., Rylander, M. N. & Davalos, R. V. A three-dimensional _in vitro_ tumor platform for modeling therapeutic irreversible
electroporation. _Biophys J_ 103, 2033–2042 (2012). CAS PubMed PubMed Central Google Scholar * Miklavcic, D. et al. Electrochemotherapy: technological advancements for efficient
electroporation-based treatment of internal tumors. _Medical & Biological Engineering & Computing_ 50, 1213–1225 (2012). CAS Google Scholar * Sersa, G. et al. Electrochemotherapy
of tumors as _in situ_ vaccination boosted by immunogene electrotransfer. _Cancer Immunol Immunother_ 64, 1315–1327 (2015). CAS PubMed PubMed Central Google Scholar * Kim, L. S., Huang,
S., Lu, W., Lev, D. C. & Price, J. E. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. _Clin Exp Metastasis_ 21, 107–118
(2004). CAS PubMed Google Scholar * Sel, D. et al. Sequential finite element model of tissue electropermeabilization. _IEEE Transactions on Biomedical Engineering_ 52, 816–827 (2005).
PubMed Google Scholar * Sano, M. B., Fan, R. E., Hwang, G. L., Sonn, G. A. & Xing, L. Production of Spherical Ablations Using Nonthermal Irreversible Electroporation: A Laboratory
Investigation Using a Single Electrode and Grounding Pad. _J Vasc Interv Radiol_ 27, 1432–1440 e1433 (2016). PubMed Google Scholar * Sano, M. B. et al. Towards the creation of
decellularized organ constructs using irreversible electroporation and active mechanical perfusion. _Biomedical Engineering Online_ 9, 83 (2010). PubMed PubMed Central Google Scholar *
Edd, J. F., Horowitz, L., Davalos, R. V., Mir, L. M. & Rubinsky, B. _In vivo_ results of a new focal tissue ablation technique: Irreversible electroporation. _Ieee T Bio-Med Eng_ 53,
1409–1415 (2006). Google Scholar * Miklavčič, D., Šemrov, D., Mekid, H. & Mir, L. M. A validated model of _in vivo_ electric field distribution in tissues for electrochemotherapy and
for DNA electrotransfer for gene therapy. _Biochimica et Biophysica Acta (BBA)-General Subjects_ 1523, 73–83 (2000). Google Scholar * Pakhomov, A. G. et al. Cancellation of cellular
responses to nanoelectroporation by reversing the stimulus polarity. _Cell Mol Life Sci_ 71, 4431–4441 (2014). CAS PubMed PubMed Central Google Scholar * Kotnik, T. & Miklavčič, D.
Theoretical evaluation of voltage inducement on internal membranes of biological cells exposed to electric fields. _Biophysical Journal_ 90, 480–491 (2006). ADS CAS PubMed Google Scholar
* Pakhomov, A. G. et al. Multiple nanosecond electric pulses increase the number but not the size of long-lived nanopores in the cell membrane. _Biochimica et Biophysica Acta
(BBA)-Biomembranes_ 1848, 958–966 (2015). CAS Google Scholar * Sweeney, D. C. et al. Quantification of cell membrane permeability induced by monopolar and high frequency bipolar bursts of
electrical pulses. _Biochimica et Biophysica Acta (BBA)-Biomembranes_ (2016). * Forde, P. F. et al. Non-viral immune electrogene therapy induces potent antitumour responses and has a
curative effect in murine colon adenocarcinoma and melanoma cancer models. _Gene Ther_ 22, 29–39 (2015). CAS PubMed Google Scholar * Kaneko, T., Sakuma, T., Yamamoto, T. & Mashimo, T.
Simple knockout by electroporation of engineered endonucleases into intact rat embryos. _Scientific reports_ 4, 6382 (2014). ADS CAS PubMed PubMed Central Google Scholar * Kos, S. et
al. Improved specificity of gene electrotransfer to skin using pDNA under the control of collagen tissue-specific promoter. _The Journal of membrane biology_ 248, 919–928 (2015). CAS PubMed
Google Scholar * Mir, L. M. et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally
and electric pulses delivered by the Cliniporator TM by means of invasive or non-invasive electrodes. _Eur J Cancer Suppl_ 4, 14–25 (2006). CAS Google Scholar * Cutrera, J., Dibra, D.,
Xia, X. & Li, S. Enhancement of reporter gene detection sensitivity by insertion of specific mini-peptide-coding sequences. _Cancer Gene Ther_ 17, 131–140 (2010). CAS PubMed Google
Scholar * Schoenbach, K. H. et al. Ion transport into cells exposed to monopolar and bipolar nanosecond pulses. _Bioelectrochemistry_ 103, 44–51 (2015). CAS PubMed Google Scholar *
Andre, F. et al. Efficiency of high-and low-voltage pulse combinations for gene electrotransfer in muscle, liver, tumor, and skin. _Hum Gene Ther_ 19, 1261–1272 (2008). CAS PubMed Google
Scholar * Glogauer, M., Lee, W. & McCulloch, C. Induced endocytosis in human fibroblasts by electrical fields. _Exp Cell Res_ 208, 232–240 (1993). CAS PubMed Google Scholar *
Escoffre, J.-M. et al. What is (still not) known of the mechanism by which electroporation mediates gene transfer and expression in cells and tissues. _Mol Biotechnol_ 41, 286–295 (2009).
CAS PubMed Google Scholar * Arena, C. B. et al. Focal blood-brain-barrier disruption with high-frequency pulsed electric fields. _Technology_ 2, 206–213 (2014). Google Scholar *
Miklavčič, D. et al. The effect of high frequency electric pulses on muscle contractions and antitumor efficiency _in vivo_ for a potential use in clinical electrochemotherapy.
_Bioelectrochemistry_ 65, 121–128 (2005). PubMed Google Scholar * Neal, R. E. et al. Treatment of breast cancer through the application of irreversible electroporation using a novel
minimally invasive single needle electrode. _Breast Cancer Res Treat_ 123, 295–301 (2010). PubMed PubMed Central Google Scholar * Neal, R. E., Garcia, P., Robertson, J. L. & Davalos,
R. V. Experimental characterization and numerical modeling of tissue electrical conductivity during pulsed electric fields for irreversible electroporation treatment planning. _Biomedical
Engineering, IEEE Transactions on_ 59, 1076–1085 (2012). Google Scholar * Neal Ii, R. E., Cheung, W., Kavnoudias, H. & Thomson, K. R. Spectrum of imaging and characteristics for liver
tumors treated with irreversible electroporation. _Journal of Biomedical Science and Engineering_ 05, 813–818 (2012). Google Scholar Download references ACKNOWLEDGEMENTS This work was
supported in part by the DOD through the Prostate Cancer Research Program (PCRP) Postdoctoral Training Award W81XWH-15-1-0137 AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Radiation Oncology, Division of Radiation Physics, Stanford University Medical Center, Stanford, CA, USA, Michael B. Sano & Lei Xing * UNC / NCSU Joint Department of Biomedical
Engineering, Chapel Hill, NC, USA., Michael B. Sano * Department of Urology, Stanford University Medical Center, Stanford, CA, USA., Richard E. Fan Authors * Michael B. Sano View author
publications You can also search for this author inPubMed Google Scholar * Richard E. Fan View author publications You can also search for this author inPubMed Google Scholar * Lei Xing View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.S. conceived of, designed, developed, and conducted the experiments. M.S., R.F. and L.X.
analyzed and interpreted the data. L.X. supervised the study. All authors contributed to writing, editing, and review of the manuscript. CORRESPONDING AUTHOR Correspondence to Michael B.
Sano. ETHICS DECLARATIONS COMPETING INTERESTS MBS and LX have pending patents related to H-FIRE technologies. The other authors disclose no potential conflicts of interest. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION (PDF 729 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative
Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sano, M., Fan, R. & Xing, L. Asymmetric Waveforms Decrease Lethal Thresholds in High Frequency Irreversible Electroporation
Therapies. _Sci Rep_ 7, 40747 (2017). https://doi.org/10.1038/srep40747 Download citation * Received: 05 May 2016 * Accepted: 12 December 2016 * Published: 20 January 2017 * DOI:
https://doi.org/10.1038/srep40747 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative