Play all audios:
ABSTRACT Exposure-based therapies are considered the state-of-the-art treatment for Posttraumatic Stress Disorder (PTSD). Yet, a substantial number of PTSD patients do not recover after
therapy. In the light of the well-known gene × environment interactions on the _risk_ for PTSD, research on individual genetic factors that influence _treatment success_ is warranted. The
gene encoding FK506-binding protein 51 (_FKBP5_), a co-chaperone of the glucocorticoid receptor (GR), has been associated with stress reactivity and PTSD risk. As _FKBP5_ single-nucleotide
polymorphism rs1360780 has a putative functional role in the regulation of _FKBP5_ expression and GR sensitivity, we hypothesized that this polymorphism influences PTSD treatment success. We
investigated the effects of _FKBP5_ rs1360780 genotype on Narrative Exposure Therapy (NET) outcome, an exposure-based short-term therapy, in a sample of 43 survivors of the rebel war in
Northern Uganda. PTSD symptom severity was assessed before and 4 and 10 months after treatment completion. At the 4-month follow-up, there were no genotype-dependent differences in therapy
outcome. However, the _FKBP5_ genotype significantly moderated the long-term effectiveness of exposure-based psychotherapy. At the 10-month follow-up, carriers of the rs1360780 risk (T)
allele were at increased risk of symptom relapse, whereas non-carriers showed continuous symptom reduction. This effect was reflected in a weaker treatment effect size (Cohen’s _D_=1.23) in
risk allele carriers compared with non-carriers (Cohen’s _D_=3.72). Genetic factors involved in stress response regulation seem to not only influence PTSD risk but also responsiveness to
psychotherapy and could hence represent valuable targets for accompanying medication. SIMILAR CONTENT BEING VIEWED BY OTHERS EPIGENETICS OF TRAUMATIC STRESS: THE ASSOCIATION OF _NR3C1_
METHYLATION AND POSTTRAUMATIC STRESS DISORDER SYMPTOM CHANGES IN RESPONSE TO NARRATIVE EXPOSURE THERAPY Article Open access 19 January 2023 LONGITUDINAL GENOME-WIDE METHYLATION STUDY OF PTSD
TREATMENT USING PROLONGED EXPOSURE AND HYDROCORTISONE Article Open access 13 July 2021 EPIGENETIC BIOTYPES OF POST-TRAUMATIC STRESS DISORDER IN WAR-ZONE EXPOSED VETERAN AND ACTIVE DUTY
MALES Article Open access 18 December 2020 INTRODUCTION Posttraumatic Stress Disorder (PTSD) is the most common mental health condition in the aftermath of traumatic stress. PTSD prevalence
rates depend on cumulative trauma exposure1 and converge around 8% in the United States,2,3 whereas the disorder occurrence is much higher in post-conflict settings.4 Without treatment, PTSD
may take a chronic course,1 associated with severe impairments in daily functioning, higher risk of physical illness5,6 and suicidality.7 However, even when treated with exposure-based
psychotherapy, considered to be the most effective treatment for PTSD,8,9 a substantial proportion of survivors does not recover.10 Therefore, the identification of individual factors that
influence PTSD treatment success is of utmost scientific and clinical importance. The formation of strong fear memories of traumatic experiences11 and a failure to extinguish the associated
reactions to trauma reminders12 are thought to be the key processes of PTSD development. Hence, exposure-based treatments aim at the modification of fear memories through extinction
learning.13 The heritability of PTSD susceptibility after trauma is ~30–40%,14,15 and studies investigating gene × environment interactions have identified several memory-related genetic
factors that moderate the influence of cumulative trauma exposure on PTSD risk.16 Hence, the response to exposure-based PTSD treatments might be also moderated by particular genetic variants
that are implicated in memory processes. To date, two studies identified genetic variations of the serotonin transporter gene17 as well as the brain-derived neurotrophic factor18 as genetic
modulators of PTSD treatment outcome. Hypothalamus–pituitary–adrenal axis regulation has been implicated in the etiology of stress-related disorders such as PTSD.19 The release of
glucocorticoids facilitates the mobilization of resources for a fight or a flight response. Concurrently, binding of cortisol to the glucocorticoid receptor (GR) is critical to terminate the
stress reaction via negative feedback.20 Hence, the functioning of the GR is necessary for an adequate stress response regulation. Several chaperones and co-chaperones, including
FK506-binding protein 51 (FKBP5), modulate GR sensitivity. Binding of FKBP5 to the GR reduces its cortisol-binding capacity and prevents nuclear translocation,21,22 which leads to impaired
negative feedback regulation of the hypothalamus–pituitary–adrenal axis and a prolonged stress response. Interestingly, _FKBP5_ gene expression can be triggered by cortisol via intronic
glucocorticoid response elements.23 Binding of cortisol to GRs leads to a rapid increase in _FKBP5_ expression, which in turn reduces GR sensitivity—an ultrashort negative feedback loop of
GR sensitivity.24 Therefore, traumatic stress and subsequent increased cortisol release could lead to enhanced _FKBP5_ gene expression and reduced GR sensitivity.25 As glucocorticoid
signaling can influence the risk of PTSD26,27 and is known to have an important role in fear memory formation and extinction,28,29 genetic variability of _FKBP5_ may influence PTSD
vulnerability as well as responsiveness to trauma-focused treatments. The _FKBP5_ gene, located on the short arm of chromosome 6, harbors several common polymorphisms in high linkage
disequilibrium, found to be associated with different psychological disorders.25 Single-nucleotide polymorphism (SNP) rs1360780 is located closest to a functional glucocorticoid response
element and has a putative functional role in the regulation of _FKBP5_ expression and GR sensitivity.30 In healthy individuals, the risk (T) allele was associated with higher _FKBP5_
expression31 and relatively reduced GR sensitivity32 as well as impaired recovery of cortisol levels in response to stress33,34 (but see also Mahon _et al._35). Furthermore, the T allele
predicted enhanced risk for depression, albeit with inconsistent findings,31,36, 37, 38 elevated probability of anxiety disorder development39 and higher suicide risk.40 Most important in
this context, _FKBP5_ genotype was also found to be associated with peritraumatic dissociation, a strong risk factor for subsequent PTSD development,41 as well as with PTSD risk.32 The
latter study investigated four _FKBP5_ polymorphisms, including rs1360780, and found the highest PTSD probability in individuals with the risk genotype who had experienced physical and
sexual child abuse. Xie _et al._42 confirmed the _FKBP5_ genotype × environment interaction effect only for one of the four SNPs investigated (rs9470080) in an African American sample. An
extension study of the original study of Binder and colleagues30 replicated the interaction effect of rs1360780 and childhood trauma on current PTSD. Interestingly, rs1360780 risk allele
carrier status was associated with a differential chromatin conformation, which promotes higher _FKBP5_ gene expression in response to early stress. Furthermore, risk allele carriers who
experienced childhood trauma showed elevated DNA demethylation near and at functional glucocorticoid response elements of the _FKBP5_ gene, further enhancing _FKBP5_ gene expression in
response to GR activation.30 Yet, in contrast to healthy subjects, _FKBP5_ risk genotype carriers with PTSD show lower _FKBP5_ gene expression43 and enhanced GR sensitivity compared with
non-carriers,32,44 a finding that is in accordance with cumulative evidence of GR hypersensitivity in PTSD.26 Summing up, rs1360780, a putative functional SNP of the _FKBP5_ gene,30 has been
shown to effect—in a PTSD disease status-dependent manner—_FKBP5_ gene expression and GR sensitivity. This is the first study to investigate whether _FKBP5_ rs1360780 genotype modulates
treatment outcome of Narrative Exposure Therapy (NET), an exposure-based short-term trauma-therapeutic treatment approach especially developed for the context of mass conflict and organized
violence.45 The efficacy of NET for the treatment of PTSD symptoms has been previously shown in multiple settings,46 including the post-war context of Northern Uganda.47 We hypothesized that
carriers of rs1360780 risk (T) allele would benefit less from treatment with NET. MATERIALS AND METHODS SUBJECTS Participants were survivors of the rebel war led by the Lord’s Resistance
Army in Northern Uganda, who had experienced numerous atrocities, including abductions and forced recruitment into the rebel forces, mutilations, forced participation in combats, killings
and sexual violence. The recruitment for the present study took place in the former Internal Displaced People camps Anaka, Pabbo and Koch Goma. Inclusion criteria were (1) a diagnosis of
PTSD according to DSM-IV, (2) age between 18 and 65, (3) absence of any signs of alcohol or substance dependence, (4) absence of psychotic symptoms, (5) no psychotropic medication and (6) no
prior trauma-focused psychotherapy. Fifty-three subjects were enrolled in the study and received treatment with NET by trained local counselors under the supervision of clinical experts in
the field of trauma therapy. Chip-based genotyping was impossible for four individuals because of low DNA concentrations, and one further participant was excluded after genotyping quality
control. In addition, three participants were excluded from the present study because of discontinuation of therapy (_N_=1, rs1360780 genotype=C/T), unavailability for both follow-up
interviews (_N_=1, genotype=C/C) and an extraordinary strength of flashbacks, which required that a clinical expert resumed the treatment (_N_=1, genotype=C/C). In the latter case, the
therapy was completed successfully; however, comparability to the other treatments conducted by trained local counselors was no longer given. Individuals who we could only trace for one
follow-up assessment (only 4 months, _N_=1, genotype=C/C; only 10 months, _N_=1, genotype=T/T) were still included in the analyses. Finally, chip-based genotyping of _FKBP5_ rs1360780 failed
for two participants. Hence, results are reported on a final sample of _N_=43 (29 females, mean age=31.91, s.d.=9.49, rs1360780 genotype C/C=13, C/T=15, T/T=15). PROCEDURE Clinical
interviews were conducted before treatment (t1) and 4 and 10 months (t2 and t3, respectively) after treatment completion. Current PTSD diagnosis and symptom severity were assessed in a
structured interview based on the Posttraumatic Diagnostic Scale (PDS).48 A 62-item event list, adapted for the context of the Lord’s Resistance Army war, cf.49 was employed to assess the
number of experienced traumatic event types (traumatic load). We assessed suicidal risk utilizing the respective section of the Mini International Neuropsychiatric Interview (M.I.N.I.).50
Local interviewers who attended 6 weeks of training on the concepts of clinical interviews, quantitative data collection and PTSD conducted the interviews. The instruments were translated
into the local language (Luo) followed by blind back-translations, group discussions and corrections by independent translators. Retest reliability and validity (consistency with expert
ratings) of the psychological assessment by trained local interviewers were previously investigated and revealed good psychometric quality.51 Participants provided saliva samples in the
first diagnostic interview using Oragene Self Collection Kits (DNA Genothek, Ottawa, Ontario, Canada). SNP-based genotyping was performed according to the Genome-Wide Human SNP Nsp/Sty 6.0
User Guide (Affymetrix Inc, Santa Clara, CA, USA). SNP rs1360780 is represented on the array (SNP ID: SNP_A-8589266). Genetic quality control was performed in PLINK v1.07.52 TREATMENT The
study participants received an average of 12 sessions of NET. Sessions took place twice a week and generally lasted between 90 and 120 min. In brief, the aim of NET is to reconstruct the
survivor’s life story with a particular focus on traumatic stressors. The first session comprises psychoeducation and serves to obtain a biographical overview of the client’s life. The
subsequent sessions involve exposure therapy to the most severe traumatic experiences in chronological order and the last session comprises the re-reading of the narrated life story.45
Intensively trained local counselors performed the therapies under the supervision of expert psychologists. Treatment adhesion was monitored in case discussions, weekly supervision meetings
as well as via reviews of detailed case documentations. After a detailed explanation of the study protocol participants gave written informed consent. All procedures followed the Declaration
of Helsinki and were approved by the Institutional Review Board of Gulu University, Uganda, the Ugandan National Council for Science and Technology and the ethics committee of the German
Psychological Society (Deutsche Gesellschaft für Psychologie). STATISTICS Statistical analyses were performed in the statistical environment R 3.0.2.53 Demographic and clinical data of the
genotype groups were compared using Fisher’s exact test for count data and one-factorial analyses of variance (ANOVA) for continuous data. If ANOVA residuals were non-normally distributed, a
non-parametric Kruskal–Wallis H test was employed. The effect of _FKBP5_ rs1360780 genotype on treatment outcome was evaluated by fitting linear mixed effect models (R package nlme
3.1-111).54 PDS score was defined as the outcome variable, genotype as a between-subject fixed factor, time as a within-subject repeated fixed factor and participants as a random effect,
with random intercepts for each participant. The correlations of the repeated measurements within participants were modeled with a general correlation structure,55,56 and the maximum
likelihood estimation method was employed to fit the model. In the model selection procedure, we fitted nested models of increasing complexity and compared their goodness-of-fit. The initial
analysis was performed including only time and _FKBP5_ rs1360780 genotype as predictors. Next, we analyzed whether a genotype model, comparing the three rs1360780 genotype groups (C/C, C/T
and T/T), or a risk allele carrier model, combining C/T and T/T genotypes in one group, represented the data best. As traumatic load is known to strongly influence PTSD symptomatology,1 and
_FKBP5_ gene × environment interactions have been observed previously,30,32 further analyses were performed with and without traumatic load as a covariate, and with and without allowing for
potential time × _ FKBP5_ genotype × traumatic load interaction effects. In addition, it was evaluated whether the inclusion of the covariates sex and age would improve model fit. As
recommended by Burnham and Anderson,57 model selection was based on Akaike’s Information Criterion, which has a profound information-theoretic foundation and aims at minimizing the expected
Kullback–Leibler divergence between the model and the true underlying data-generating process. We expected T allele carriers to benefit less from NET treatment, which would result in a
significant time × _FKBP5_ rs1360780 interaction. More specifically, we hypothesized that the difference between the pretreatment PDS score (time point t1) and the follow-up PDS scores (time
points t2 or t3) would be higher in individuals with the protective genotype (C/C) than in carriers of the T (risk) allele. We tested this specific hypothesis by evaluating the statistical
significance of planned contrasts while adjusting for multiple comparisons (R package multcomp 1.3-1).58 RESULTS _FKBP5_ rs1360780 genotype groups showed no differences in age, gender
distribution, traumatic load, number of NET sessions, PTSD symptom severity, PTSD symptom scores and suicidality before treatment (Table 1). Confirming our main hypothesis, we found a
significant time × _ FKBP5_ rs1360780 genotype interaction effect. This effect was present in every estimated linear mixed effect model. Our successive model selection procedure strongly
suggested that a risk allele carrier model, including traumatic load and sex as covariates, represented the data best (Table 2). We therefore report statistical inference from this model.
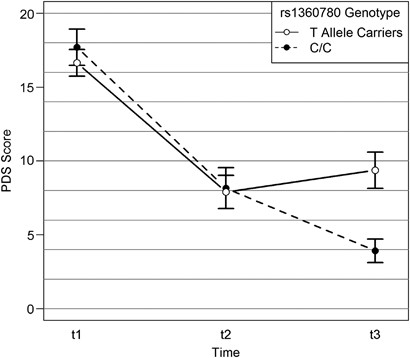
The selected model revealed a significant treatment effect (main effect time, F2,80=96.84, _P_<0.001), no significant main _FKBP5_ rs1360780 genotype effect, but a significant time × _
FKBP5_ rs1360780 genotype interaction effect (F2,80=5.40, _P_=0.006, Figure 1). Furthermore, the two included covariates, traumatic load (F1,39=18.42, _P_<0.001) and sex (F1,39=6.08,
_P_=0.018) significantly predicted PTSD symptom severity. To further examine the nature of the time × _ FKBP5_ rs1360780 genotype interaction effect, we next calculated three planned
orthogonal contrasts while adjusting P-values for multiple testing. There was no genotype-dependent difference in treatment success rates 4 months after treatment (comparison t1–t2). Yet, 10
months after the end of the treatment (comparison t1–t3), non-carriers of the risk allele had significantly higher symptom improvements than risk allele carriers (_Z_=3.37, _P_<0.001,
Figure 1). Furthermore, there was a significant difference in the symptom development between the two follow-up assessments (difference t2–t3); whereas risk allele carriers had a greater
risk for symptom relapse, non-carriers continued to show symptom improvements (_Z_=2.71, _P_=0.009, Figure 1). To obtain a better understanding of the substantial main effect of traumatic
load, we plotted the fitted values of the PDS score against traumatic load separately for the three time points and genotype groups (Figure 2). We observed a dose-dependent relationship
between traumatic load and PDS score at all time points, yet T allele carriers showed a higher intercept 10 months post treatment. We next analyzed the clinical significance of the genotype
effect on therapy outcome by investigating the mean change scores between the 10-month follow-up and the pretreatment assessment, as well as the treatment effect sizes for each genotype
group. Both genotype groups presented with large treatment effects according to the conventions of Cohen; however, for individuals with the protective (C/C) genotype the treatment effect
size was approximately three times higher than for carries of the risk allele (Table 3). Furthermore, 10 months after treatment, no individual in the C/C genotype group fulfilled the
diagnosis of PTSD, whereas 43% of T allele carriers still met the diagnostic criteria according to DSM-IV (Table 3). Finally, we exploratively investigated whether genotype differences in
treatment success would be also reflected in the three PTSD symptom clusters and suicidality risk. T Allele carriers scored higher on all variables 10 months after treatment (Table
3,Supplementary Figures S1 and 2). These effects resulted in a significant rs1360780 × time interaction for avoidance (F2,80=5.74, _P_=0.005) and hyperarousal symptoms (F2,80=4.65,
_P_=0.012), but not for intrusion symptoms (F2,80=2.10, _P_=0.130). The interaction was marginally significant for the outcome suicidality (F2,80=3.06, _P_=0.053). DISCUSSION Confirming our
hypothesis, we found a significant effect of _FKBP5_ rs1360780 genotype on therapeutic outcome of NET. This effect was present at 10 months, but not 4 months after treatment completion,
indicating that the _FKBP5_ genotype predominantly influences long-term therapeutic outcome. Psychotherapy—in contrast to pharmacological treatment—initializes a process of recovery that
continues over time. Confirming this, evidence from Northern Uganda shows that NET treatment effects increase over time (that is, treatment effects assessed 1 year after therapy completion
were more pronounced than 6 months post treatment).47 Hence, genetic variants that are linked to psychotherapeutic outcome should show stronger effects on the long-term course of symptoms
rather than on immediate psychotherapeutic outcomes. This is in line with the findings of Bryant _et al._,17 reporting a moderating influence of genetic variation at the serotonin
transporter locus on trauma therapy outcome at the follow-up assessment, but not immediately post treatment. The empirical data favored a risk allele carrier model: carriers of one or two
copies of the T allele showed enhanced risk for symptom relapse 10 months after treatment, whereas individuals with the protective C/C genotype showed continuous symptom improvement. The
genotype effect had significant clinical implications: individuals with the protective genotype had three times higher treatment effect sizes than risk allele carriers. Whereas 43% of risk
allele carriers fulfilled the diagnostic criteria of PTSD, the entire C/C genotype group showed clinical remission 10 months post treatment. In addition, the strong effect of the _FKBP5_
genotype on treatment success was descriptively reflected in all PTSD symptom clusters as well as in suicidality, with significant effects on the avoidance and hyperarousal symptom cluster.
Hence, trauma-related symptoms of distress and arousal seem to be more resistant to modification through trauma-focused therapy in carriers of the rs1360780 T allele. How might _FKBP5_
rs1360780 genotype influence psychotherapy success? Extensive evidence indicates that the stress hormone cortisol facilitates memory formation, impairs retrieval and enhances memory
extinction processes.28 Both the formation of traumatic memories and their modification through psychotherapy rely on learning and memory processes: whereas fear conditioning is thought to
be responsible for the onset of PTSD, extinction learning is the basis of exposure therapy.16 Indeed, stress and associated elevated cortisol levels increase extinction memory
consolidation,59 and the administration of glucocorticoids enhances the effects of exposure therapy for anxiety disorders.60, 61, 62 Accordingly, _FKBP5_ genotype might influence the initial
fear memory strength as well as the process of extinction learning. The _FKBP5_ rs1360780 T allele is characterized by a different three-dimensional structure of the _FKBP5_ gene, which
allows direct interaction between the glucocorticoid response element at intron 2 and the promoter region.30 This leads to higher _FKBP5_ induction by glucocorticoids, reduced GR sensitivity
and consequently to a prolonged cortisol response following stress exposure.30 This corresponds well with findings of reduced GR sensitivity32,44 and enhanced cortisol responses to stress
