Play all audios:
ABSTRACT The 15q13.3 microdeletion syndrome is caused by a 1.5-MB hemizygous microdeletion located on 15q13.3 affecting seven genes: _FAN1_; _MTMR10_; _TRPM1_; _miR-211_; _KLF13_; _OTUD7A_;
and _CHRNA7_. The 15q13.3 microdeletion increases the risk of intellectual disability, epilepsy, autism spectrum disorder and schizophrenia, though the clinical profile varies considerably.
Two mouse models of this syndrome, with hemizygous deletion of the orthologous region in the murine genome, have recently been shown to recapitulate a number of the behavioral and
physiological deficits that characterize the human condition. Still, little is known of the underlying biological mechanisms. Eleven human cases with homozygous deletion of the 15q13.3
region have been reported, all with severe functional and physiological impairments. We therefore hypothesized that a 15q13.3 homozygous knockout would confer more pronounced behavioral and
physiological deficits in mice than the 15q13.3 hemizygous deletion. Here we report the characterization of a 15q13.3 knockout mouse. We observed marked deficits including altered seizure
susceptibility, autistic behavior-related phenotypes, and auditory sensory processing. Several of these deficits, albeit less pronounced, were also found in the 15q13.3 hemizygous
littermates indicating a gene-dosage dependency. Our findings strongly indicate that studies of the hemi- and homozygous 15q13.3 mouse strains will facilitate understanding of the biological
mechanisms of severe mental disorders. SIMILAR CONTENT BEING VIEWED BY OTHERS _CUX1_-RELATED NEURODEVELOPMENTAL DISORDER: DEEP INSIGHTS INTO PHENOTYPE-GENOTYPE SPECTRUM AND UNDERLYING
PATHOLOGY Article Open access 30 August 2023 WILLIAMS SYNDROME Article 17 June 2021 FUNCTIONAL BRAIN DEFECTS IN A MOUSE MODEL OF A CHROMOSOMAL T(1;11) TRANSLOCATION THAT DISRUPTS _DISC1_ AND
CONFERS INCREASED RISK OF PSYCHIATRIC ILLNESS Article Open access 19 February 2021 INTRODUCTION Decades of intensive research have provided little knowledge and understanding of the
biological changes underlying severe psychiatric disorders, in particular schizophrenia and autism. One advancement has come from genome-wide association studies that identified hemizygous
microdeletions and microduplications termed copy-number variants (CNVs), which confer high risk of these disorders.1 The 15q13.3 hemizygous microdeletion increases the risk of intellectual
disability, epilepsy, autism spectrum disorder and schizophrenia 10-fold or more.2, 3, 4, 5 The microdeletion encompasses a region of ~1.5 MB from break-point (BP) 4 to BP5, with seven
genes; _MTMR10_; _FAN1_; _TRPM1_; _miR-211_; _KLF13_; _OTUD7A_; and _CHRNA7_ (OMIM #612001). The clinical phenotype varies greatly among individuals with the 15q13.3 microdeletion, ranging
from asymptomatic to severe neuropsychiatric disorders. In addition, the microdeletion is present in ~1% of patients with idiopathic generalized epilepsy compared with 0.019% in the general
population.1, 4, 6 Epileptic carriers that have been phenotyped in detail mainly suffer from absence seizures and not from generalized seizures.7, 8 Disorder-associated CNVs provide an
opportunity to gain insight into underlying mechanisms of the pathogenesis. We, and others, recently generated and characterized mouse models of the 15q13.3 microdeletion syndrome by
deleting the orthologous genomic region on mouse chromosome 7.9, 10 Behavioral characterization of the 15q13.3 mouse models, Df(h15q13)+/−, revealed schizophrenia-, autism- and
epilepsy-related phenotypes.9, 10 The 15q13.3 CNV is distinct from most known high-risk CNVs as relatively few genes are involved, simplifying mechanistic evaluation. Furthermore, humans
with homozygous deletions are viable, as 11 cases of homozygous deletion of the 15q13.3 region have been reported, all of them with severe physical and mental handicaps.11 The viability of
humans with homozygous 15q13.3 deletion suggests that the generation of a homozygous mouse model for this syndrome is feasible. We hypothesize that a 15q13.3 homozygous mouse model will
provide both new and stronger phenotypes than those previously observed in the hemizygous mouse models, and that the combined study of wildtype (WT), hemizygous and homozygous 15q13.3 mouse
strains will inform on the molecular and physiological mechanisms underlying schizophrenia, autism and epilepsy. Here we present, to the best of our knowledge, the first study of homozygous
15q13.3 knockout mice, Df(h15q13)−/− mice, in which we compare Df(h15q13)−/−, Df(h15q13)+/− and WT littermates. We focus on behavioral assays related to the neuropsychiatric disorders known
to affect human 15q13.3 microdeletion carriers. MATERIALS AND METHODS ANIMALS Df(h15q13)−/− mice were bred by mating Df(h15q13)+/− mice. The generation of Df(h15q13)+/− mice was described by
Fejgin _et al._9 At weaning, when mice were 3 or 4 weeks of age, biopsies were collected for PCR-based genotyping (see next paragraph). Mice were then split by gender and group housed
(mixed genotypes from the same litter, up to seven animals per cage), except during the Nest-building assay where animals were single housed. Mice were bred, genotyped and housed until the
age of 8 weeks at Taconic MB (Lille Skensved, Denmark), except for the pups used for ultrasonic vocalization recordings. As the genotype assigned animals to groups, randomization of animals
to experimental groups was not relevant. At 8 weeks of age, the mice were transferred to the Lundbeck facility. Animals were housed in Macrolon (type II) cages with standard sawdust bedding
and environmental enrichment (plastic igloo and wooden chew blocks) in a 12-h light cycle starting at 0600 hours and a temperature of 21±2 °C; and humidity of 55±5%. Food (Altromin 1323
pills, Brogaarden, Denmark) and tap water were available _ad libitum_. Animals were allowed to acclimatize to the facility for at least 5 days before any test. Experimenters were blinded to
the genotype. Testing was conducted using male and female mice between 10 and 22 weeks of age unless otherwise stated. All the data shown is from males unless otherwise stated. Sample sizes
for each experiment can be seen in Table 1. Sample sizes were generally determined by how many homozygous mice could be bred (and survived). All studies were carried out in accordance with
Danish legislation, granted by the animal welfare committee, appointed by the Danish Ministry of Food, Agriculture and Fisheries—Danish Veterinary and Food Administration. GENOTYPING AND
CONFIRMATION OF GENE DELETION PCR was carried out as described in Fejgin _et al._9 Tissue was isolated from whole brain. Total RNA was purified using the NucleoSpin RNA and Protein kit
according to manufacturer’s instructions (Macherey-Nagel, Düren, Germany) followed by first strand complementary DNA synthesis using TaqMan reverse transcription reagents according to
manufacturer (Life Technologies, Paisley, UK). Primers designed and validated by Fejgin _et al._9 were used. Quantitative PCR was performed on a BioRad C1000 Touch thermal cycler with a
CFX384 optical reaction module using SsoFast EvaGreen Supermix according to manufacturer (BioRad, Hercules, CA, USA). Eight reference genes were included of which three were selected
(_Actb_, _Gapdh_ and _H3f3a_) for geometric normalization according to geNorm software version 3.5.12 Transcripts with Cq values >35 were considered below the detection limit and removed.
Genes close to the deleted region were included for detection of possible effects on bordering genes. The number of genes upstream and downstream of the deleted region was increased until
transcripts could be detected. IRWIN TEST Basic behavioral functions and neurological reflexes, such as vision, fear responses, muscle coordination and so on were tested by a simplified
version of the original screening described by Irwin.13 We measured: (1) undisturbed behavioral observation in a small observation cage (following 10-min habituation to the cage)—body
position, bizarre behavior (compulsive/self-bite, circling and head movements) and tremors. (2) Response to finger approach (withdrawal and explorative responses to a slow finger approach).
(3) Touch escape (response to the gentle pressure over the sides and back of the mouse). (4) Grip strength (resistance of the animal to pull when holding on a wire-mesh grid). (5) Visual
placing response (animal is lifted vertically by mid-tail about 15 cm above the wire-mesh grid and lowered slowly to elicit limb extension). (6) Corneal response (response to the slow
approach of a toothpick to the eye, on both sites). (7) Toe-pinch response (elicited by light compression of the lateral surface of the mid-digit of each foot with a forceps). (8)
Wire-maneuver (animal is brought to the horizontal wire, allowed to grasp it with its forelimbs, then released and observed). (9) Limb and abdominal tone (detected by palpation). (10)
Tail-pinch response (response to a moderate pressure applied with a forceps, ~2.5 cm below the base of the tail). GRIP STRENGTH Grip strength was measured using a grip strength meter and
peak amplifier (47105-001 Ugo Basile, Varese, Italy) with a metal wire. Mice were held by the tail and lowered toward the wire to allow grasping by the forelimbs. With the body perpendicular
to the wire, the mouse was pulled steadily backwards until the grip was released, and the maximal grip strength was recorded. OPEN FIELD Individual mice were placed in a circular open-field
arena (74 cm diameter) for 1 h with low illumination (14 lux). Movement was automatically video tracked and quantified using the Ethovision 7.0 software (Noldus Information Technologies,
Wageningen, The Netherlands). PTZ-INDUCED SEIZURE To assess seizure susceptibility in the Df(h15q13)−/− mice we scored 4 seizure behaviors after administration of the convulsant drug
pentylene tetrazole (PTZ); (1) early-stage seizures, (2) myoclonic jerks, (3) clonic seizures and (4) clonic–tonic seizures.9 After the injection of Pentylene tetrazol (PTZ) (Sigma-Aldrich,
Brøndby, Denmark; 40 mg kg−1, subcutaneously), each mouse was observed and video recorded for 30 min by an observer blinded to genotype. Video recordings were scored using the Observer
software (Noldus Information Technologies). Early-stage seizure was scored as the first four stages of the Racine scale: (1) mouth and facial movements; (2) head nodding; (3) forelimb
clonus; (4) rearing.14 BEHAVIOR QUANTIFICATION Animals were placed in separate cages and video recorded for 10 min. Grooming, digging, rearing, jumping/crawling in the corner of the cage and
crawling in the lid was manually scored and quantified off-line using the Observer software (Noldus Information Technologies). THREE-CHAMBERED SOCIAL APPROACH TASK The three-chambered
social approach task was carried out following the protocol presented by Yang _et al._15 In this test, the mice can move freely between three chambers; an empty chamber in the middle, a
chamber with an empty cylinder on one side and a chamber with a novel mouse in a cylinder in the other side. Briefly, the mice were first habituated to the center chamber for 10 min, second,
they were habituated to all three chambers for 10 min. Finally, an empty cylinder was put on one of the side chambers and a cylinder with a novel mouse was put on the other side chamber,
after which the test for social sniffing was carried out for 10 min. ULTRASONIC VOCALIZATION Ultrasonic vocalization was recorded as described by Scattoni _et al._16 Pregnant dams were
shipped from Taconic to the Lundbeck facility 2 weeks after conception. At postnatal day 6, the pups were put into a sound-isolated box one by one where vocalization was recorded for 3 min
with an ultrasound microphone (CM16/CMPA, Avisoft Bioacoustics, Glienicke, Germany) and preamplifier (116H, Avisoft Bioacoustics). After recording, the pup was marked and put back to the dam
and littermates. When all pups had been recorded, tail biopsies were obtained and genotyped by PCR. Ultrasonic calls were quantified using the Avisoft-SASLab Pro software (Avisoft
Bioacoustics). NEST BUILDING Mice were habituated for 2 weeks without nesting material in the home cages. On the day of testing, mice were divided into single cages with an igloo with three
openings in one corner and a single square of nesting material (Nestlets, Ancare, Belmont, NY, USA) in the opposite corner of the cage. Nest building was scored every hour during the first
12 h of testing and after 24 h, using the ‘Nesting Index Score’, (NIS), introduced by Pedersen _et al._17 NIS score has two components: (1) how much nest-building material is used (0–5
points); (2) how many openings of the igloo are covered (0–3). Maximal score is 8. PROGRESSIVE ACOUSTIC STARTLE Progressive acoustic startle was tested using the SM100 Startle Monitor System
(Kinder Scientific, Poway, CA, USA), consisting of eight sound-attenuated startle chambers and StartleMonitor software (Kinder Scientific). Animals were placed in an adjustable Plexiglas
holder, providing limited movement but not restraint, positioned directly above a sensing platform registering the animals startle response. Each test session consisted of a 5-min
acclimatization period with only background white noise (62 dB), followed by startle pulses at intensities varying from 95 to 120 dB, each presented 12 times in a balanced manner. Intertrial
intervals (ITIs) varied between 9 and 21 s (average ITI 15 s). The full acoustic startle test lasted ~25 min. PREPULSE INHIBITION Prepulse inhibition (PPI) was tested using the same
equipment as for the progressive acoustic startle test and as described by Fejgin _et al._9 Each test session consisted of a 5-min acclimatization period with only background white noise (62
dB), followed by a brief habituation setting where 32 regularly occurring startle pulses of 105 dB (ITI: 10 s) were presented to the animals to maximize habituation prior to the PPI part of
the session. Animals were then subjected to 5 types of trials presented 12 times each in a balanced manner: pulse alone; prepulse+pulse (5, 10 or 15 dB above background); or highest
prepulse intensity (77 dB) alone. ITI varied between 9 and 21 s (average ITI 15 s) and interstimulus interval was set to 100 ms with a prepulse length of 20 ms. Each PPI session ended with
eight startle pulses of 105 dB to estimate habituation across PPI trials. The full PPI test lasted about 28 min. PPI was calculated as % PPI for each prepulse intensity as:
100−((prepulse+pulse/pulse alone) × 100), that is, a lower percentage score indicates a decrease in PPI. Startle magnitude was calculated as an average of pulse alone trials. MORRIS WATER
MAZE The apparatus has been described earlier.18 One week prior to water maze testing the animals were habituated for 1 min (20 s in 1-cm water and 40 s of handling) for 5 consecutive days.
Animals were subjected to four trials per day for 6 consecutive days, with ITI of 30 min. Before the first trial on the first day, the animals were placed on the platform for 10 s. The first
trial started immediately thereafter. During any trial, if the mice did not find the platform they were guided to it and left there for 5 s before being lifted out of the maze. For each
trial, animals started from randomized positions and were allowed a maximum time of 60 s to find the hidden platform placed in the middle of the northern quadrant. On the seventh day, a 60-s
probe test was performed with the platform removed, followed by a visual test where the platform was marked with a visible flag. After each trial, mice were dried with paper towels and
allowed to heat under a heating lamp for 3–4 min before resting in their home cage. Time spent before finding the platform was analyzed using the Ethovision 3.0 software (Noldus). CONTEXTUAL
AND CUED FEAR CONDITIONING Training and contextual testing was conducted in a sound-proof chamber (30 × 20 × 40 cm) connected to a ventilation system in an isolated room. The floor of the
chamber consisted of a metal grid attached to an electric shock generator. Prior to training and testing, the chamber was cleaned with 70% ethanol. A video camera was used for recording of
sessions for subsequent off-line analysis. The test program (shock, sound and light) was run by FCONwin (Ellegaard Systems, Faaborg, Denmark). The training session lasted 6 min. During the
first and last minute of the training there was only white light. After 1 min and 40 s, a tone was presented for 20 s (80 db, 2000 Hz). During the last 2 s of the tone the mice received a
shock through the grid floor (0.6 mA). The context testing was performed 24 h later. The animals were placed in the same box as previously. The white light was on and the animal was video
recorded for 3 min. The cue test was performed 4 h after the context test. The animals were placed in a sound-proof chamber similar to the training and context chamber, but with a white
inner box instead of the metal one with grid floor. When an animal was inside the box, the red light was turned on. The animal was left in the box for 1 min before the tone was initiated.
The tone (80 dB, 2000 Hz) was on for 2 min after which the animal was left in the box for another minute. Freezing during training, context test and cue test was scored manually using the
Observer software (Noldus Information Technologies). DATA AND STATISTICAL ANALYSIS Statistical tests are indicated in the respective figure legends. All tests were carried out in Graphpad
Prism (La Jolla, CA, USA) and excel, and _P_<0.05 was considered statistically significant. The three genotypes were generally compared by one-way or two-way analysis of variances, if the
variance did not differ between the three genotype groups. If the variance did differ between the three groups, they were compared by the non-parametric Kruskal–Wallis test. If overall
comparison of genotype groups was significant multiple comparisons were computed. Data shown are from males, unless otherwise stated. RESULTS DECREASED SURVIVAL AND BODY WEIGHT OF
DF(H15Q13)−/− MICE Expression of deleted genes was examined in whole brain by quantitative PCR. All six protein-coding genes in the deleted region were absent in the Df(h15q13)−/− mice,
whereas all except Trpm1, which is not normally expressed in the brain, were expressed in WT littermates (data not shown). At birth, the fraction of pups that were homozygous for the
deletion, Df(h15q13), was 0.20; slightly, but significantly below the expected ratio of 0.25 (binomial test, _P_<0.05). Survival into adulthood (8 weeks of age) was also reduced, but
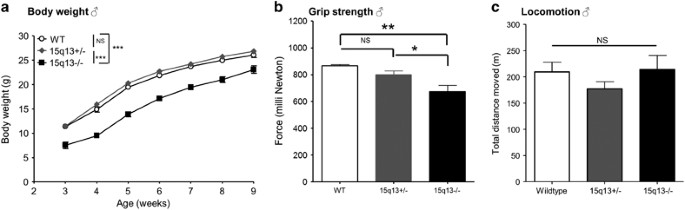
could be improved by later weaning and addition of nutritional supplements (Table 2). We observed decreased body weight persisting into adulthood (Figure 1a), and a small decrease in adult
brain weight of Df(h15q13)−/− mice (Supplementary Figure S1). DF(H15Q13)−/− MICE BASIC BEHAVIOR Homozygous Df(h15q13)−/− mice were compared with hemizygous Df(h15q13)+/− and WT littermates
in the Irwin test, which assesses basic behavior and physiological function.13 During undisturbed observation Df(h15q13)−/− mice had normal body posture and displayed normal behavior
(active, exploring, rearing, sniffing, climbing and grooming). However, some Df(h15q13)−/− mice displayed repetitive behavior (jumping or crawling in the corner of the cage). This
observation was followed up by a formalized test in which this repetitive behavior was quantified (see later section ‘Df(h15q13)-/- mice exhibit alterations in autism-related tests’). We
found that Df(h15q13)−/− mice had decreased grip strength and performed less well in the wire maneuver test, compared with Df(h15q13)+/− and WT littermates (data not shown). Subsequent
quantification of grip strength with a Newton meter confirmed that Df(h15q13)−/− mice had decreased grip strength (Figure 1b). Df(h15q13)−/− mice were normal in all other characteristics
assessed by the Irwin test; visual placing response, corneal response, limb and abdominal tone, touch escape, and responses to tail-pinch, toe-pinch and finger approach (data not shown). To
investigate motor activity and anxiety of the Df(h15q13)−/− mice, they were tested in the open-field assay. Df(h15q13)−/− mice moved the same distance as their WT littermates during the 1-h
trial (Figure 1c). Other parameters such as velocity, time spent in inner, middle or outer zone and so on, did not differ between genotypes either (data not shown). Several of the following
behavioral assays have been conducted in more than one batch of animals and in both male and female mice. Unless otherwise stated, the data shown in the primary figures are from the batch of
males with most animals, and the additional data sets are shown in the Supplementary Figures. Table 3 summarizes the behavioral phenotypes observed in the Df(h15q13)+/− mice. DF(H15Q13)−/−
MICE SHOW ABNORMAL SUSCEPTIBILITY TO PTZ-INDUCED SEIZURES To assess seizure susceptibility in the Df(h15q13)−/− mice we scored four seizure behaviors after pentylene tetrazole (PTZ)
administration; (1) early-stage seizures, (2) myoclonic jerks, (3) clonic seizures and (4) clonic–tonic seizures. EARLY-STAGE SEIZURES The Df(h15q13)−/− mice consistently had at least a
twofold increase in time spent in early-stage seizures than their WT littermates. There was also an increase in the time spent in early-stage seizures for the Df(h15q13)+/− mice compared
with WT littermates (_t_-test _P_<0.01), although this was not statistically significant when correcting for multiple testing (Figure 2a and Supplementary Figures S2A and D). To clarify
if the genotype difference in time in early-stage seizures was because the WT mice stopped having early-stage seizure behavior after progressing into clonic seizures, we compared time spent
in early-stage seizures before any of the animals had developed clonic seizures. During this period the Df(h15q13)−/− mice also spent more time in early-stage seizures (Supplementary Figure
S3). MYOCLONIC JERKS As previously reported, the Df(h15q13)+/− mice had significantly increased propensity for myoclonic jerks compared with WT littermates after PTZ administration.9
However, the Df(h15q13)−/− mice had few (Figure 2b and Supplementary Figures S2B and E). CLONIC SEIZURES We observed increased latency and decreased incidence of clonic seizures induced by
PTZ in Df(h15q13)+/− mice, similar to what have previously been reported.9 No Df(h15q13)−/− mice had clonic seizures after PTZ administration, indicating that this phenotype is also
gene-dosage dependent (Figure 2c and Supplementary Figures S2C and F). CLONIC–TONIC SEIZURES In both the batches of animals 0–33% of the WT mice with clonic seizures progressed to
clonic–tonic seizures, whereas we did not observe clonic–tonic seizures in any of the Df(h15q13)+/− or Df(h15q13)−/− mice (data not shown). There was no difference in PTZ blood levels
between genotypes (average=10651 ng ml−1, _P_=0.96, measured 1 h after dosing). We have not observed spontaneous convulsive seizures in home cages or during behavioral tests. DF(H15Q13)−/−
MICE EXHIBIT ALTERATIONS IN AUTISM-RELATED TESTS Stereotypic repetitive movements, in the form of jumping or crawling in the corner of the cage were observed in several homozygotes during
the Irwin test and in home cages, primarily in females. Therefore, this behavior was quantified, and Df(h15q13)−/− mice displayed more repetitive crawling or jumping in the corner of the
cage than WT and Df(h15q13)+/− littermates (Figure 3a). This was replicated in a separate batch of animals and while the phenotype appeared stronger in female mice a similar pattern was
observed in male mice (Supplementary Figure S4). We also quantified other behaviors such as grooming, rearing, digging and so on, but found no difference between the genotypes (data not
shown). In order to address social behavior in the Df(h15q13)−/− mice, we tested them in the three-chambered social approach task.15 All animals spent more time sniffing the cylinder with
the novel mouse compared with the empty cylinder. Df(h15q13)−/− mice spent 20% more of total sniffing time at the cylinder with the novel mouse than their WT littermates (Figure 3b). There
was also a change in the same direction for the Df(h15q13)+/− mice (_t_-test, _P_<0.05), although this was not statistically significant when accounting for multiple testing. There was no
difference in total sniffing time (time spent sniffing either of the two cylinders) between genotypes. We tested ultrasonic vocalization of Df(h15q13)−/− pups, and found that they had a
twofold decrease in number of vocalizations Df(h15q13)+/− and WT littermates (Figure 3c). There was also a trend towards a decrease in vocalization for the Df(h15q13)+/− mice, although this
was not statistically significant. DF(H15Q13)−/− MICE PERFORM LESS NEST BUILDING We scored nest building in Df(h15q13)−/−, Df(h15q13)+/− and WT female mice. In a gene-dosage dependent
manner, Df(h15q13) mice performed less nest building than their WT littermates (Figure 4). DF(H15Q13)−/− MICE SHOW ALTERATIONS IN PREATTENTIVE INFORMATION PROCESSING We assessed acoustic
startle response at varying intensities, and PPI of the acoustic startle response in Df(h15q13)−/− mice compared with Df(h15q13)+/− and WT littermates. In a gene-dosage-dependent manner
Df(h15q13), mice startled less in response to the acoustic stimulus than WT mice (Figure 5a). This pattern was seen in both males and females in two separate batches of animals
(Supplementary Figure S5). Df(h15q13)−/− mice displayed decrease in PPI, that is, impaired sensorimotor gating, compared to WT littermates (Figure 5b). This pattern was also seen in both
sexes in two separate batches of animals (Supplementary Figure S6). COGNITION IN DF(H15Q13)−/− MICE To assess spatial memory in the Df(h15q13)−/− mice, we tested them in the Morris water
maze and compared their performance with Df(h15q13)+/− and WT littermates. As observed previously, the Df(h15q13)+/− mice were as good as the WT mice at learning where the platform was9
(Figure 6a). In contrast, the Df(h15q13)−/− mice did not learn where the platform was (Figure 6a). In the subsequent visual trial, where a flag was placed on top of the platform, the
Df(h15q13)+/− mice and WT littermates reached the platform in about 10 s as seen previously,9 but the Df(h15q13)−/− mice did not find the platform (Figure 6b). It was not clear whether the
inability of the Df(h15q13)−/− mice to learn the location of the platform was due to cognitive and/or visual impairment. Therefore, we chose to perform a memory test that does not rely
strongly on vision—the auditory fear-conditioning test. Contextual fear memory did not differ significantly between the genotypes (Figure 6c). In contrast, auditory cued fear memory was
significantly decreased in Df(h15q13)−/− mice compared with WT littermates. There was a change in the same direction for the Df(h15q13)+/− mice, although this was not significant (Figure
6d). DISCUSSION We have shown that Df(h15q13)−/− mice display strong phenotypes related to epilepsy, autism and schizophrenia. Several of these phenotypes were also present in the
Df(h15q13)+/− mice, although weaker, indicating a gene-dosage dependency. Df(h15q13)−/− mice exhibited a strong but complex seizure threshold phenotype in response to the GABA(A) antagonist
PTZ with increased propensity for early-stage seizures and a decreased propensity for full clonic seizures. We also found similar, but weaker effects in mice with hemizygous deletion in
accordance with earlier work.9 In humans, there is also an apparent gene-dosage effect on seizure phenotypes, as humans with hemizygous 15q13.3 deletion have a highly increased risk of
epilepsy, while all 11 humans with homozygous deletion developed epilepsy in early childhood. In this context, it may seem unexpected that spontaneous clonic seizures were not observed in
the mice, which instead display this complex seizure threshold phenotype. However, human 15q13.3 deletion carriers mainly display absence seizures and some rat models of absence epilepsy are
protected from clonic seizures.7, 8, 19, 20 Furthermore, the PTZ-induced seizure assay tests the excitability of neurocircuitry involved in seizures, but does not directly model epilepsy.
We have not observed spontaneous convulsive seizures; however, it would be relevant in future studies to examine whether seizures develop in mice older than 6 months, and whether spontaneous
absence seizures develop as previous EEG studies in hemizygous mice, indicating a lowered threshold to absence seizures.9 It is striking that seizure-related alterations are prominent
phenotypes in both humans and mice with the deletion and that they show gene-dosage-dependent effects in both. Therefore, we think that these alterations are very likely to be related and
that further exploration of the seizure phenotypes in the mice might provide an understanding of the epilepsy phenotypes in humans. We also observed changes relevant to the three hallmarks
of autism; (1) stereotypic repetitive behavior, (2) change in social behavior, and (3) communication abnormalities.21 The repetitive jumping that we observed in Df(h15q13)−/− mice indicates
that these mice mimic the restricted and repetitive behaviors seen in patients suffering from autism. Similarly, the decrease in ultrasonic vocalization that we observed in Df(h15q13)−/−
pups indicates that the abnormal communication observed in patients suffering from autism is mirrored in the Df(h15q13)−/− mice.22 For both repetitive behavior and ultrasonic vocalization we
observed changes in the same direction in Df(h15q13)+/− mice that were not significant after correcting for multiple testing. A previous report of similar changes in another hemizygous
deletion model further supports that there is an effect in the hemizygotes and thus a gene-dosage dependent effect on these phenotypes.10 In contrast, the increase in social sniffing that we
observed in the three-chambered social approach assay for Df(h15q13)−/− mice, is unexpected given the impairment of social behavior characteristic for patients with autism.21 There was a
nonsignificant trend in the same direction in Df(h15q13)+/− mice, but this is not supported by previous work in another hemizygous 15q13.3 deletion mouse model.10 The three-chambered
social-approach assay quantifies how much time the mice use exploring a novel mouse, which can be interpreted as social interest, but it does not qualitatively assess social behavior.23
Thus, it indicates a social behavior abnormality of Df(h15q13)−/− mice, but further studies are needed to describe this in detail. The social phenotype of the Df(h15q13)−/− mice could be
further investigated in reciprocal interaction, where Kogan _et al._10 observed decreased social interaction for their hemizygous 15q13 mouse model. Nest building has been proposed as a
behavior with relevance to negative symptoms in schizophrenia.24 And we observed a gene-dosage-dependent nest-building impairment in the Df(h15q13)−/− and Df(h15q13)+/− mice.17 We also
detected a decrease in acoustic startle response and prepulse inhibition in Df(h15q13)−/− mice, whereas Df(h15q13)+/− only had decreased acoustic startle response in accordance with previous
characterization of the hemizygotes.9 Without direct tests of hearing, it cannot be ruled out that impaired hearing contribute to this phenotype in Df(h15q13)-/- mice. However, this finding
further support the Df(h15q13)−/− mice as a model for aspects of schizophrenia, as impairments in PPI have been reported in several psychiatric disorders and particularly in
schizophrenia.25 Cognitive disabilities are another relevant group of phenotypes in relation to both schizophrenia and autism. As a measure of cognitive function we tested spatial learning
and memory in the Morris water maze test, where Df(h15q13)+/− mice were previously shown to have a deficit in long-term spatial memory.9 The Df(h15q13)−/− mice were unable to learn the
location of the hidden platform, and were unable to find the platform in the visual test where a flag is placed on top of the platform. Possible reasons for this might be; (1) cognitive
impairments that prevent the mice from using the visual cues to guide them to the platform, (2) visual impairments that prevent the mice from seeing the visual cues and the flag, (3)
repetitive movements or absence seizures and (4) a combination of these. In the present study, we observed normal visual placing response and corneal response in the Df(h15q13)−/− mice, so
the mice are not blind. However, the _Trpm1_ gene in the 15q13.3 region has been linked to deficits in visual light response,26, 27 and mild visual impairment might prevent the Df(h15q13)−/−
mice from detecting the visual cues in the Morris water maze test. Further assessment of visual function in the Df(h15q13)−/− mice is needed to elucidate this. Owing to the difficult
interpretation of the Morris water maze data, we performed a cognitive test that did not rely strongly on visual function—the fear-conditioning test. In the cue test the Df(h15q13)−/− mice
displayed a deficit in auditory cued fear memory (Figure 6d), indicating that memory is indeed impaired in Df(h15q13)−/− mice, consistent with the high penetrance of intellectual disability
in the 15q13.3 microdeletion syndrome.2 For most of the observed behavioral Df(h15q13)−/− phenotypes there was a similar but less pronounced change in the Df(h15q13)+/− mice (Table 2). Clear
examples of such gene-dosage-dependent phenotypes were the reduced incidence of PTZ-induced clonic seizures, impaired nest building and reduced acoustic startle response. A few phenotypes
such as PPI deficits were only present in the Df(h15q13)−/− mice. Such phenotypes may be irrelevant to the hemizygous deletion, but considering the variable phenotype in the human hemizygous
microdeletion syndrome, some may be dependent on interaction with environmental or additional genetic factors. Thus, it could be interesting to test whether hemizygous Df(h15q13)+/− mice
had, for example, PPI phenotypes if subjected to environmental challenge or bred in a different genetic background. It is unknown which gene(s) in the 15q13.3 region that drives the
phenotypes seen in humans and mice affected by the hemizygous and homozygous microdeletions. CHRNA7 has been the most popular candidate, as several cases of deletions encompassing only
CHRNA7 have been described in patients with neuropsychiatric disorders.3, 28, 29, 30 The characterization of Df(h15q13)−/− mice allows comparison with mice with single homozygous gene
deletions. Single gene knockout mice have been described for three of the genes in the 15q13.3 region, _KLF13_, _TRPM1_ and _CHRNA7_. Klf13−/− mice are reported as normal overall and do not
have reduced weight like the Df(h15q13)−/− mice.31, 32 Klf13−/− mice characterization has focused on blood composition and no behavioral tests of the Klf13−/− mice have been described.
Trpm1−/− mice display reduced visual sensitivity to light.26, 27 As Trpm1 is mainly expressed in the retina and essentially not in the brain, it is unlikely to be a major driver of the
behavioral and physiological phenotypes of the Df(h15q13)−/− mice. However, it might contribute to the impaired water-maze performance through effects on vision. Chrna7−/− mice have been
thoroughly studied, but have very subtle phenotypes.33, 34, 35 In contrast to Df(h15q13)−/− mice, Chrna7−/− mice develop normally, have unaltered seizure threshold, display normal acoustic
startle response and normal prepulse inhibition, do not display deficits in memory assessed by the Morris water maze and auditory fear-conditioning tests.35 Consequently, it seems unlikely
that Chrna7 alone accounts for the phenotypes that we observed in the Df(h15q13)+/− and Df(h15q13)−/− mice. Thus, one or more of the other genes in the region must be important for the
phenotypes in Df(h15q13)−/− mice and by inference in the human 15q13.3 deletion. In conclusion, the behavioral phenotypes of the Df(h15q13)−/− mice reported here demonstrate that
Df(h15q13)−/− mice are relevant as a model of both the hemizygous and the rare homozygous 15q13.3 microdeletion syndromes. The phenotypes related to epilepsy, autism and schizophrenia that
we observed in the Df(h15q13)−/− mice support that they can be used to explore how 15q13.3 deletion can lead to these disorders. Importantly, the Df(h15q13)−/− mice might also be useful for
studying the biological overlap between the disorders. Mechanistic exploration is much simpler when phenotypes are strong, and the many gene-dosage-dependent phenotypes indicate that the
Df(h15q13)−/− model may be used to complement the Df(h15q13)+/− model to explore the underlying biology of 15q13.3-associated disorders. Currently, the treatment options for epilepsy, autism
and schizophrenia are not optimal and drug development efforts have been hampered by the lack of biological understanding of these disorders. If the Df(h15q13)−/− mice can contribute to a
better mechanistic understanding of biological changes underlying epilepsy, autism and schizophrenia they might ultimately facilitate drug discovery efforts in relation to these disorders.
REFERENCES * Malhotra D, Sebat J . CNVs: harbingers of a rare variant revolution in psychiatric genetics. _Cell_ 2012; 148: 1223–1241. Article CAS Google Scholar * Lowther C, Costain G,
Stavropoulos DJ, Melvin R, Silversides CK, Andrade DM _et al_. Delineating the 15q13.3 microdeletion phenotype: a case series and comprehensive review of the literature. _Genet Med_ 2014;
17: 149–157. Article Google Scholar * Ben-Shachar S, Lanpher B, German JR, Qasaymeh M, Potocki L, Nagamani SCS _et al_. Microdeletion 15q13.3: a locus with incomplete penetrance for
autism, mental retardation, and psychiatric disorders. _J Med Genet_ 2009; 46: 382–388. Article CAS Google Scholar * Helbig I, Mefford HC, Sharp AJ, Guipponi M, Fichera M, Franke A _et
al_. 15Q13.3 microdeletions increase risk of idiopathic generalized epilepsy. _Nat Genet_ 2009; 41: 160–162. Article CAS Google Scholar * Sharp A, Mefford H, Li K, Baker C, Skinner C,
Stevenson R _et al_. A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. _Nat Genet_ 2008; 40: 322–328. Article CAS Google Scholar * Rees E,
Walters JTR, Georgieva L, Isles AR, Chambert KD, Richards AL _et al_. Analysis of copy number variations at 15 schizophrenia-associated loci. _Br J Psychiatry_ 2014; 204: 108–114. Article
Google Scholar * Muhle H, Mefford HC, Obermeier T, von Spiczak S, Eichler EE, Stephani U _et al_. Absence seizures with intellectual disability as a phenotype of the 15q13.3 microdeletion
syndrome. _Epilepsia_ 2011; 52: e194–e198. Article Google Scholar * Coppola A, Bagnasco I, Traverso M, Brusco A, Di Gregorio E, Del Gaudio L _et al_. Different electroclinical picture of
generalized epilepsy in two families with 15q13.3 microdeletion. _Epilepsia_ 2013; 54: 69–73. Article Google Scholar * Fejgin K, Nielsen J, Birknow MR, Bastlund JF, Nielsen V, Lauridsen JB
_et al_. A Mouse Model that Recapitulates Cardinal Features of the 15q13.3 Microdeletion Syndrome Including Schizophrenia- and Epilepsy-Related Alterations. _Biol Psychiatry_ 2013; 76:
128–137. Article Google Scholar * Kogan JH, Gross AK, Featherstone RE, Shin R, Chen Q, Heusner CL _et al_. Mouse model of chromosome 15q13.3 microdeletion syndrome demonstrates features
related to autism spectrum disorder. _J Neurosci_ 2015; 35: 16282–16294. Article CAS Google Scholar * Masurel-Paulet A, Drumare I, Holder M, Cuisset J-M, Vallée L, Defoort S _et al_.
Further delineation of eye manifestations in homozygous 15q13.3 microdeletions including TRPM1: a differential diagnosis of ceroid lipofuscinosis. _Am J Med Genet A_ 2014; 164A: 1537–1544.
Article Google Scholar * Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A _et al_. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging
of multiple internal control genes. _Genome Biol_ 2002; 3: RESEARCH0034. Article Google Scholar * Irwin S . Comprehensive observational assessment: ia. a systematic, quantitative procedure
for assessing the behavioral and physiologic state of the mouse. _Pharmacologia_ 1968; 13: 222–257. CAS Google Scholar * Racine RJ . Modification of seizure activity by electrical
stimulation. II. Motor seizure. _Electroencephalogr Clin Neurophysiol_ 1972; 32: 281–294. Article CAS Google Scholar * Yang M, Silverman JL, Crawley JN . Automated three-chambered social
approach task for mice. _Curr Protoc Neurosci_ 2011 Chapter 8: Unit 8.26. * Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L _et al_. Reduced ultrasonic
vocalizations in vasopressin 1b knockout mice. _Behav Brain Res_ 2008; 187: 371–378. Article CAS Google Scholar * Pedersen CS, Sørensen DB, Parachikova AI, Plath N . PCP-induced deficits
in murine nest building activity: Employment of an ethological rodent behavior to mimic negative-like symptoms of schizophrenia. _Behav Brain Res_ 2014; 273: 63–72. Article CAS Google
Scholar * Podhorna J, Didriksen M . Performance of male C57BL/6 J mice and Wistar rats in the water maze following various schedules of phencyclidine treatment. _Behav Pharmacol_ 2005; 16:
25–34. Article CAS Google Scholar * Aker RG, Yananli HR, Gurbanova AA, Özkaynakçi AE, Ateş N, Van Luijtelaar G _et al_. Amygdala kindling in the WAG/Rij rat model of absence epilepsy.
_Epilepsia_ 2006; 47: 33–40. Article Google Scholar * Carcak N, Zheng T, Ali I, Abdullah A, French C, Powell KL _et al_. The effect of amygdala kindling on neuronal firing patterns in the
lateral thalamus in the GAERS model of absence epilepsy. _Epilepsia_ 2014; 55: 654–665. Article Google Scholar * King BH, Navot N, Bernier R, Webb SJ . Update on diagnostic classification
in autism. _Curr Opin Psychiatry_ 2014; 27: 105–109. Article Google Scholar * Takahashi T, Okabe S, Broin PÓ, Nishi A, Ye K, Beckert MV _et al_. Structure and function of neonatal social
communication in a genetic mouse model of autism. _Mol Psychiatry_ 2015 15 December 2015; doi: 10.1038/mp.2015.190 (e-pub ahead of print). * Shah CR, Forsberg CG, Kang J-Q,
Veenstra-VanderWeele J . Letting a typical mouse judge whether mouse social interactions are typical. _Autism Res_ 2013; 29: 212–220. Article Google Scholar * Powell CM, Miyakawa T .
Uniquely human disorder? _Biol Psychiatry_ 2014; 59: 1198–1207. Article Google Scholar * Takahashi H, Hashimoto R, Iwase M, Ishii R, Kamio Y, Takeda M . Prepulse inhibition of startle
response: Recent advances in human studies of psychiatric disease. _Clin Psychopharmacol Neurosci_ 2011; 9: 102–110. Article CAS Google Scholar * Hughes S, Pothecary Ca, Jagannath A,
Foster RG, Hankins MW, Peirson SN . Profound defects in pupillary responses to light in TRPM-channel null mice: a role for TRPM channels in non-image-forming photoreception. _Eur J Neurosci_
2012; 35: 34–43. Article Google Scholar * Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM _et al_. TRPM1 is required for the depolarizing light response in retinal
ON-bipolar cells. _Proc Natl Acad Sci USA_ 2009; 106: 19174–19178. Article CAS Google Scholar * Shinawi M, Schaaf CP, Bhatt SS, Xia Z, Patel A, Cheung SW _et al_. A small recurrent
deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. _Nat Genet_ 2009; 41: 1269–1271. Article CAS Google Scholar * Hoppman-Chaney N, Wain K, Seger P,
Superneau D, Hodge J . Identification of single gene deletions at 15q13.3: further evidence that CHRNA7 causes the 15q13.3 microdeletion syndrome phenotype. _Clin Genet_ 2013; 83: 345–351.
Article CAS Google Scholar * Gillentine Ma, Schaaf CP . The human clinical phenotypes of altered CHRNA7 copy number. _Biochem Pharmacol_ 2015; 97: 352–362. Article CAS Google Scholar *
Zhou M, McPherson L, Feng D, Song a, Dong C, Lyu S-C _et al_. Kruppel-like transcription factor 13 regulates T lymphocyte survival _in vivo_. _J Immunol_ 2007; 178: 5496–5504. Article CAS
Google Scholar * Gordon AR, Outram SV, Keramatipour M, Goddard Ca, Colledge WH, Metcalfe JC _et al_. Splenomegaly and modified erythropoiesis in KLF13-/- mice. _J Biol Chem_ 2008; 283:
11897–11904. Article CAS Google Scholar * Orr-Urtreger a, Göldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M _et al_. Mice deficient in the alpha7 neuronal nicotinic acetylcholine
receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. _J Neurosci_ 1997; 17: 9165–9171. Article CAS Google Scholar * Franceschini D, Paylor R, Broide R,
Salas R, Bassetto L, Gotti C _et al_. Absence of alpha7-containing neuronal nicotinic acetylcholine receptors does not prevent nicotine-induced seizures. _Brain Res Mol Brain Res_ 2002; 98:
29–40. Article CAS Google Scholar * Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A . α7 nicotinic receptor subunits are not necessary for hippocampal-dependent
learning or sensorimotor gating: a behavioral characterization of acra7-deficient mice. _Learn Mem_ 1998; 5: 302–316. CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS We thank Annette Bjørn and Dorte Clausen for skillful technical assistance, and Lars Arvastson for statistical guidance. This work was supported by an industrial PhD grant
to from the Ministry of Higher Education and Science, Denmark (AF). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Synaptic Transmission, In Vitro, Neuroscience Research DK, H. Lundbeck A/S,
Valby, Denmark A Forsingdal, K Fejgin, V Nielsen & J Nielsen * Institute of Biological Psychiatry, Mental Health Center, Sct. Hans, Mental Health Services, Roskilde, Denmark A Forsingdal
& T Werge * Institute of Clinical Sciences, Faculty of Medicine and Health Sciences, University of Copenhagen, Copenhagen, Denmark A Forsingdal & T Werge * iPSYCH, The Lundbeck
Foundation’s Initiative for Integrative Psychiatric Research, Denmark T Werge Authors * A Forsingdal View author publications You can also search for this author inPubMed Google Scholar * K
Fejgin View author publications You can also search for this author inPubMed Google Scholar * V Nielsen View author publications You can also search for this author inPubMed Google Scholar *
T Werge View author publications You can also search for this author inPubMed Google Scholar * J Nielsen View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to J Nielsen. ETHICS DECLARATIONS COMPETING INTERESTS AF, KF, VN and JN are employed by H. Lundbeck A/S. The remaining author declares no conflict of
interest. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the _Translational Psychiatry_ website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES (DOC 33 KB)
SUPPLEMENTARY FIGURE 1 (JPG 500 KB) SUPPLEMENTARY FIGURE 2 (JPG 1068 KB) SUPPLEMENTARY FIGURE 3 (JPG 614 KB) SUPPLEMENTARY FIGURE 4 (JPG 630 KB) SUPPLEMENTARY FIGURE 5 (JPG 741 KB)
SUPPLEMENTARY FIGURE 6 (JPG 839 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users
will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Forsingdal, A., Fejgin, K., Nielsen, V. _et al._ 15q13.3 homozygous knockout mouse model display epilepsy-, autism- and schizophrenia-related phenotypes.
_Transl Psychiatry_ 6, e860 (2016). https://doi.org/10.1038/tp.2016.125 Download citation * Received: 18 February 2016 * Revised: 18 May 2016 * Accepted: 01 June 2016 * Published: 26 July
2016 * Issue Date: July 2016 * DOI: https://doi.org/10.1038/tp.2016.125 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative