Play all audios:
ABSTRACT Oxidative stress is thought to be involved in the development of behavioral and histopathological alterations in animal models of psychosis. Here we investigate the causal
contribution of reactive oxygen species generation by the phagocyte NADPH oxidase NOX2 to neuropathological alterations in a rat model of chronic psychosocial stress. In rats exposed to
social isolation, the earliest neuropathological alterations were signs of oxidative stress and appearance of NOX2. Alterations in behavior, increase in glutamate levels and loss of
parvalbumin were detectable after 4 weeks of social isolation. The expression of the NOX2 subunit p47phox was markedly increased in pyramidal neurons of isolated rats, but below detection
threshold in GABAergic neurons, astrocytes and microglia. Rats with a loss of function mutation in the NOX2 subunit p47phox were protected from behavioral and neuropathological alterations
induced by social isolation. To test reversibility, we applied the antioxidant/NOX inhibitor apocynin after initiation of social isolation for a time period of 3 weeks. Apocynin reversed
behavioral alterations fully when applied after 4 weeks of social isolation, but only partially after 7 weeks. Our results demonstrate that social isolation induces rapid elevations of the
NOX2 complex in the brain. Expression of the enzyme complex was strongest in pyramidal neurons and a loss of function mutation prevented neuropathology induced by social isolation. Finally,
at least at early stages, pharmacological targeting of NOX2 activity might reverse behavioral alterations. SIMILAR CONTENT BEING VIEWED BY OTHERS NUCLEAR GAPDH IN CORTICAL MICROGLIA MEDIATES
CELLULAR STRESS-INDUCED COGNITIVE INFLEXIBILITY Article 13 April 2024 CHANGES AT GLUTAMATE TRIPARTITE SYNAPSES IN THE PREFRONTAL CORTEX OF A NEW ANIMAL MODEL OF RESILIENCE/VULNERABILITY TO
ACUTE STRESS Article Open access 18 February 2023 MICROGLIAL P2Y12 MEDIATES CHRONIC STRESS-INDUCED SYNAPSE LOSS IN THE PREFRONTAL CORTEX AND ASSOCIATED BEHAVIORAL CONSEQUENCES Article 14
December 2022 INTRODUCTION There is increasing evidence that psychosocial stress leads to oxidative stress in the brain, thereby contributing to the development of mental disorders such as
anxiety and psychosis.1 Data from psychotic patients have shown a key role of oxidative stress in the pathogenesis of mental disorders (reviewed in Yao and Ravinder2). NOX enzymes are
proteins that transfer electrons across biological membranes to catalyze the reduction of molecular oxygen and generate the superoxide anion O2−.3 In the central nervous system, NOX isoforms
are heterogeneously distributed in different regions and cell types,4 and thought to be involved in redox regulation of cell fate and neuronal activity.5 From a pathological point of view,
NOX enzymes have been implicated in the generation of oxidative stress seen in a variety of brain disorders, from psychiatric to neurodegenerative diseases.4 Some progress in the
understanding of the mechanistic link between oxidative stress and psychiatric diseases has come from animal models. In the ketamine-induced model of psychosis,6 we and others have
previously shown that NOX2 is a major source of reactive oxygen species (ROS) in the brain, controlling glutamate release and behavioral alterations,7, 8 and that decrease of parvalbumin in
GABAergic neurons is prevented by NOX2 deficiency and by the treatment with the antioxidant/NOX inhibitor compound apocynin.8, 9, 10 The social isolation rearing of young adult rats is
defined as a model of chronic psychosocial stress11, 12 and provides a non-pharmacological tool to study long-term alterations reminiscent of several symptoms seen in schizophrenic
patients.13 These include hyper-reactivity to novel environments,14 cognitive impairment15 as well as decrease in parvalbumin-positive neurons.16 We previously described a possible
involvement of NOX2 in isolation-induced neuropathology.17 However, in the absence of NOX2 knockout rats and specific NOX2 inhibitors, causal relationships have not yet been established and
the possibility that NOX2 activation is a late epiphenomenon could not be excluded. A natural polymorphism of the _Ncf1_ gene controlling NOX2-dependent ROS has recently been reported in
wild rats and in inbred laboratory strains.18, 19 Importantly, a one nucleotide difference determines the functional effects. Indeed, DA.Ncf1DA rats with lower capacity for ROS production18,
20 differ only at the _Ncf1_ locus from the congenic strain DA.Ncf1E3 coding for the p47phox protein, which is an essential component of the NOX2/NADPH oxidase complex, and a methionine
instead of a threonine at position 153 reduces the capacity of oxidative burst by 40%.20 For simplicity we will refer to this polymorphism throughout the text as ‘loss-of-function mutation’.
Dysfunctions of cortical glutamatergic neurotransmission are observed in schizophrenic patients and in animal models of schizophrenia,21 and thought to be involved in the development of
behavioral alterations.22 These dysfunctions are reflected by an increased cortical release of glutamate with consequent alterations in the _N_-methyl-D-aspartate (NMDA) receptor.23 More
specifically, the subunit 2A of the receptor is the most affected.24 Here, we have investigated the role of NOX2 in the development of the neuropathology induced by social isolation. Using
time-course analysis, animals with NOX2 loss of function mutation as well as pharmacological tools, we demonstrate a crucial role of NOX2 in this experimental model of psychosis. MATERIALS
AND METHODS Note that for space reasons, details are provided in Supplementary Materials and methods. Also, behavioral tests and immunohistochemical/western blot assays were performed on the
same sets of animals. ANIMALS A total number of 30 wistar (Harlan, S Pietro al Natisone, Udine, Italy), 10 DA (with the ROS low-responder _Ncf1__DA_ allele) and 10 DA.Pia4 (with the ROS
high-responder _Ncf1_E3 allele) dams provided offspring for inclusion in the study. For simplicity, DA.Pia4 rats and DA rats will be indicated as Ncf1E3 and Ncf1DA, respectively. The Ncf1E3
and Ncf1DA rats were from the Medical Inflammation Research animal house, Karolinska Institute (Stockholm, Sweden). Animals were housed as described previously.17 This study was performed in
conformity with ethical guidelines, and national and international laws.7, 17 Details regarding genotyping of animals are provided in Supplementary Materials and methods. SOCIAL ISOLATION
PROTOCOL The social isolation procedure was performed on male rats, as described previously.17 The experimental protocol lasted 2, 4 or 7 weeks for wistar rats and 7 weeks for Ncf1E3 and
Ncf1DA rats. Details are provided in Supplementary Materials and methods. APOCYNIN TREATMENT Animals were firstly exposed to behavioral tests after 4 or 7 weeks of social isolation. Then,
control and isolated rats received approximately 5 mg/kg per day of apocynin (Sigma-Aldrich, Buchs, Switzerland)8, 17, 25 in drinking water from week 4 to week 7 (protocol 1, _n_=6 controls
and 6 isolated) or from week 7 to week 10 (protocol 2, _n_=6 controls and 6 isolated). Behavioral tests were repeated at the end of each protocol. BEHAVIORAL TESTS OPEN FIELD TEST The open
field test was performed as described previously.17, 26, 27 Details are provided in Supplementary Materials and methods. NOVEL OBJECT RECOGNITION TEST The novel object recognition test was
performed as described previously.17, 28, 29, 30 The discrimination index was calculated using the following formula: (_N_−_F_)/(_N_+_F_) (_N_=time spent in exploration of the novel object
during the choice trial; _F_=time spent in exploration of the familiar object in the choice trial).31 Details are provided in Supplementary Materials and methods. MICRODIALYSIS Intracerebral
microdialysis in awake freely moving rats was performed as described previously.7, 30 A vertical probe was positioned in the medial prefrontal cortex of rats: AP +3.7 mm from bregma, L ±0.7
mm from midline and DV −2.8 from dura.32, 33 Three baseline samples were collected to evaluate cortical basal levels of glutamate. Details are provided in Supplementary Materials and
methods. QUANTIFICATION OF GLUTAMATE IN THE DIALYSATE Glutamate concentrations were determined by high-performance liquid chromatography as described previously.8, 34 Details are provided in
Supplementary Materials and methods. IMMUNOHISTOCHEMISTRY Immunohistochemical analysis were performed as described previously,7, 17 using monoclonal or polyclonal antibodies against
8-hydroxy-2-deoxyguanosine (8-OHdG; 1:10; JaICA, Shizuoka, Japan), parvalbumin (1:1000; Calbiochem, Zug, Switzerland), p47phox (1:250; Santa Cruz Biotechnology, Santa Cruz, CA, USA), GAD67
(1:2000; Chemicon, Zug, Switzerland) glial fibrillary acidic protein (1:4000; Chemicon), ionized calcium binding adapter molecule 1 (IBA-1) (1:500; Wako, Neuss, Germany) and CD68 (1:100; AbD
Serotec, Dusseldorf, Germany). Fluorescence images were obtained using an epifluorescence system. Merged images of single fluorescence channels were obtained using the AxioVision Rel. 4.5
software (Carl Zeiss, Jena, Germany). Specificity of the antibody against p47phox was tested comparing the staining obtained on the spleen and brain tissues of wild-type and p47phox knockout
mice (kindly provided by Prof. Ralph Brandes, Faculty of Medicine, Goethe-University, Frankfurt, Germany) (Supplementary Figure 1). Quantifications of immunohistochemistry have been
performed using the Metamorph software (Molecular Devices, Biberach an der Riss, Germany). WESTERN BLOTTING Western blotting was performed as described previously,18 using polyclonal or
monoclonal antibodies against c-fos (1:500; Santa Cruz Biotechnology), hypoxia-inducible factor-1α (HIF-1α; 1:500; Santa Cruz Biotechnology), phospho-JNK (1:200; Cell Signaling, Allschwil,
Switzerland), JNK (1:200; Santa Cruz Biotechnology), phospho-ERK (1:200; Cell Signaling), ERK (1:200; Cell Signaling), phospho-p38 (1:1000; Cell Signaling) and p38 (1:1000; Santa Cruz
Biotechnology), IBA-1 (1:1000; Dako, Glostrup, Denmark), NOX2 (1:1000; Biosciences Pharmingen, Erembodegem, Belgium), parvalbumin (1:4000; kindly provided by Professor Do Kim, Centre
Hospitalier Universitaire Vaudois, Lausanne, Switzerland), calretinin (1:1000; Abcam, Cambridge, UK), NMDA receptor subunit 2A (1:500; Abcam), NMDA receptor subunit 2B (1:500; Abcam),
p47phox (1:250; Santa Cruz Biotechnology), GAD67 (1:1000; Chemicon) and α-actin (1:4000; Sigma-Aldrich). Optical densities of the bands were measured using the ImageJ software
(http://rsb.info.nih.gov/ij/) and normalized with α-actin. REVERSE TRANSCRIPTASE-POLYMERASE CHAIN REACTION Reverse transcriptase-polymerase chain reaction methods, and primer and product
size have been described previously.17, 35 DATA ANALYSIS Data were analyzed using the Sigma Stat 3.1 software (Systat Software Inc., San Jose, CA, USA). The statistical tests are indicated
in the figure legends. For all tests, a _P_-value <0.05 was considered statistically significant. Results are expressed as means±standard error (s.e.m.). RESULTS TIME DEPENDENCE OF
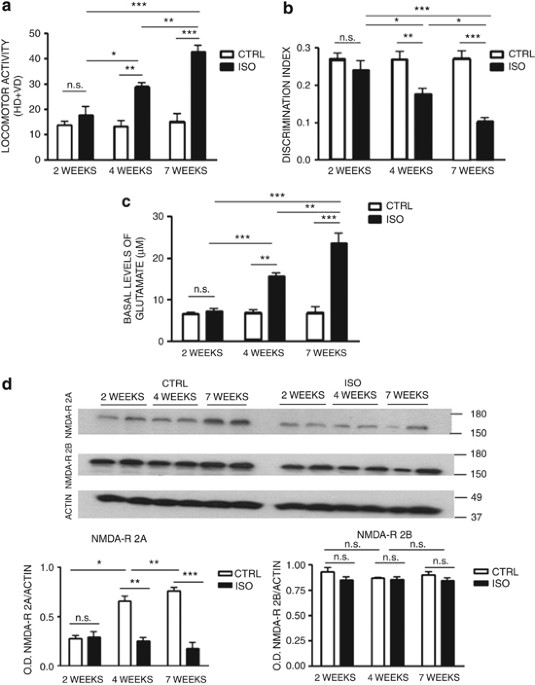
BEHAVIORAL ALTERATIONS AND GLUTAMATE ELEVATIONS IN RESPONSE TO SOCIAL ISOLATION To define the time course of behavioral alterations induced by social isolation, we isolated rats for 2, 4 or
7 weeks. At the end of the isolation period, we performed the open field test and the novel object recognition test. We focused on behavioral functions that are regulated by nucleus
accumbens and prefrontal cortex, such as spontaneous locomotor activity and novel object discrimination.23 These two brain regions appear to be most affected by social isolation, in terms of
neurochemistry, neuronal organization and stress-induced injury.34, 36 Results from the open field test showed no significant difference in locomotor activity between control and isolated
rats after 2 weeks of social isolation (Figure 1a). Increased locomotor activity in isolated rats was detected after 4 weeks of social isolation with respect to control animals (Figure 1a)
and became more significant after 7 weeks of social isolation (Figure 1a). Similar results were obtained in the novel object recognition test. Thus, decrease in discrimination index started
around 4 weeks after the beginning of social isolation, and worsened after 7 weeks (Figure 1b). To correlate these behavioral alterations with disorders in neurotransmission and alterations
in NMDA receptors, we evaluated cortical basal levels of glutamate by intracerebral microdialysis and NMDA receptors subunit 2A and 2B levels by western blotting in control and isolated rats
(_n_=6 for each time point). Glutamate levels were stable in control animals over the 7-week period. In contrast, in isolated animals, glutamate levels progressively increased after 4 and 7
weeks of social isolation (Figure 1c). Western blotting results showed a physiological time-dependent increase in the subunit 2A of the NMDA receptor in the prefrontal cortex and nucleus
accumbens from week 2 to week 7, whereas no increase in this subunit was observed in isolated animals during the same period of the brain development (Figure 1d and Supplementary Figure 2A
and B). The levels of the subunit 2B of the NMDA receptor remained stable over the considered time points in control and isolated animals (Figure 1d). IMPACT OF SOCIAL ISOLATION ON DNA
OXIDATION IN SPECIFIC BRAIN REGIONS Alterations in behavior induced by social isolation and impairment in extracellular release of glutamate in rodent model of schizophrenia depend on
oxidative stress.7, 17 Therefore, to correlate the profile of behavior and neurotransmission alterations with oxidative stress, we performed immunohistochemistry in the nucleus accumbens and
prefrontal cortex of control and isolated rats (_n_=4, controls _n_=4, isolated for each time point), using an antibody raised against 8-OHdG, a readily detectable marker of oxidative
stress to DNA.37 Both in the nucleus accumbens and in the prefrontal cortex of control rats, no signs of oxidative stress were detected at 2, 4 or 7 weeks of social isolation (Supplementary
Figure 3A, B, E, F, I, J, M, N, Q, R and U, V). The nucleus accumbens, but not the prefrontal cortex, of isolated rats already showed an increase in 8-OHdG after 2 weeks of social isolation
(Supplementary Figure 3C, D and O, P). After 4 weeks of social isolation, both the nucleus accumbens (Supplementary Figure 3G, H) and the prefrontal cortex (Supplementary Figure 3S, T)
stained positively for 8-OHdG. This staining was further enhanced by 7 weeks of social isolation (Supplementary Figure 3K, L and W, X). Quantitative analysis, obtained through a count of
8-OHdG-positive cells in the nucleus accumbens and in the prefrontal cortex (Metamorph software), confirmed the visual impression (Figures 2a and b). We next investigated possible
isolation-induced changes in the levels of some indirect markers of oxidative stress, such as the immediate-early gene and redox-sensitive transcription factor c-fos, the HIF-1α protein, as
well as the phosphorylated form of the MAP kinase JNK (p-JNK) (Figures 2c and d). In control animals, only weak levels of these indirect markers of oxidative stress were found. In contrast,
in isolated animals there was a strong and time-dependent increase of these markers in the nucleus accumbens and the prefrontal cortex (Figures 2c and d). We also detected an increase of the
phosphorylated form of the MAP kinase ERK and a weak increase of the phosphorylated form of p38 MAP kinase in isolated rats compared with controls. However, the expression of these two
proteins did not seem to follow the time dependence of the increase observed for the other proteins (Figures 2c and d). Neither in the nucleus accumbens nor in the prefrontal cortex there
were social isolation-induced differences in the expression of the total form of JNK, ERK and p38 (Figures 2c and d). LOSS OF PARVALBUMIN IMMUNOREACTIVITY We next investigated the time
dependence of the loss of parvalbumin protein in specific brain regions of isolated rats. This decrease reflects a loss of inhibitor phenotype of GABAergic neurons.8 In the prefrontal cortex
and in the nucleus accumbens of control rats, parvalbumin protein amount (evaluated by immunohistochemistry and western blotting) was stable (Supplementary Figure 4A, B, E, F, I, J and M).
In contrast, a statistically significant loss of parvalbumin content was detected starting from 2 weeks of social isolation in the nucleus accumbens (Supplementary Figure 4M) and from 4
weeks of social isolation in the prefrontal cortex (Supplementary Figure 4G, H). The loss of parvalbumin became even more marked after 7 weeks (Supplementary Figure 4K, L and M).
Quantification of western blotting results in the nucleus accumbens (Supplementary Figure 4N) and quantitative analysis of immunohistochemistry in the prefrontal cortex (Figure 3), obtained
through a count of parvalbumin-positive neurons (Metamorph software), confirmed these results. SOCIAL ISOLATION INDUCES EARLY NOX2 ELEVATIONS IN THE NUCLEUS ACCUMBENS AND THE PREFRONTAL
CORTEX The NOX2 complex is an important source of oxidative stress in the brain.7, 8, 9, 17 We have previously shown that after 7 weeks of social isolation, there are marked elevations of
NOX2 in critical brain regions.17 To evaluate whether these NOX2 elevations are early or late events in the brain pathology induced by social isolation, we investigated the time course of
NOX2 elevations. In the nucleus accumbens and the prefrontal cortex of control rats, expression levels of NOX2 were below detection threshold as assessed by reverse transcriptase-polymerase
chain reaction (30 cycles) (Figure 4a). In contrast, in isolated rats, a marked expression of NOX2 mRNA was observed already after 2 weeks in the nucleus accumbens and after 4 weeks in the
prefrontal cortex (Figure 4a). To verify whether there were indeed elevations in NOX2 protein levels, we analyzed the expression of NOX2 by western blotting. No or only faint bands
corresponding to the NOX2 protein were detected in control rats (Figure 4b). In contrast, the NOX2 protein was readily detectable in the nucleus accumbens after 2 weeks and after 4 weeks in
the prefrontal cortex (Figure 4b). Quantification of NOX2 protein (ImageJ software; normalized with actin control) revealed that the expression of NOX2 in the nucleus accumbens progressively
increased from week 2 to week 7 of social isolation (Figure 4c), whereas in the prefrontal cortex, NOX2 protein began to be expressed at 4 weeks and increased up to 7 weeks (Figure 4c).
SOCIAL ISOLATION INDUCES AN INCREASE IN THE EXPRESSION OF P47PHOX SUBUNIT IN PYRAMIDAL NEURONS To determine which cell subpopulation of the brain was responsible of the NOX2 complex-derived
increase of oxidative stress, we performed a double staining for the NOX2 subunits p47phox GAD67, a marker of GABAergic neurons, glial fibrillary acidic protein, a marker of astrocytes, and
IBA-1, a marker of microglia in the prefrontal cortex of control and isolated animals. A weak red staining, corresponding to p47phox, was detected in controls (Figure 4d). Social isolation
(7 weeks) induced a significant increase of the p47phox staining (Figure 4g and Supplementary Figure 5A, D and G). This was also confirmed by the analysis of the p47phox protein levels by
western blotting in control and isolated animals (Supplementary Figure 6A and B). Merged images of the red and green staining GAD67 showed that the staining for p47phox was distinct from the
GAD67 staining (Figures 4f–i and Supplementary Figure 5C, F and I). More specifically, the p47phox staining was located in cells whose density, distribution, anatomical connections with
other neurons (in particular GABAergic neurons) and soma shapes unequivocally identify the p47phox-positive cells as pyramidal neurons.38, 39 The small green dots surrounding the cell body
of the pyramidal neurons are dendrites from GABAergic neurons. These results indicate that the large majority of cells in which an increased p47phox subunit expression was observed were
pyramidal neurons. Neither astrocytes nor microglia showed an increase of p47phox (data not shown). To quantify the expression levels of GAD67 (green staining; Figures 4e–h), we performed
western blotting analysis. Results showed a decrease in GAD67 expression in isolated animals compared with controls (Supplementary Figure 7A and B). IMPACT OF SOCIAL ISOLATION ON MICROGLIAL
CELLS Microglia is strongly affected in individuals with social stress-induced psychotic disorders40 and in animal models of chronic psychosocial stress.17 To evaluate the effects of social
isolation on microglia cell number during brain development, we investigated possible alterations in IBA-1 protein expression in the nucleus accumbens and prefrontal cortex of controls and
animals isolated for 2, 4 or 7 weeks by western blotting. In control animals, levels of IBA-1 protein were stable during the three considered time points both in the nucleus accumbens and in
the prefrontal cortex (Figure 5). We could not observe any differences in IBA-1 expression between controls and isolated animals after 2 or 4 weeks of social isolation. Thus, only rats
exposed to 7 weeks of social isolation showed an increase in the microglial cell number compared with controls (Figure 5). Analysis of activation of microglia, performed by
immunohistochemistry for CD68, revealed that social isolation did not affect activation of microglia. Indeed, no staining for CD68 was observed in the nucleus accumbens and prefrontal cortex
of control and isolated animals after 2, 4 or 7 weeks (data not shown for the absence of staining at week 2 and 4, absence of staining at 7 weeks shown in Supplementary Figure 8A–H),
indicating that the increase of NOX2-derived oxidative stress was not mainly due to microglia activation. Note that CD68-positive microglial cells were found in other brain regions of the
same animals (such as the forceps minor and the anterior commissure), indicating the validity of the antibody (Supplementary Figure 8I–L). NCF1 POLYMORPHISM INFLUENCE THE DEVELOPMENT OF
BEHAVIORAL AND NEUROPATHOLOGICAL ALTERATIONS INDUCED BY SOCIAL ISOLATION To investigate the functional role of NOX2, we used DA rats that differed only in the _Ncf1_ locus: Ncf1E3 that has a
normal NOX2 activity and Ncf1DA that has a strongly reduced NOX2 activity. After 7 weeks of social isolation rearing, we investigated the effect of this polymorphism on locomotor activity.
Under control conditions, we did not observe any difference in locomotor activity, parvalbumin expression and subunit 2A of the NMDA receptor in Ncf1E3 rats and in Ncf1DA rats (data not
shown). Note also that we did not observe any marked difference in the basal behavior, levels of parvalbumin and NMDA receptor subunit 2A and the social isolation response between Ncf1E3 and
wistar rats (Supplementary Figure 9A–C). As wistar rats may also have the polymorphism, we genotyped all wistar rats used in our experiments and found that all of them had at least one
_Ncf1_E3 allele, and therefore had normal NOX2 activity (data not shown). Similarly as described above for wistar rats, social isolation led to increased motor activity as well as decreased
central nervous system expression of parvalbumin and subunit 2A of the NMDA receptor (Figures 6a–c). In contrast, social isolation had no impact on motor activity of Ncf1DA rats (Figure 6a).
Similarly, there was no decrease in protein expression of parvalbumin and subunit 2A of the NMDA receptor in isolated Ncf1DA rats (Figures 6b and c). Importantly, calretinin, a Ca2+-binding
protein found in another subpopulation of GABAergic neurons, was stable for all conditions (including social isolation of Ncf1E3 rats), showing the specificity of the loss of parvalbumin
(Figure 6b). TREATMENT WITH THE ANTIOXIDANT/NOX INHIBITOR APOCYNIN IS MORE EFFICIENT WHEN STARTED EARLY DURING SOCIAL ISOLATION We have previously demonstrated that apocynin treatment during
7 weeks of social isolation prevents the development of behavioral and neuropathological alterations.17 To determine if apocynin might also have therapeutic effects, we treated isolated
rats according to two different protocols. A first set of control and isolated rats was treated with apocynin during the second half of the social isolation period (weeks 4–7). Another set
of animals was treated with apocynin 3 weeks starting from week 7, and were kept under isolation conditions until week 10 (Figure 7a). For both protocols, we evaluated the increase of
locomotor activity and decrease in discrimination index in isolated animals with respect to controls. Results show that apocynin treatment fully reversed the increase of locomotor activity
and the decrease of discrimination index if the treatment started at 4 weeks of social isolation, whereas only a partial reversal could be achieved if treatment was started later (Figure
7b). DISCUSSION In this study, we investigated the development of neuropathology in a rat social isolation model and its causal relationship with increased oxidative stress in specific brain
areas. We demonstrate that social isolation leads to early neuropathology and increased expression of the NOX2 subunit p47phox in cortical pyramidal neurons followed by altered behavior.
Both loss of function polymorphism in the NOX2 subunit p47phox or a treatment with the antioxidant/NOX inhibitor apocynin after apparition of the symptoms largely prevents behavioral and
neuropathological alterations induced by social isolation. To our knowledge, the results shown here are the first time course of behavioral changes in the rat model of social isolation. Our
study demonstrates that increased NOX2 expression, oxidative stress and neuropathological alterations occur first in the nucleus accumbens and only at later time points in the prefrontal
cortex. Thus, behavioral alterations correlate best with oxidative stress and neuropathological alterations in the prefrontal cortex. This does however not exclude a role of the nucleus
accumbens. The nucleus accumbens and the prefrontal cortex are particularly sensitive to social stress in humans and rodents.41, 42, 43, 44, 45 An early involvement of the nucleus accumbens
in response to social isolation was described previously: already after 3 weeks of social isolation, expression of the corticotropin-releasing factor in the nucleus accumbens is increased46
and the monoaminergic innervation in areas connecting to the nucleus accumbens are modified.47 In our study, we have shown that important neuropathological changes occur in the nucleus
accumbens even before behavioral alterations are detectable. This might reflect a time delay in the response to oxidative stress; alternatively, it may indicate that behavioral alterations
are due to concomitant neurochemical changes in the nucleus accumbens and in the prefrontal cortex.48, 49 Another novelty of this work is the demonstration of a causal involvement of p47phox
in the progression of the brain pathology induced by social isolation. This conclusion is based on two observations: (i) the early neuronal elevations of p47phox and oxidative stress in
response to social isolation; and (ii) the prevention of neuropathological alterations in rats with a decrease of function polymorphism in the _Ncf1_ gene coding for the NOX2 subunit
p47phox. The fact that NOX2 mRNA and protein are increased in the early phase strongly suggests that NOX2 is a major source of oxidative stress in the onset of psychosis. Early oxidative
stress during the maturation window of normal brain development has been observed in perinatal pharmacological mouse model of psychosis induction, and has been implicated in
schizophrenia-like behavioral dysfunctions in adulthood.10 Several observations suggest that oxidative stress is an important feature of human psychosis. This concept is mostly based on
measurements of putative biomarkers of oxidative stress, such as reduction of reduced GSH in cerebrospinal fluid and brain tissue in schizophrenic patients,50 increased levels of plasma
thioredoxin-1 at the onset of schizophrenia51 or low total plasma antioxidant levels in first-episode drug-naive patients with schizophrenia.52 As NOX enzymes are major ROS generator, an
increased activity of NOX2 or possibly other NOX isoforms may represent an important cause of redox modifications in psychosis. The social isolation model is an important tool in psychosis
research. Social isolation represents a profound psychological stressor for rodents,53 leading to reproducible neuropathology that mimics certain aspects of the response of the human brain
to stressful situations.54 Rats are the best suited rodent model for this type of research, presumably because they have evolved different behavioral attitudes to react to external stimuli,
such as alterations in its own social organization and stress-induced emotionality.55 Thus, rat behavioral responses to social deprivation are stronger than those observed in mice,
presumably because the development of normal behavior in rats very strongly depends on social interactions.15 Despite the usefulness of the rat model, the required genetic proof of principle
in rats is challenging, given the difficulties to obtain knockout rats. However, recent work has demonstrated the existence of a loss of function mutation in the _Ncf1_ gene coding for the
p47phox subunit of NOX2. Through gene mapping experiments, it was found that an _Ncf1_ allele exchanging a single amino acid, threonine to methionine at position 153, leads to lower
oxidative burst.18, 20 Importantly, the locus is highly polymorphic in the wild rat population, with a dominant effect of the fully functional _E3_ allele with threonine at position 153. It
has been approximated that 60% of wild rats in northern Europe are homozygous for the _Ncf1__DA_ alleles and therefore have a low ROS response.18, 19 Rat laboratory strains, including
wistar, are also polymorphic for the _Ncf1_ locus.19 In our study, we have used inbred DA rats that were polymorphic only with respect to the _Ncf1_ locus. The effects observed in Figure 6,
showing the impact of homozygosis for the low oxidative burst allele _Ncf1__DA_ on social isolation, are therefore due only to a single-nucleotide polymorphism.20 Indeed, rats with low
oxidative burst are protected from behavioral and neuropathological alterations induced by social isolation. As this polymorphism is widespread in wild rats, it might have been selected by
evolution. Indeed, the polymorphism could confer resistance to stress to a subpopulation of rats. The balancing selection could be that rats with higher oxidative burst response might be
protected from infectious and autoimmune diseases. In humans, an inverse correlation between schizophrenia and arthritis and rheumatoid arthritis has indeed been described.56, 57, 58
Together with the above-described genetic proof of concept, a pharmacological approach was performed. There are presently no specific NOX2 inhibitors; however, the anti-oxidant/NOX inhibitor
apocynin shows neuroprotective properties in several brain disorders, such as cerebral ischemia59, 60 or Alzheimer disease.61 We and others have previously demonstrated that prophylaxis
with apocynin prevents brain neuropathology in models of psychosis.8, 17 Here we demonstrate that apocynin is also efficacious when started after induction of behavioral alterations.
However, complete curative effects, leading to normalization of altered behavior in isolated animals, are only observed when treatment is started relatively early. This is an important
finding of this study as it may open new therapeutic approaches in human psychosis. The data presented here provide the first investigation on the localization of the NOX2 complex in
specific neuronal subpopulations. The large majority of GABAergic neurons did not show an increase in the expression of the NOX2 subunit p47phox induced by social isolation. Indeed,
virtually all p47phox-expressing cells were pyramidal neurons, which are the predominant population of glutamatergic neurons in the cerebral cortex.38, 39 In control animals, the p47phox
staining was weak, but after social isolation, it was intense. Several questions arise from this observation: * i) Why does the increase in the NOX2 complex occur selectively in
glutamatergic pyramidal neurons, but the GABAergic neurons appear mostly affected? Our working hypothesis at this point is a diffusion of hydrogen peroxide from glutamatergic to GABAergic
neurons. Indeed, this neuronal subpopulation is particularly vulnerable to oxidative stress.62, 63, 64 Such a paracrine action of ROS has been previously suggested in neuron–glia signaling
in the hippocampus.65 * ii) What is the stimulus leading to increase of the NOX2 complex? At this point, the mechanisms leading to NOX2 upregulation in neurons are not understood. In the
ketamine mouse model, neuronal production of interleukin-6 is necessary for the activation of NADPH oxidase in the brain.9 * iii) How could the increase in the NOX2 complex be related to
glutamate levels? Basically we can consider two options: a cell autonomous effect of NOX2-derived ROS in glutamatergic neurons or a paracrine effect on GABAergic neurons (see above) or glial
cells. In glutamatergic neurons, specific pathways leading to glutamate release may be inhibited by redox mechanisms, including specific enzymes involved in glutamate production, transport,
exocytosis66 or redox-sensitive kinases or phosphatases regulating molecular pathways of glutamate release,67 or due to redox-sensitive ion channels, such as ryanodine receptors.68
Alternatively, a paracrine effect of NOX2-derived ROS might be due to an acute inhibition of GABAergic neurons, thereby blocking the inhibitory effect on glutamatergic neurons and
subsequently leading to overproduction of glutamate. Social isolation induced a decrease in the expression levels of GAD67, which is a marker for all GABAergic neurons and not related to a
specific subtype. These results are in line with previous finding showing a consistent reduction of this global GABAergic marker in post-mortem brain samples from psychotic patients69, 70,
71 and other animal models of psychosis.72, 73, 74 Note however that the extent of GAD67 decrease (∼45%) was markedly smaller than the parvalbumin decrease (∼85%) after 7 weeks of social
isolation. And we did not observe any decrease in specific markers of other GABAergic subtypes, namely calretinin (Figure 6b) and calbindin (data not shown). Thus, our results suggest that
the main target cells in the social isolation model are parvalbumin-positive neurons; however, we cannot exclude some changes in gene expression also in other GABAergic subtypes.
Contradictory evidence exists about the effects of social isolation on the cortical expression of the subunit 2A of NMDA receptor. Thus, either increase75 or decrease24 in this brain region
has been described previously. Our results are clearly in favor of the latter. Indeed, as expected, in the cortex of control animals, the expression of the NMDA receptor subunit 2A
progressively increased from the second week to the seventh week after weaning.76, 77, 78 In contrast, NMDA receptor subunit 2A levels in the prefrontal cortex of isolated animals did not
increase during this critical age period of brain development. Importantly, in rats with a decrease of function _Ncf1_ allele, the NMDA receptor subunit 2A shows a normal age-dependent
increase after 7 weeks. These findings suggest that NOX2-derived oxidative stress is responsible for abnormal development of NMDA glutamate receptors. Many reports describe the presence of
NOX2 and its subunit p47phox in cells other than neurons in the brain, in particular microglia.4 Yet, there are also studies reporting the presence of p47phox in neurons, but not in the
glia.79, 80 It is thought that increased NOX2 occurs in inflammatory conditions in microglia and regulates microglia activation.81, 82 However, under our experimental conditions, in spite of
microglia proliferation at week 7, no or little activated microglia (as evidenced by the absence of CD68 staining) was observed. This might explain why p47phox staining was below detection
level in the microglia. In conclusion, this study provides strong evidence that psychosocial stress leads to a rapid upregulation of the NOX2 complex in neurons with subsequent generation of
oxidative stress, ultimately leading to the development of neuropathological alterations. The NOX2-derived oxidative stress leads to increased glutamate levels, interferes with normal brain
development and reduces the number of parvalbumin-positive inhibitory neurons. Thus, our results provide new elements for the understanding of the early neuropathological processes leading
to stress-induced psychosis. Our data suggest that NOX2 is central in stress-induced central nervous system signaling and that NOX2-related pathways might provide new diagnostic and curative
approaches for psychosis-related mental disorders. REFERENCES * van Winkel R, Stefanis N, Myin-Germeys I . Psychosocial stress and psychosis. A review of the neurobiological mechanisms and
the evidence for gene–stress interaction. _Schizophr Bull_ 2008; 34: 1095–1105. Article PubMed PubMed Central Google Scholar * Yao J, Ravinder R . Oxidative stress in schizophrenia:
pathogenetic and therapeutic implications. _Antioxid Redox Signal_ 2011; 15: 1999–2002. Article CAS PubMed PubMed Central Google Scholar * Bedard K, Krause K . The NOX family of
ROS-generating NADPH oxidases: physiology and pathophysiology. _Physiol Rev_ 2007; 87: 245–313. Article CAS PubMed Google Scholar * Sorce S, Krause K-H . NOX enzymes in the central
nervous system: from signaling to disease. _Antioxid Redox Signal_ 2009; 11: 2481–2504. Article CAS PubMed Google Scholar * Infanger D, Sharma R, Davisson R . NADPH oxidases of the
brain: distribution, regulation, and function. _Antioxid Redox Signal_ 2006; 8: 1583–1596. Article CAS PubMed Google Scholar * Bubeníková-Valesová V, Horácek J, Vrajová M, Höschl C .
Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. _Neurosci Biobehav Rev_ 2008; 32: 1014–1023. Article CAS PubMed Google Scholar * Sorce S, Schiavone
S, Tucci P, Colaianna M, Jaquet V, Cuomo V _et al_. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. _J Neurosci_ 2010; 30:
11317–11325. Article CAS PubMed PubMed Central Google Scholar * Behrens M, Ali S, Dao D, Lucero J, Shekhtman G, Quick K _et al_. Ketamine-induced loss of phenotype of fast-spiking
interneurons is mediated by NADPH-oxidase. _Science_ 2007; 318: 1645–1647. Article CAS PubMed Google Scholar * Behrens M, Ali S, Dugan L . Interleukin-6 mediates the increase in
NADPH-oxidase in the ketamine model of schizophrenia. _J Neurosci_ 2008; 28: 13957–13966. Article CAS PubMed PubMed Central Google Scholar * Powell SB, Sejnowski TJ, Behrens MM .
Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin–interneuron maturation in rodent models of schizophrenia. _Neuropharmacology_ 2012; 62: 1322–1331.
Article CAS PubMed Google Scholar * Weiss I, Feldon J . Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. _Psychopharmacology (Berl)_ 2001;
156: 305–326. Article CAS Google Scholar * Leng A, Feldon J, Ferger B . Long-term social isolation and medial prefrontal cortex: dopaminergic and cholinergic neurotransmission. _Pharmacol
Biochem Behav_ 2004; 77: 371–379. Article CAS PubMed Google Scholar * King M, Seeman P, Marsden C, Fone K . Increased dopamine D2High receptors in rats reared in social isolation.
_Synapse_ 2009; 63: 476–483. Article CAS PubMed Google Scholar * Geyer MA, Ellenbroek B . Animal behavior models of the mechanisms underlying antipsychotic atypicality. _Progr
Neuro-Psychopharmacol Biol Psychiatry_ 2003; 27: 1071–1079. Article CAS Google Scholar * Lapiz M, Fulford A, Muchimapura S, Mason R, Parker T, Marsden C . Influence of postweaning social
isolation in the rat on brain development, conditioned behavior, and neurotransmission. _Neurosci Behav Physiol_ 2003; 33: 13–29. Article CAS PubMed Google Scholar * Harte M, Powell S,
Swerdlow N, Geyer M, Reynolds G . Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. _J Neural Transm_ 2007; 114: 893–898. Article CAS
PubMed Google Scholar * Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M _et al_. Involvement of NOX2 in the development of behavioral and pathologic alterations in
isolated rats. _Biol Psychiatry_ 2009; 66: 384–392. Article CAS PubMed Google Scholar * Olofsson P, Holmberg J, Tordsson J, Lu S, Akerström B, Holmdahl R . Positional identification of
Ncf1 as a gene that regulates arthritis severity in rats. _Nat Genet_ 2003; 33: 25–32. Article CAS PubMed Google Scholar * Olofsson P, Johansson Å, Wedekind D, Klöting I, Klinga-Levan K,
Lu S _et al_. Inconsistent susceptibility to autoimmunity in inbred LEW rats is due to genetic crossbreeding involving segregation of the arthritis-regulating gene Ncf1. _Genomics_ 2004;
83: 765–771. Article CAS PubMed Google Scholar * Hultqvist M, Sareila O, Vilhardt F, Norin U, Olsson L, Olofsson P _et al_. Positioning of a polymorphic quantitative trait nucleotide in
the Ncf1 gene controlling oxidative burst response and arthritis severity in rats. _Antioxid Redox Signal_ 2011; 14: 2373–2383. Article CAS PubMed Google Scholar * Léna I, Chessel A, Le
Pen G, Krebs M, Garcia R . Alterations in prefrontal glutamatergic and noradrenergic systems following MK-801 administration in rats prenatally exposed to methylazoxymethanol at gestational
day 17. _Psychopharmacology (Berl)_ 2007; 192: 373–383. Article CAS Google Scholar * da Silva FCC, do Carmo de Oliveira Cito M, da Silva MIG, Moura BA, de Aquino Neto MR, Feitosa ML _et
al_. Behavioral alterations and pro-oxidant effect of a single ketamine administration to mice. _Brain Res Bull_ 2010; 83: 9–15. Article CAS PubMed Google Scholar * Fone KCF, Porkess MV
. Behavioural and neurochemical effects of post-weaning social isolation in rodents—relevance to developmental neuropsychiatric disorders. _Neurosci Biobehav Rev_ 2008; 32: 1087–1102.
Article CAS PubMed Google Scholar * Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N _et al_. Isolation rearing induces social and emotional function abnormalities and alters glutamate and
neurodevelopment-related gene expression in rats. _Progr Neuro-Psychopharmacol Biol Psychiatry_ 2009; 33: 1173–1177. Article CAS Google Scholar * Harraz M, Marden J, Zhou W, Zhang Y,
Williams A, Sharov V . SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. _J Clin Invest_ 2008; 18: 659–670. Google Scholar * Arakawa H . Age
dependent effects of space limitation and social tension on open-field behavior in male rats. _Physiol Behav_ 2005; 84: 429–436. Article CAS PubMed Google Scholar * Carratù M, Borracci
P, Coluccia A, Giustino A, Renna G, Tomasini M . Acute exposure to methylmercury at two developmental windows: focus on neurobehavioral and neurochemical effects in rat offspring.
_Neuroscience_ 2006; 141: 1619–1629. Article CAS PubMed Google Scholar * Ennaceur A, Delacour J . A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data.
_Behav Brain Res_ 1988; 31: 47–59. Article CAS PubMed Google Scholar * King M, Sleight A, Woolley M, Topham I, Marsden C, Fone K . 5-HT6 receptor antagonists reverse delay-dependent
deficits in novel object discrimination by enhancing consolidation—an effect sensitive to NMDA receptor antagonism. _Neuropharmacology_ 2004; 47: 195–204. Article CAS PubMed Google
Scholar * Trabace L, Cassano T, Colaianna M, Castrignanò S, Giustino A, Amoroso S . Neurochemical and neurobehavioral effects of ganstigmine (CHF2819), a novel acetylcholinesterase
inhibitor, in rat prefrontal cortex: an _in vivo_ study. _Pharmacol Res_ 2007; 56: 288–294. Article CAS PubMed Google Scholar * Giovannini M, Bartolini L, Kopf S, Pepeu G . Acetylcholine
release from the frontal cortex during exploratory activity. _Brain Res_ 1998; 784: 218–227. Article CAS PubMed Google Scholar * Carli M, Calcagno E, Mainolfi P, Mainini E, Invernizzi R
. Effects of aripiprazole, olanzapine, and haloperidol in a model of cognitive deficit of schizophrenia in rats: relationship with glutamate release in the medial prefrontal cortex.
_Psychopharmacology (Berl)_ 2011; 214: 639–652. Article CAS Google Scholar * Paxinos G, Watson C . _The Rat Brain in Stereotaxic Coordinates_. Academic Press: New York, 1986. Google
Scholar * Czéh B, Perez-Cruz C, Fuchs E, Flügge G . Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: does hemisphere
location matter? _Behav Brain Res_ 2008; 190: 1–13. Article PubMed Google Scholar * Uchizono Y, Takeya R, Iwase M, Sasaki N, Oku M, Imoto H _et al_. Expression of isoforms of NADPH
oxidase components in rat pancreatic islets. _Life Sci_ 2006; 80: 133–139. Article CAS PubMed Google Scholar * Alquicer G, Morales-Medina JC, Quirion R, Flores G . Postweaning social
isolation enhances morphological changes in the neonatal ventral hippocampal lesion rat model of psychosis. _J Chem Neuroanat_ 2008; 35: 179–187. Article PubMed Google Scholar * Breen A,
Murphy J . Reactions of oxyl radicals with DNA. _Pharmacol Res_ 1995; 18: 1033–1077. CAS Google Scholar * Parnavelas J, Lieberman A, Webster K . Organization of neurons in the visual
cortex, area 17, of the rat. _J Anat_ 1977; 124: 305–322. CAS PubMed PubMed Central Google Scholar * Miller M . Development of projection and local circuit neurons in neocortex. In:
_Cerebral Cortex_. Peters and Jones (eds). vol. 7. Development and maturation of cerebral cortex. Plenum Press: New York, 1988, pp 133–175. Chapter Google Scholar * Wohleb E, Hanke M,
Corona A, Powell N, Stiner L, Bailey M _et al_. Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. _J Neurosci_ 2011;
31: 6277–6288. Article CAS PubMed PubMed Central Google Scholar * Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ . Glucose metabolic changes in the prefrontal cortex
are associated with HPA axis response to a psychosocial stressor. _Psychoneuroendocrinology_ 2008; 33: 517–529. Article CAS PubMed PubMed Central Google Scholar * Oei N, Everaerd W,
Elzinga B, van Well S, Bermond B . Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. _Stress_ 2006; 9: 133–141. Article CAS
PubMed Google Scholar * Gibb J, Hayley S, Gandhi R, Poulter MO, Anisman H . Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: circulating and brain
cytokines, plasma corticosterone and behavioral changes in mice. _Brain Behav Immun_ 2008; 22: 573–589. Article CAS PubMed Google Scholar * Amato J, Bankson M, Yamamoto B . Prior
exposure to chronic stress and MDMA potentiates mesoaccumbens dopamine release mediated by the 5-HT(1B) receptor. _Neuropsychopharmacology_ 2007; 32: 946–954. Article CAS PubMed Google
Scholar * Perry JC, D'Almeida V, Antunes IB, Tufik S . Distinct behavioral and neurochemical alterations induced by intermittent hypoxia or paradoxical sleep deprivation in rats.
_Progr Neuro-Psychopharmacol Biol Psychiatry_ 2008; 32: 87–94. Article CAS Google Scholar * Lukkes JL, Mokin MV, Scholl JL, Forster GL . Adult rats exposed to early-life social isolation
exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. _Hormones Behav_ 2009; 55: 248–256. Article CAS Google Scholar * Gos T, Becker K, Bock J,
Malecki U, Bogerts B, Poeggel G _et al_. Early neonatal and postweaning social emotional deprivation interferes with the maturation of serotonergic and tyrosine hydroxylase-immunoreactive
afferent fiber systems in the rodent nucleus accumbens, hippocampus and amygdala. _Neuroscience_ 2006; 140: 811–821. Article CAS PubMed Google Scholar * Han X, Wang W, Shao F, Li N .
Isolation rearing alters social behaviors and monoamine neurotransmission in the medial prefrontal cortex and nucleus accumbens of adult rats. _Brain Res_ 2011; 1385: 175–181. Article CAS
PubMed Google Scholar * Fabricius K, Steiniger-Brach B, Helboe L, Fink-Jensen A, Wörtwein G . Socially isolated rats exhibit changes in dopamine homeostasis pertinent to schizophrenia.
_Int J Dev Neurosci_ 2011; 29: 347–350. Article CAS PubMed Google Scholar * Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M . Redox dysregulation, neurodevelopment, and schizophrenia.
_Curr Opin Neurobiol_ 2009; 19: 220–230. Article CAS PubMed Google Scholar * Owe-Larsson B, Ekdahl K, Edbom T, Ösby U, Karlsson H, Lundberg C _et al_. Increased plasma levels of
thioredoxin-1 in patients with first episode psychosis and long-term schizophrenia. _Progr Neuro-Psychopharmacol Biol Psychiatry_ 2011; 35: 1117–1121. Article CAS Google Scholar * Li XF,
Zheng YL, Xiu MH, Chen DC, Kosten TR, Zhang XY . Reduced plasma total antioxidant status in first-episode drug-naive patients with schizophrenia. _Progr Neuro-Psychopharmacol Biol
Psychiatry_ 2011; 35: 1064–1067. Article CAS Google Scholar * Karelina K, DeVries A . Modeling social influences on human health. _Psychosom Med_ 2011; 73: 67–74. Article PubMed Google
Scholar * Marsden CA, King MV, Fone KCF . Influence of social isolation in the rat on serotonergic function and memory—relevance to models of schizophrenia and the role of 5-HT6 receptors.
_Neuropharmacology_ 2011; 61: 400–407. Article CAS PubMed Google Scholar * Bohus B, Benus R, Fokkema D, Koolhaas J, Nyakas C, van Oortmerssen G _et al_. Neuroendocrine states and
behavioral and physiological stress responses. _Prog Brain Res_ 1987; 72: 57–70. Article CAS PubMed Google Scholar * Torrey EF, Yolken RH . The schizophrenia–rheumatoid arthritis
connection: infectious, immune, or both? _Brain Behav Immunity_ 2001; 15: 401–410. Article CAS Google Scholar * Vinogradov S, Gottesman I, Moises H, Nicol S . Negative association between
schizophrenia and rheumatoid arthritis. _Schizophr Bull_ 1991; 17: 669–678. Article CAS PubMed Google Scholar * Gorwood P, Pouchot J, Vinceneux P, Puéchal X, Flipo R, De Bandt M _et
al_. Rheumatoid arthritis and schizophrenia: a negative association at a dimensional level. _Schizophr Res_ 2004; 66: 21–29. Article CAS PubMed Google Scholar * Tang LLYK, Yang XF, Zheng
JS . Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. _J Int Med Res_ 2007; 35: 517–522. Article CAS PubMed Google Scholar * Wang Q, Tompkins KD, Simonyi
A, Korthuis RJ, Sun AY, Sun GY . Apocynin protects against global cerebral ischemia–reperfusion-induced oxidative stress and injury in the gerbil hippocampus. _Brain Res_ 2006; 1090:
182–189. Article CAS PubMed Google Scholar * Lull M, Levesque S, Surace M, Block M . Chronic apocynin treatment attenuates beta amyloid plaque size and microglial number in hAPP(751)(SL)
mice. _PLoS One_ 2011; 6: e20153. Article CAS PubMed PubMed Central Google Scholar * Zeevalk G, Derr-Yellin E, Nicklas W . Relative vulnerability of dopamine and GABA neurons in
mesencephalic culture to inhibition of succinate dehydrogenase by malonate and 3-nitropropionic acid and protection by NMDA receptor blockade. _J Pharmacol Exp Ther_ 1995; 257: 1124–1130.
Google Scholar * Guentchev M, Voigtländer T, Haberler C, Groschup MH, Budka H . Evidence for oxidative stress in experimental prion disease. _Neurobiol Dis_ 2000; 7: 270–273. Article CAS
PubMed Google Scholar * Mei Y, Gawai KR, Nie Z, Ramkumar V, Helfert RH . Age-related reductions in the activities of antioxidant enzymes in the rat inferior colliculus. _Hearing Res_ 1999;
135: 169–180. Article CAS Google Scholar * Atkins C, Sweatt J . Reactive oxygen species mediate activity-dependent neuron–glia signaling in output fibers of the hippocampus. _J Neurosci_
1999; 19: 7241–7248. Article CAS PubMed PubMed Central Google Scholar * Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F _et al_. Schizophrenia and oxidative stress: glutamate
cysteine ligase modifier as a susceptibility gene. _Am J Hum Genet_ 2006; 79: 586–592. Article CAS PubMed PubMed Central Google Scholar * Pantano C, Reynaert N, van der Vliet A,
Janssen-Heininger Y . Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. _Antioxid Redox Signal_ 2006; 8: 1791–1806. Article CAS PubMed Google Scholar * Prosser BL,
Ward CW, Lederer WJ . X-ROS signaling: rapid mechano-chemo transduction in heart. _Science_ 2011; 333: 1440–1445. Article CAS PubMed Google Scholar * Guidotti A, Auta J, Davis JM,
Gerevini VD, Dwivedi Y, Grayson DR _et al_. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. _Arch Gen
Psychiatry_ 2000; 57: 1061–1069. Article CAS PubMed Google Scholar * Hossein Fatemi S, Stary JM, Earle JA, Araghi-Niknam M, Eagan E . GABAergic dysfunction in schizophrenia and mood
disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and reelin proteins in cerebellum. _Schizophr Res_ 2005; 72: 109–122. Article PubMed Google Scholar
* Thompson M, Weickert CS, Wyatt E, Webster MJ . Decreased glutamic acid decarboxylase67 mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. _J
Psychiatr Res_ 2009; 43: 970–977. Article PubMed Google Scholar * Lodge D, Behrens M, Grace AA . A loss of parvalbumin-containing interneurons is associated with diminished oscillatory
activity in an animal model of schizophrenia. _J Neurosci_ 2009; 29: 2344–2354. Article CAS PubMed PubMed Central Google Scholar * Kilts CD . The changing roles and targets for animal
models of schizophrenia. _Biol Psychiatry_ 2001; 50: 845–855. Article CAS PubMed Google Scholar * Kalkman HO, Loetscher E . GAD(67): the link between the GABA-deficit hypothesis and the
dopaminergic- and glutamatergic theories of psychosis. _J Neural Transm_ 2003; 110: 803–812. CAS PubMed Google Scholar * Turnock-Jones J, Jennings C, Robbins M, Cluderay J, Cilia J, Reid
J _et al_. Increased expression of the NR2A NMDA receptor subunit in the prefrontal cortex of rats reared in isolation. _Synapse_ 2009; 63: 836–884. Article CAS PubMed Google Scholar *
Morris R, Anderson E, Lynch G, Baudry M . Selective impairment of learning and blockade of long-term potentiation by an _N_-methyl-D-aspartate receptor antagonist, AP5. _Nature_ 1986; 319:
774–776. Article CAS PubMed Google Scholar * Steele RJ, Stewart MG, Rose SPR . Increases in NMDA receptor binding are specifically related to memory formation for a passive avoidance
task in the chick: a quantitative autoradiographic study. _Brain Res_ 1995; 674: 352–356. Article CAS PubMed Google Scholar * Carmignoto G, Vicini S . Activity-dependent decrease in NMDA
receptor responses during development of the visual cortex. _Science_ 1992; 258: 1007–1011. Article CAS PubMed Google Scholar * Ju Kim M, Shin K-S, Chung Y-B, Jung KW, Cha CI, Hoon Shin
D . Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. _Brain Res_ 2005; 1040: 178–186. Article CAS Google Scholar * Serrano F, Kolluri NS, Wientjes FB, Card
JP, Klann E . NADPH oxidase immunoreactivity in the mouse brain. _Brain Res_ 2003; 988 (1–2): 193–198. Article CAS PubMed Google Scholar * Yoshioka H, Niizuma K, Katsu M, Okami N, Sakata
H, Kim G _et al_. NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. _J Cereb Blood Flow Metab_ 2011; 31: 868–880. Article CAS PubMed Google
Scholar * Carrano A, Hoozemans J, van der Vies S, Rozemuller A, van Horssen J, de Vries H . Amyloid beta induces oxidative stress-mediated blood–brain barrier changes in capillary amyloid
angiopathy. _Antioxid Redox Signal_ 2011; 15: 1167–1178. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Olivier Plastre, Dr Paolo Tucci, Dr Margherita
Zotti and Dr MariaGrazia Morgese for excellent technical support for western blotting and behavioral test and Katharine Brieger for help with editing. This work has been supported by the
Swiss National Foundation (Grant No. 3200A0-103725), the Italian PRIN to LT, the Swedish Medical Research Council, the European Union Grants MASTERSWITCH (HEALTH-F2-2008-223404) and
EURATRANS (HEALTH-F4-2010-241504). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pathology and Immunology, University of Geneva and Department of Genetic and Laboratory
Medicine, Geneva University Hospitals, Geneva, Switzerland S Schiavone, V Jaquet, S Sorce, M Dubois-Dauphin & K-H Krause * Redoxis AB, Lund, Sweden M Hultqvist * Department of Medical
Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden L Bäckdahl & R Holmdahl * Department of Biomedical Sciences, University of Foggia, Foggia, Italy M Colaianna & L
Trabace * Department of Physiology and Pharmacology ‘Vittorio Erspamer’, University of Rome, La Sapienza, Italy V Cuomo Authors * S Schiavone View author publications You can also search
for this author inPubMed Google Scholar * V Jaquet View author publications You can also search for this author inPubMed Google Scholar * S Sorce View author publications You can also search
for this author inPubMed Google Scholar * M Dubois-Dauphin View author publications You can also search for this author inPubMed Google Scholar * M Hultqvist View author publications You
can also search for this author inPubMed Google Scholar * L Bäckdahl View author publications You can also search for this author inPubMed Google Scholar * R Holmdahl View author
publications You can also search for this author inPubMed Google Scholar * M Colaianna View author publications You can also search for this author inPubMed Google Scholar * V Cuomo View
author publications You can also search for this author inPubMed Google Scholar * L Trabace View author publications You can also search for this author inPubMed Google Scholar * K-H Krause
View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to S Schiavone. ETHICS DECLARATIONS COMPETING INTERESTS KHK and VJ
are founding members of the startup company GenKyoTex (http://www.genkyotex.com), which develops NOX inhibitors. The other authors declare no potential conflicts of interest relevant to the
subject matter of this work. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Translational Psychiatry website SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION (DOC 35 KB) SUPPLEMENTARY FIGURE 1 (DOC 1204 KB) SUPPLEMENTARY FIGURE 2 (DOC 319 KB) SUPPLEMENTARY FIGURE 3 (DOC 2449 KB) SUPPLEMENTARY FIGURE 4 (DOC 12836 KB) SUPPLEMENTARY
FIGURE 5 (DOC 896 KB) SUPPLEMENTARY FIGURE 6 (DOC 165 KB) SUPPLEMENTARY FIGURE 7 (DOC 154 KB) SUPPLEMENTARY FIGURE 8 (DOC 1291 KB) SUPPLEMENTARY FIGURE 9 (DOC 114 KB) RIGHTS AND PERMISSIONS
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Schiavone, S., Jaquet, V., Sorce, S. _et al._ NADPH oxidase elevations in
pyramidal neurons drive psychosocial stress-induced neuropathology. _Transl Psychiatry_ 2, e111 (2012). https://doi.org/10.1038/tp.2012.36 Download citation * Received: 13 October 2011 *
Revised: 15 March 2012 * Accepted: 05 April 2012 * Published: 08 May 2012 * Issue Date: May 2012 * DOI: https://doi.org/10.1038/tp.2012.36 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative KEYWORDS * apocynin * behavior * brain * parvalbumin * psychosis * social isolation