in healthy individuals carrying the high induction risk allele.33,34 Furthermore, higher _FKBP5_ mRNA expression in peripheral blood hours after the traumatic experience predicted PTSD
symptom development in survivors 4 months later.63 However, the described relationship between _FKBP5_ risk genotype, higher _FKBP5_ expression, relative GR resistance and prolonged cortisol
response seems to be only valid for PTSD-unaffected individuals. In contrast, carriers of the _FKBP5_ risk genotype with PTSD show enhanced GR sensitivity compared with non-carriers,32,44
corresponding well to repeated findings of GR supersensitivity in PTSD.26 In addition, lower _FKBP5_ gene expression has been reported in individuals with PTSD,43,64,65 and was associated
with _FKBP5_ risk genotype.43 Furthermore, two recent studies reported an increase in _FKBP5_ gene expression as a marker for successful exposure-based trauma therapy,65,66 which was
accompanied by an increase in plasma cortisol in one of the studies.66 In line with these findings, cortisol administration has been shown to improve exposure therapy success60, 61, 62 and
to reduce symptoms in PTSD.67 The present study is the first to show that the _FKBP5_ genotype modulates psychotherapeutic outcome. Reasons for the reduced long-term therapeutic benefits in
risk allele carriers may include stronger stress reactions at the time of trauma and hence stronger memories of the encountered traumata, which are more resistant to modification. On the
other hand, initial evidence indicates that once PTSD is developed, _FKBP5_ risk genotype may be associated with higher GR sensitivity and hence a rapid reduction in cortisol levels
following stress.32,44 Assuming that exposure to the traumatic experiences during psychotherapy triggers the stress response, a shortened cortisol response may impair the process of
extinction learning or extinction memory consolidation,28 which could explain the resurgence of symptoms in T allele carriers. We did not find a three-way interaction effect of time, _FKBP5_
genotype and traumatic load. In other words, genotype influenced the symptom improvement following NET; however, this effect was not moderated by traumatic load. Instead, the number of
traumatic events experienced increased PTSD symptom severity at all time points. This result replicates earlier findings of a dose-dependent effect of traumatic load on PTSD symptom
severity1,68 and extends them by illustrating that higher trauma load also leads to higher PTSD symptoms after treatment. Several explanations may account for the lack of an interaction
effect. First of all, genetic risk × trauma exposure interaction effects might be more central to the onset of PTSD than to its treatment. However, our results do not exclude the possibility
of an interaction with childhood trauma, which was found in previous investigations.30,32,69 Our trauma questionnaire only includes four items of experienced or witnessed physical childhood
abuse and we did not find any interaction effect with this assessment of childhood trauma. A more detailed assessment of childhood trauma and the investigation of a larger sample with
greater variations in childhood trauma might be required to detect potential interactions. The relatively small sample size clearly represents a limitation of this study, as it prevents the
assessment of potential underlying population stratification. Replication studies with much larger samples are warranted to confirm the moderating role of _FKBP5_ on trauma therapy outcome.
Nevertheless, this investigation provides initial evidence of a strong effect of the _FKBP5_ genotype that influences long-term treatment outcome at all investigated levels of traumatic
load. What do these results imply for PTSD treatment development? According to a meta-analysis performed by Bradley _et al._10 approximately one-third of clients treated with exposure-based
therapies still meet diagnostic criteria for PTSD. Comparable rates (32%) were found 12 months after treatment with NET in survivors of the Lord’s Resistance Army war in Northern Uganda.47
Similarly, 31% of our total study population still met DSM-IV criteria for PTSD 10 months after treatment completion; however, all of them were carriers of the _FKBP5_ rs1360780 T allele.
Given the strong effects of the _FKBP5_ genotype on treatment outcome in this study, in combination with the central role of FKBP5 in the regulation of the stress response, this molecule
seems to be an interesting drug target for the treatment of stress-related disorders69,70 or for the pharmacological enhancement of psychotherapeutic effectiveness. REFERENCES * Kolassa IT,
Ertl V, Kolassa S, Onyut LP, Elbert T . The probability of spontaneous remission from PTSD depends on the number of traumatic event types experienced. _Psychol Trauma_ 2010; 3: 169–174.
Article Google Scholar * Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB . Posttraumatic stress disorder in the National Comorbidity Survey. _Arch Gen Psychiatry_ 1995; 52: 1048–1060.
Article CAS PubMed Google Scholar * Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE . Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the
National Comorbidity Survey Replication. _Arch Gen Psychiatry_ 2005; 62: 593–602. Article PubMed Google Scholar * Neuner F, Schauer M, Karunakara U, Klaschik C, Robert C, Elbert T .
Psychological trauma and evidence for enhanced vulnerability for posttraumatic stress disorder through previous trauma among West Nile refugees. _BMC Psychiatry_ 2004; 4: 34. Article PubMed
PubMed Central Google Scholar * Kubzansky LD, Bordelois P, Jun HJ, Roberts AL, Cerda M, Bluestone N _et al_. The weight of traumatic stress: a prospective study of posttraumatic stress
disorder symptoms and weight status in women. _JAMA Psychiatry_ 2013; 71: 44–51. Article Google Scholar * Glaesmer H, Brahler E, Gundel H, Riedel-Heller SG . The association of traumatic
experiences and posttraumatic stress disorder with physical morbidity in old age: a German population-based study. _Psychosom Med_ 2011; 73: 401–406. Article PubMed Google Scholar *
Jakupcak M, Cook J, Imel Z, Fontana A, Rosenheck R, McFall M . Posttraumatic stress disorder as a risk factor for suicidal ideation in Iraq and Afghanistan War veterans. _J Trauma Stress_
2009; 22: 303–306. Article PubMed Google Scholar * Bisson JI, Ehlers A, Matthews R, Pilling S, Richards D, Turner S . Psychological treatments for chronic post-traumatic stress disorder.
Systematic review and meta-analysis. _Br J Psychiatry_ 2007; 190: 97–104. Article PubMed Google Scholar * Ehlers A, Bisson J, Clark DM, Creamer M, Pilling S, Richards D _et al_. Do all
psychological treatments really work the same in posttraumatic stress disorder? _Clin Psychol Rev_ 2010; 30: 269–276. Article PubMed PubMed Central Google Scholar * Bradley R, Greene J,
Russ E, Dutra L, Westen D . A multidimensional meta-analysis of psychotherapy for PTSD. _Am J Psychiatry_ 2005; 162: 214–227. Article PubMed Google Scholar * Elbert T, Schauer M . Burnt
into memory. _Nature_ 2002; 419: 883. Article CAS PubMed Google Scholar * Jovanovic T, Ressler KJ . How the neurocircuitry and genetics of fear inhibition may inform our understanding of
PTSD. _Am J Psychiatry_ 2010; 167: 648–662. Article PubMed PubMed Central Google Scholar * Rothbaum BO, Davis M . Applying learning principles to the treatment of post-trauma reactions.
_Ann N Y Acad Sci_ 2003; 1008: 112–121. Article PubMed Google Scholar * True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ _et al_. A twin study of genetic and environmental
contributions to liability for posttraumatic stress symptoms. _Arch Gen Psychiatry_ 1993; 50: 257–264. Article CAS PubMed Google Scholar * Stein MB, Jang KL, Taylor S, Vernon PA,
Livesley WJ . Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. _Am J Psychiatry_ 2002; 159: 1675–1681. Article PubMed
Google Scholar * Wilker S, Kolassa IT . The formation of a neural fear network in posttraumatic stress disorder: Insights from molecular genetics. _Clin Psychol Sci_ 2013; 1: 452–469.
Article Google Scholar * Bryant RA, Felmingham KL, Falconer EM, Pe Benito L, Dobson-Stone C, Pierce KD _et al_. Preliminary evidence of the short allele of the serotonin transporter gene
predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. _Biol Psychiatry_ 2010; 67: 1217–1219. Article CAS PubMed Google Scholar * Felmingham KL,
Dobson-Stone C, Schofield PR, Quirk GJ, Bryant RA . The brain-derived neurotrophic factor Val66Met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. _Biol
Psychiatry_ 2013; 73: 1059–1063. Article CAS PubMed PubMed Central Google Scholar * Pervanidou P, Chrousos GP . Neuroendocrinology of post-traumatic stress disorder. _Prog Brain Res_
2010; 182: 149–160. Article CAS PubMed Google Scholar * Sapolsky RM, Romero LM, Munck AU . How do glucocorticoids influence stress responses? Integrating permissive, suppressive,
stimulatory, and preparative actions. _Endocr Rev_ 2000; 21: 55–89. CAS PubMed Google Scholar * Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T . FK506-binding proteins 51 and
52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. _J Biol Chem_ 2005; 280: 4609–4616. Article CAS PubMed Google
Scholar * Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG . Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. _Endocrinology_ 2000;
141: 4107–4113. Article CAS PubMed Google Scholar * Hubler TR, Scammell JG . Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. _Cell
Stress Chaperones_ 2004; 9: 243–252. Article CAS PubMed PubMed Central Google Scholar * Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M .
Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. _J Clin Endocrin
Metab_ 2003; 88: 277–284. Article CAS Google Scholar * Binder EB . The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and
anxiety disorders. _Psychoneuroendocrinology_ 2009; 34: S186–S195. Article CAS PubMed Google Scholar * Yehuda R . Status of glucocorticoid alterations in post-traumatic stress disorder.
_Ann N Y Acad Sci_ 2009; 1179: 56–69. Article CAS PubMed Google Scholar * van Zuiden M, Kavelaars A, Geuze E, Olff M, Heijnen CJ . Predicting PTSD: pre-existing vulnerabilities in
glucocorticoid-signaling and implications for preventive interventions. _Brain Behav Immun_ 2013; 30: 12–21. Article CAS PubMed Google Scholar * de Quervain DJ-F, Aerni A, Schelling G,
Roozendaal B . Glucocorticoids and the regulation of memory in health and disease. _Front Neuroendocrinol_ 2009; 30: 358–370. Article CAS PubMed Google Scholar * Pitman RK, Rasmusson AM,
Koenen KC, Shin LM, Orr SP, Gilbertson MW _et al_. Biological studies of post-traumatic stress disorder. _Nat Rev Neurosci_ 2012; 13: 769–787. Article CAS PubMed PubMed Central Google
Scholar * Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM _et al_. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. _Nat
Neurosci_ 2013; 16: 33–41. Article CAS PubMed Google Scholar * Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B _et al_. Polymorphisms in FKBP5 are associated with
increased recurrence of depressive episodes and rapid response to antidepressant treatment. _Nat Genet_ 2004; 36: 1319–1325. Article CAS PubMed Google Scholar * Binder EB, Bradley RG,
Liu W, Epstein MP, Deveau TC, Mercer KB _et al_. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. _JAMA_ 2008; 299:
1291–1305. Article CAS PubMed PubMed Central Google Scholar * Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S _et al_. Polymorphisms in the FKBP5 gene region modulate
recovery from psychosocial stress in healthy controls. _Eur J Neurosci_ 2008; 28: 389–398. Article PubMed Google Scholar * Buchmann AF, Holz N, Boecker R, Blomeyer D, Rietschel M, Witt SH
_et al_. Moderating role of FKBP5 genotype in the impact of childhood adversity on cortisol stress response during adulthood. _Eur Neuropsychopharmacol_ 2013; 24: 837–845. Article PubMed
Google Scholar * Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS . Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults.
_Psychopharmacology (Berl)_ 2013; 227: 231–241. Article CAS Google Scholar * Zimmermann P, Bruckl T, Nocon A, Pfister H, Binder EB, Uhr M _et al_. Interaction of FKBP5 gene variants and
adverse life events in predicting depression onset: results from a 10-year prospective community study. _Am J Psychiatry_ 2011; 168: 1107–1116. Article PubMed Google Scholar * Lekman M,
Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ _et al_. The FKBP5-gene in depression and treatment response—an association study in the Sequenced Treatment Alternatives to Relieve
Depression (STAR*D) Cohort. _Biol Psychiatry_ 2008; 63: 1103–1110. Article CAS PubMed PubMed Central Google Scholar * Papiol S, Arias B, Gasto C, Gutierrez B, Catalan R, Fananas L .
Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. _J Affect Disord_ 2007; 104: 83–90. Article CAS PubMed Google Scholar * Minelli A,
Maffioletti E, Cloninger CR, Magri C, Sartori R, Bortolomasi M _et al_. Role of allelic variants of FK506-binding protein 51 (fkbp5) gene in the development of anxiety disorders. _Depress
Anxiety_ 2013; 30: 1170–1176. Article CAS PubMed Google Scholar * Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA . Interaction of FKBP5, a stress-related gene, with childhood trauma
increases the risk for attempting suicide. _Neuropsychopharmacology_ 2010; 35: 1674–1683. Article CAS PubMed PubMed Central Google Scholar * Koenen KC, Saxe G, Purcell S, Smoller JW,
Bartholomew D, Miller A _et al_. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. _Mol Psychiatry_ 2005; 10: 1058–1059. Article CAS
PubMed Google Scholar * Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA _et al_. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder.
_Neuropsychopharmacology_ 2010; 35: 1684–1692. Article CAS PubMed PubMed Central Google Scholar * Sarapas C, Cai G, Bierer LM, Golier JA, Galea S, Ising M _et al_. Genetic markers for
PTSD risk and resilience among survivors of the World Trade Center attacks. _Dis Markers_ 2011; 30: 101–110. Article PubMed PubMed Central Google Scholar * Mehta D, Gonik M, Klengel T,
Rex-Haffner M, Menke A, Rubel J _et al_. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression
studies. _Arch Gen Psychiatry_ 2011; 68: 901–910. Article CAS PubMed PubMed Central Google Scholar * Schauer M, Neuner F, Elbert T . _Narrative Exposure Therapy. A Short- Term
intervention for Traumatic Stress Disorders after War, Terror or Torture_. 2nd edn, Hogrefe & Huber: Göttingen, Germany, 2011. Google Scholar * Robjant K, Fazel M . The emerging
evidence for narrative exposure therapy: a review. _Clin Psychol Rev_ 2010; 30: 1030–1039. Article PubMed Google Scholar * Ertl V, Pfeiffer A, Schauer E, Elbert T, Neuner F .
Community-implemented trauma therapy for former child soldiers in Northern Uganda: a randomized controlled trial. _JAMA_ 2011; 306: 503–512. Article CAS PubMed Google Scholar * Foa EB,
Cashman L, Jaycox L, Perry K . The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. _Psychol Assess_ 1997; 9: 445–451. Article
Google Scholar * Wilker S, Kolassa S, Vogler C, Lingenfelder B, Elbert T, Papassotiropoulos A _et al_. The role of memory-related gene WWC1 (KIBRA) in lifetime posttraumatic stress
disorder: evidence from two independent samples from African conflict regions. _Biol Psychiatry_ 2013; 74: 664–671. Article CAS PubMed Google Scholar * Sheehan DV, Lecrubier Y, Sheehan
KH, Amorim P, Janavs J, Weiller E _et al_. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for
DSM-IV and ICD-10. _J Clin Psychiatry_ 1998; 59: 22–33. PubMed Google Scholar * Ertl V, Pfeiffer A, Saile R, Schauer E, Elbert T, Neuner F . Validation of a mental health assessment in an
African conflict population. _Psychol Assess_ 2010; 22: 318–324. Article PubMed Google Scholar * Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D _et al_. PLINK: a tool
set for whole-genome association and population-based linkage analyses. _Am J Hum Genet_ 2007; 81: 559–575. Article CAS PubMed PubMed Central Google Scholar * R Core Team. _R: A
Language and Environment for Statistical Computing_. R Foundation for Statistical Computing: Vienna, Austria, 2013. * Pinheiro J, Bates D, DebRoy S, Sarkar D and the R Development Core Team
. nlme: Linear and Nonlinear Mixed Effects Models. _R package version 3.1-111_ 2013. * Pinheiro J, Bates D . Unconstrained parametrizations for variance-covariance matrices. _Stat Comput_
1996; 6: 289–296. Article Google Scholar * Pinheiro J, Bates D . _Mixed-Effects Models in S and S-PLUS_. Statistics and Computing: Springer: New York, NY, USA, 2000. Book Google Scholar
* Burnham KP, Anderson DR . _Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach_. 2nd edn, Springer: New York, NY, USA, 2002. Google Scholar * Hothorn T,
Bretz F, Westfall P . Simultaneous inference in general parametric models. _Biom J_ 2008; 50: 346–363. Article PubMed Google Scholar * Hamacher-Dang TC, Engler H, Schedlowski M, Wolf OT .
Stress enhances the consolidation of extinction memory in a predictive learning task. _Front Behav Neurosci_ 2013; 7: 108. Article PubMed PubMed Central Google Scholar * Soravia LM,
Heinrichs M, Winzeler L, Fisler M, Schmitt W, Horn H _et al_. Glucocorticoids enhance _in vivo_ exposure-based therapy of spider phobia. _Depress Anxiety_ 2013; 31: 429–435. Article PubMed
Google Scholar * de Quervain DJ-F, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J _et al_. Glucocorticoids enhance extinction-based psychotherapy. _Proc Natl Acad Sci USA_ 2011;
108: 6621–6625. Article CAS PubMed PubMed Central Google Scholar * Bentz D, Michael T, de Quervain DJ, Wilhelm FH . Enhancing exposure therapy for anxiety disorders with
glucocorticoids: from basic mechanisms of emotional learning to clinical applications. _J Anxiety Disord_ 2010; 24: 223–230. Article PubMed Google Scholar * Segman RH, Shefi N,
Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY . Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. _Mol
Psychiatry_ 2005; 10: 500–513, 425. Article CAS PubMed Google Scholar * Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M _et al_. Gene expression patterns associated with
posttraumatic stress disorder following exposure to the World Trade Center attacks. _Biol Psychiatry_ 2009; 66: 708–711. Article CAS PubMed Google Scholar * Levy-Gigi E, Szabo C, Kelemen
O, Keri S . Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy.
_Biol Psychiatry_ 2013; 74: 793–800. Article CAS PubMed Google Scholar * Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E _et al_. Epigenetic biomarkers as
predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. _Front Psychiatry_ 2013; 4: 118. Article PubMed PubMed Central Google Scholar *
Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A _et al_. Low-dose cortisol for symptoms of posttraumatic stress disorder. _Am J Psychiatry_ 2004; 161: 1488–1490.
Article PubMed Google Scholar * Mollica RF, McInnes K, Poole C, Tor S . Dose-effect relationships of trauma to symptoms of depression and post-traumatic stress disorder among Cambodian
survivors of mass violence. _Br J Psychiatry_ 1998; 173: 482–488. Article CAS PubMed Google Scholar * Zannas AS, Binder EB . Gene-environment interactions at the FKBP5 locus: sensitive
periods, mechanisms and pleiotropism. _Genes Brain Behav_ 2014; 13: 25–37. Article CAS PubMed Google Scholar * Schmidt MV, Paez-Pereda M, Holsboer F, Hausch F . The prospect of FKBP51 as
a drug target. _ChemMedChem_ 2012; 7: 1351–1359. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This research was funded by a grant of the German Research
Foundation (DFG) awarded to ITK, DQ and AP (D-CH programme), by a doctoral scholarship of the German National Academic Foundation (Studienstiftung des deutschen Volkes) awarded to SW and by
the Swiss National Science Foundation (Sinergia grant CRSI33_130080 to DQ and AP). We would like to thank the team of Ugandan therapists for conducting therapies and diagnostic interviews of
highest quality, and for their commitment, empathy and professionalism in the work with traumatized survivors. We thank the non-governmental organization _vivo international_ for the
fruitful cooperation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Clinical and Biological Psychology, Institute for Psychology and Education, University of Ulm, Ulm, Germany S Wilker &
I-T Kolassa * Clinical Psychology and Neuropsychology, University of Konstanz,, Konstanz, Germany A Pfeiffer, T Elbert & B Lingenfelder * SAP Switzerland AG,, Tägerwilen, Switzerland S
Kolassa * Faculty of Medicine, Gulu University, Gulu, Uganda E Ovuga * Division of Molecular Neuroscience, University of Basel, Basel, Switzerland A Papassotiropoulos * Division of Cognitive
Neuroscience, University of Basel, Basel, Switzerland D de Quervain Authors * S Wilker View author publications You can also search for this author inPubMed Google Scholar * A Pfeiffer View
author publications You can also search for this author inPubMed Google Scholar * S Kolassa View author publications You can also search for this author inPubMed Google Scholar * T Elbert
View author publications You can also search for this author inPubMed Google Scholar * B Lingenfelder View author publications You can also search for this author inPubMed Google Scholar * E
Ovuga View author publications You can also search for this author inPubMed Google Scholar * A Papassotiropoulos View author publications You can also search for this author inPubMed Google
Scholar * D de Quervain View author publications You can also search for this author inPubMed Google Scholar * I-T Kolassa View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to S Wilker. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION
Supplementary Information accompanies the paper on the Translational Psychiatry website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 40 KB) RIGHTS AND PERMISSIONS This work is
licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license
holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Wilker, S., Pfeiffer, A., Kolassa, S. _et al._ The role of _FKBP5_ genotype in moderating long-term effectiveness of exposure-based psychotherapy for posttraumatic stress disorder. _Transl
Psychiatry_ 4, e403 (2014). https://doi.org/10.1038/tp.2014.49 Download citation * Received: 13 March 2014 * Revised: 29 April 2014 * Accepted: 22 May 2014 * Published: 24 June 2014 * Issue
Date: June 2014 * DOI: https://doi.org/10.1038/tp.2014.49 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative