Play all audios:
ABSTRACT Previous studies have indicated that schizophrenia is linked to abnormal lipid metabolism. Free fatty acids (FFAs) in peripheral blood can reflect the status of lipid metabolism in
human body. The purpose of this study was to scan the FFA pattern and elucidate the characteristics of lipid metabolic abnormality in schizophrenia patients. One hundred and ten patients
with schizophrenia (SCZs) and 109 healthy controls (HCs) were included in the study and divided into a discovery set and a validation set. Forty-seven serum FFAs were detected by
UPLC-QTOF-MS and 39 of them were absolutely quantified by establishing standard curves. Monounsaturated fatty acids (MUFAs) and ω-6 polyunsaturated fatty acids (ω-6 PUFAs) were significantly
increased in SCZs compared with HCs. Desaturation from saturated fatty acids to MUFAs and β-oxidation were enhanced, as estimated by the ratios of products to precursors. These results
suggest that lipolysis and β-oxidation are upregulated in SCZ, presumably resulting from insufficient brain energy supply. SIMILAR CONTENT BEING VIEWED BY OTHERS PLASMA LIPID METABOLITES AS
POTENTIAL BIOMARKERS FOR IDENTIFYING INDIVIDUALS AT RISK OF OBESITY-INDUCED METABOLIC COMPLICATIONS Article Open access 20 July 2023 TARGETED METABOLOMICS ANALYSIS OF AMINO ACIDS AND
ACYLCARNITINES AS RISK MARKERS FOR DIABETES BY LC–MS/MS TECHNIQUE Article Open access 19 May 2022 IDENTIFICATION OF METABOLIC MARKERS PREDICTIVE OF PREDIABETES IN A KOREAN POPULATION Article
Open access 15 December 2020 INTRODUCTION Schizophrenia is a psychiatric disease associated with delusions, hallucinations, thought disorders and cognitive deficits.1 Schizophrenia affects
approximately 0.5 to 1.0% of the population worldwide and is disastrous for affected individuals and their families. Schizophrenia is also a serious burden on the social healthcare system.1,
2 Unfortunately, our current understanding of schizophrenia remains limited. Accumulating evidence indicates that schizophrenia is linked to abnormal lipid metabolism and related pathways
in both the central and peripheral nervous systems. Prabakaran demonstrated that the lipid biosynthetic pathway was down-regulated in the brains of schizophrenia patients, whereas several
fatty acid beta-oxidation enzymes were significantly increased.3 Free fatty acids (FFAs) and phosphatidylcholines were significantly changed in the prefrontal cortex (both gray matter and
white matter) of schizophrenia patients.4 In 2013, another study showed a number of statistically significant changes in prefrontal cortex lipid concentrations, including changes in total
lipids, phospholipids, triglycerides and cholesteryl esters, in schizophrenia patients compared with these concentrations in controls.5 This metabolic dysfunction is also reflected in the
peripheral blood. Higher levels of serum triglycerides and lower levels of serum HDL have been detected in schizophrenia patients,6 and associations between serum triglycerides and positive
psychotic symptoms and between polyunsaturated membrane fatty acids (PUFAs) in RBCs and negative symptoms have been demonstrated.7 We previously noted that fatty acids and ketone bodies were
elevated in the serum or urine of schizophrenia patients, suggesting upregulated fatty acid catabolism.8 Compared with brain tissue, peripheral blood samples are more accessible and can be
controlled to collect a medication-free group of samples that better reflects the disease state without the interference of antipsychotic drugs. Fasting plasma FFAs have been reported to be
closely related to adipose tissue fatty acids,9 which might reflect the energy supply. A comprehensive scan of FFAs might facilitate the elucidation of the characteristic lipid metabonomics
abnormality of schizophrenia and may contribute to a better understanding of this disease. In many previous studies, the reported fatty acid profile was incomplete or lacked absolute
quantification data. To better investigate the lipid metabolism dysfunction in schizophrenia patients, we enrolled 110 schizophrenia patients and 109 normal healthy subjects as controls,
utilizing a UPLC-QTOF-MS platform to achieve targeted FFA quantitative analysis. MATERIALS AND METHODS SUBJECTS Our sample set was consistent with our previous research.8 We recruited 110
patients from the Anhui Province, China, diagnosed as schizophrenic (SCZ) according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Of the 110
patients, 63 were experiencing first-onset psychosis, and 47 were hospitalized for relapse after at least 1 month without any antipsychotic drugs. A total of 109 healthy controls (HC) were
recruited in the same district and were matched for age, body mass index (BMI) and gender. In both the patient and control groups, we excluded participants with metabolic disorders (such as
type I or type II diabetes) and/or with heavy consumption of alcohol. The enrolled subjects were separated into a discovery set (60 SCZ & 61 HC) and a validation set (50 SCZ & 48 HC;
Supplementary Table 1). Written informed consent was obtained from all participants. All samples were collected following the guidelines of the local ethics committee. The overall sampling
was finished within 1 year. SERUM SAMPLE COLLECTION Venous blood was collected in polypropylene tubes in the early morning after overnight fasting. The fresh blood samples were stored at
room temperature and clotted naturally after approximately 2 h. Serum was then obtained after centrifugation at 10 000 r.p.m. for 10 min and immediately stored at −80 °C. REAGENTS AND
INSTRUMENTATION Reference standards of FFAs (purity>90%) and deuterated internal standards (IS) were acquired from Sigma-Aldrich and NU-CHEK. HPLC-grade methanol, _n_-hexane,
acetonitrile, and water were obtained from Merck. A Waters ACQUITY ultraperformance LC system equipped with a binary solvent delivery manager and a sample manager (Waters, Milford, MA, USA)
was used throughout the study. The mass spectrometer was a Waters XEVO TQ-S instrument with an ESI source (Waters, Milford, MA, USA). The entire LC−MS system was controlled by MassLynx 4.1
software. All chromatographic separations were performed with an ACQUITY BEH C18 column (1.7 μm, 100 mm × 2.1 mm internal dimensions; Waters, Milford, MA, USA). SERUM SPECIMEN PREPARATION A
30-μl aliquot of serum sample was extracted for UPLC-QTOF-MS analysis. Each sample was spiked with the internal standard (10 μl of C19:0-d37) and added to 500 μl of a mixture of
isopropanol/_n_-hexane/2 M phosphoric acid (40:10:1, v/v/v). The resulting samples were vortexed for 2 min and incubated at room temperature for 20 min. After the incubation, 400 μl of
_n_-hexane and 300 μl of water were added, and the mixtures were vortexed for 2 min and centrifuged at 12 000 r.p.m., 4 °C for 5 min. An aliquot of 400 μl of supernatant was collected and
transferred into a clean tube. The remaining mixture was further extracted with additional 400 μl of _n_-hexane by vortexing and centrifuging at 12 000 r.p.m., 4 °C for 5 min, after which
the second 400 μl of supernatant was collected. The two supernatants were pooled together and vacuum-dried at room temperature. The residue was re-dissolved in 80 μl of methanol.10, 11 After
centrifugation, the supernatant was used for UPLC−QTOF-MS analysis (Waters, Manchester, UK). UPLC-QTOF-MS SPECTRAL ACQUISITION A 5- μl aliquot of the sample was injected into an ACQUITY BEH
C18 column with the column temperature set at 40 °C. The elution solvents were water (A) and acetonitrile/isopropyl (v/v=80/20, B) with a flow rate of 400 μl/min. The elution gradient was
as follows: 0−2 min (70% B), 2−5 min (75% B), 5−10 min (80% B), 13−16 min (90% B), and 16−24 min (99% B). Samples from healthy controls and schizophrenic individuals were alternately
injected. In addition to the internal standard, a blank vial and a mixture of all the samples were prepared and run after every 10 serum samples for quality control. The mass spectrometer
was operated in negative ion mode with the following optimal conditions: capillary voltage 2.5 kV, cone voltage 55 V, and extractor voltage 4 V. The desolvation and cone gas flow rates were
650 and 50 l h−1, respectively. The source temperature was 120 °C, and the temperature for the desolvation gas was set at 450 °C. MassLynx software (Waters, Manchester, UK) was used to
collect the data with a mass range of 50 to 1000 Da. The scan time was set to 0.35 s, and the interscan delay was set to 0.02 s. Leucine (encephalin) was used as the lock mass
(_m/z_=554.2615). DATA PROCESSING The UPLC-QTOF-MS raw data were analyzed by the MarkerLynx applications (manager version 4.1). A list of the ion intensities of each detected peak was
generated using the RT and _m/z_ data pairs as the identifier for each ion. The resulting three-dimensional matrix contained arbitrarily assigned peak indices (retention time–_m/z_ pairs),
sample names (observations), and ion intensity information (variables). The missing values were imputed with 50. The internal standard and QC were used for data quality control
(reproducibility), and the QC was used for data normalization. The ion peaks generated by the internal standard were removed. To obtain more information, we conducted the statistical
analysis based on the relative quantitative data and also presented the absolute quantitative values for reference. STATISTICAL ANALYSIS All data analyses were performed using SIMCA-P 11.5
(Umetrics, Umea, Sweden) and R 3.2.1 software (Stanford University, Stanford, CA, USA). The well-matched discovery set and the validation set both followed the procedure for basic analysis.
The Shapiro-Wilk normality test was performed first to evaluate the normality of our data. Then, the Mann−Whitney _U_-test was chosen to investigate differences between the SCZ and HC in FFA
measurements. The resultant _P_-values for all FFAs were subsequently adjusted to account for multiple testing by the Benjamin–Hochberg method. We regarded _P_-values of <0.05 as
significant. For multivariable analysis, PLS-DA was conducted, and we obtained the VIP (variable importance for the projection) values. According to the fatty acid metabolism pathways, we
analyzed 18 pairs of interconverted FFAs. A Mann−Whitney _U_-test was used to evaluate the differences in the product/substrate ratios between SCZ and HC. _P_-values were also adjusted by
the Benjamin–Hochberg method. Geometric averages were used in the progress. Pearson correlation analysis was performed to evaluate the interactions between the FFAs and age, gender and BMI,
as well as the correlations between mutual transformed FFAs. RESULTS DEMOGRAPHIC CHARACTERISTICS The study included 110 patients with schizophrenia and 109 healthy controls, consistent with
our previous work.8 We selected 60 patients and 61 healthy controls matched by age, gender and BMI before statistical analysis as the discovery set, and the remaining subjects composed the
validation set (Supplementary Table 1). UNIVARIATE ANALYSIS OF 47 FREE FATTY ACIDS Forty-seven FFAs were detected by UPLC-QTOF-MS, as shown in Table 1, and were classified into 8 types:
saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), ω-6 polyunsaturated fatty acids (ω-6 PUFAs), ω-3 polyunsaturated fatty acids (ω-3 PUFAs), trans-fatty acids (TFAs),
branched-chain fatty acids (BCFAs), odd-carbon fatty acids (OCFAs) and others. The first four types participate in the metabolism of fatty acids in the human body. We refer to these FFAs as
the FFAs of most concern, and a total of 26 FFAs belonging to the first four types were detected. All detected FFAs deviated from normality according to the Shapiro-Wilk test, and we
therefore applied the Mann-Whitney _U_-test to compare the 47 FFAs between SCZ and HC. Sixteen FFAs differed significantly (adjusted _P_-values <0.05; Table 1) between SCZ and HC in the
discovery set. Fifteen FFAs were significantly increased in SCZ, with changes ranging from 1.34- to 3.7-fold, and only one (C24:0) was decreased, with a change of 1.22-fold. All differential
FFAs were verified in the validation set (Supplementary table 2). As noted previously, the 26 FFAs of most concern included the following four groups according to saturation: 8 SFAs, 7
MUFAs, 7 ω-6 PUFAs and 4 ω-3 PUFAs. Among the 8 SFAs, one was significantly increased (C16:0, FC=1.49), and one was significantly decreased (C24:0, FC=-1.22). All 7 MUFAs were elevated in
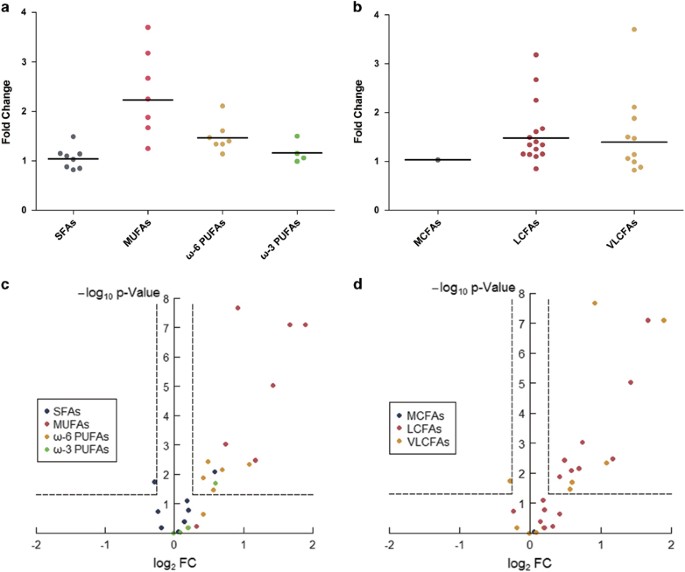
SCZ (FC>1.2), 6 significantly. C22:1(_cis_.13), C20:1(_cis_.11), C16:1(_cis_.9) and C14:1(_cis_.9) had the top four fold changes (FCs) of 3.7, 3.18, 2.67 and 2.25, respectively. The
geometric mean of the FCs of the 7 MUFAs of most concern was 2.234 (Figure 1a). Five of the 7 ω-6 PUFAs were significantly increased in SCZ compared with their levels in HC, with a geometric
mean of the FCs of 1.46. By contrast, only one of the four ω-3 PUFAs (C22:5) showed a significant increase, with a geometric mean of the FC of 1.16. With respect to carbon (C) chain-length,
the 26 FFAs of most concern were classified into the following three groups: medium-chain fatty acids (MCFAs), long-chain fatty acids (LCFAs) and very long-chain FAs (VLCFAs). The only MCFA
of most concern, octanoic acid, did not differ between SCZ and HC. Fifteen LCFAs of most concern were detected, and 8 were significantly increased in SCZ, with FCs ranging from 1.34 to
3.18. C20:1(_cis_.11), C16:1(_cis_.9), and C14:1(_cis_.9) had FC values of 3.18, 2.67 and 2.25, respectively. The geometric mean of the FCs of the 15 LCFAs of most concern was 1.475 (Figure
1b). Of the 10 VLCFAs of most concern detected in our study, 5 were significantly increased in SCZ, with FCs ranging from 1.47 to 3.7. C22:1(_cis_.13) and C22:2(_cis_.13, 16) had FC values
of 3.7 and 2.11, respectively. C24:0 was the only one that decreased significantly in SCZ (Table 1). The geometric mean of the FCs of the 10 VLCFAs of most concern was 1.395 (Figure 1b). In
addition to the 26 FFAs of most concern, twelve odd-carbon FAs were detected in the study. C17:1(_cis_.10) and C19:1(_cis_.10) were increased significantly in SCZ. The elevation of the
contents of these FAs was verified in the validation set (Supplementary Table 2). MULTIVARIATE ANALYSIS OF 47 FREE FATTY ACIDS The PLS-DA score plot showed a good separation between SCZ and
HC corresponding to the first 3 PLS-DA components (Supplementary Figure 1). The explained variation in _X_ (_R_2_X_) was 60.1%, while the explained variation in _Y_ (_R_2_Y_) was 61.5%. A
permutation test with a permutation number of 200 was also performed to test the overfitting status of the model (Supplementary Figure 2), which indicated the success of the model for
differentiating SCZ from HC. The VIP scores of the 47 FFAs are listed in Table 1. The top five VIP scores were C24:1(_cis_.15), C20:4(_cis_.5.8.11.14), C22:1(_cis_.13), C20:1(_cis_.11), and
C16:1(_cis_.9), which all belong to the unsaturated FFA group. FATTY ACID METABOLIC PATHWAY ANALYSIS BASED ON THE PRODUCT/PRECURSOR RATIO To further explore the changes in FFAs in SCZ
patients, we investigated the ratios of product to precursor that were transformed unidirectionally or bidirectionally. There were 18 pairs of transformations among the 47 FFAs we detected
(Figure 2). The ratios of product to precursor in HC and SCZ were calculated. Analysis using the Mann-Whitney test followed by Benjamin–Hochberg adjustment indicated that the ratios of 14
pairs of FFAs differed significantly between the two groups (Figure 2 and Supplementary Table 3). These results demonstrate that the regulation of FFA equilibrium differs between SCZ and HC.
Δ9-Desaturase catalyzes the one-way reaction from SFAs to MUFAs, including C14:0 to C14:1, C16:0 to C16:1 and C18:0 to C18:1, and these reactions were enhanced in SCZ according to our data
(Supplementary Table 3). As an example, the correlation coefficient for C18:0 and C18:1 was 0.32 in HC and 0.69 in SCZ. The geometric average of the ratio of C18:1/C18:0 was 0.72 in HC and
increased significantly to 0.99 in SCZ. Similar phenomena were observed for C14:0/C14:1 and C16:0/C16:1. These results are consistent with the general increase in the MUFAs we mentioned
previously. Therefore, our data support an enhanced tendency of desaturation from SFAs to MUFAs as one of the characteristics of serum FFAs in schizophrenia patients. With respect to carbon
number, C16:0 and C22:1 (_cis_.13) accumulated among SFAs and MUFAs, respectively. Elongation from C12:0 to C14:0 and from C14:0 to C16:0 was enhanced. In addition, β-oxidation from C24:0 to
C22:0, from C22:0 to C20:0 and from C18:0 to C16:0 was increased in SCZ. Both of these phenomena were reflected in the trend to produce more C16:0. Among MUFAs, the ratios of product to
precursor indicated a tendency to accumulate C22:1 (_cis_.13) (Figure 2 and Supplementary Table 3). ABSOLUTE QUANTITATION OF 39 FFAS IN SERUM FROM SCHIZOPHRENIA PATIENTS AND HEALTHY CONTROLS
We performed absolute quantitation of 39 FFAs using standard curves according to a method described previously.11, 12 The concentrations of the 39 FFAs are presented in μg/ml (Supplementary
Table 4). The most abundant classes of FFAs in SCZ were MUFAs, SFAs and ω-6 PUFAs, accounting for 98.6% of all quantitated FFAs (Supplementary figure 3). In HC, these three classes were
also the most abundant, accounting for 97.7% of the total FFAs, whereas SFAs were greater than MUFAs and came first. With respect to individual fatty acids, the most highly concentrated FFAs
were C18:1, C18:2(ω-6) and C16:0. The pattern of FFAs in human bodies presented in our research is consistent with the work of other groups but depends on the technology platform.13 We
computed the coefficients of variation (CVs) of the concentration data. Ninety-seven percent of FFAs (38/39) in SCZ and 95% (37/39) in HC in the discovery set had CV values of <15%, while
in the validation set, there were 87% FFAs with CV<15% both in SCZ and HC group, indicating that the content of FFAs was relatively stable for all individuals with the same health
status. DISCUSSION Schizophrenia, a mental disease that affects approximately 30 million people globally, has devastating consequences for patients and their families.1 Accumulating evidence
indicates that abnormal lipid metabolism is the main characteristic of schizophrenia and may contribute to its pathology. Previous studies generally focused on ω-3 and ω-6 PUFAs in samples
of patients with schizophrenia. In the present study, we were able to compare the concentrations of not only the ω-3 and ω-6 PUFA series but also MUFAs, SFAs, TFAs, BCFAs, and OCFAs in the
serum of patients experiencing their first episodes or drug-free schizophrenia with those of matched control subjects. Moreover, we estimated the endogenous metabolic enzyme activities, as
well as changes in metabolic progress. FACTORS INFLUENCING ALTERATIONS OF THE FREE FATTY ACID PATTERN IN SCHIZOPHRENIA PATIENTS CHANGES IN THE LIPID METABOLIC PROCESS FFAs can be used for
fat synthesis or as fuel through β-oxidation. Previous studies have provided converging evidence for an insufficient energy supply in schizophrenia patients and elevated lipolysis and
β-oxidation as compensatory mechanisms.3, 8 We observed significant accumulation of 13 even-carbon FFAs, whereas only one even-carbon FFA decreased significantly (Table 1, Figure 1). The
activities of lipid metabolic enzymes can be estimated using indices based on product to precursor fatty acid ratios.13, 14 Fasting plasma FFAs are closely correlated with adipose tissue.9
The ratios of FFAs may reflect the desaturase activity in adipose tissue. By comparing the ratios of products to precursors between patients and controls, we determined that β-oxidation
prevailed from C24:0 to C16:0 and from C24:1 to C22:1 (Figure 2, blue arrows indicate statistical significance). This result provides evidence in the level of free fatty acid of the
hypothesis that lipid mobilization and β-oxidation are enhanced to provide more energy in schizophrenia patients. The activities of the delta-9 and delta-5 desaturases were also estimated
using the product to precursor fatty acid ratios. Delta-9 activity was estimated from the ratios of 16:1/16:0 and 18:1/18:0.15, 16 In the present study, delta-9 activity was significantly
enhanced in SCZ compared with its activity in HC, with _P_-values of 1.2684E−07 and 0.0037 for 16:1/16:0 and 18:1/18:0, respectively (Figure 2a). Delta-5 desaturase activity is reflected by
the 20:4/20:3 ratio.13, 15, 17 The desaturation progress catalyzed by delta-5 (20:4/20:3(ω-6) and 20:5/20:4(ω-6)) did not differ appreciably between SCZ and HC in the present study (Figures
2b and c). Since using metabolite ratios to estimate biochemical equilibrium status or metabolic enzyme activity is numerical and indirect, direct measurement of the characteristics of
related enzymes participating in lipid metabolism in schizophrenia patients is highly encouraged in future studies. HIGHER LEVELS OF OXIDATIVE STRESS The increased production of ROS and/or
decreased occurrence of antioxidant protection indicates that oxidative stress is involved in the pathophysiology of schizophrenia.18 Increased oxidative stress in schizophrenia patients has
been observed in the central nervous system3, 18, 19 and peripheral blood.20, 21 Many factors can cause an imbalance of ROS and antioxidants. Enhanced β-oxidation can increase ROS and
oxidative stress significantly.14 As mentioned above, our findings suggest increased lipid mobilization and β-oxidation. The elevated levels of FFAs may be a factor causing oxidative stress.
PUFAs, the main material of membranes, are vulnerable to damage by free radicals due to their double-bond structure. This process is called lipid peroxidation and damages cell membrane
fluidity and permeability, signal transduction and mitochondrial function.22, 23 Membrane PUFAs are significantly decreased in both the central nervous system and RBCs of patients with
schizophrenia.24, 25 There may be some links between plasma or serum FFA abnormalities and changes in membrane fatty acids in schizophrenia patients. Therefore, we hypothesize that in
schizophrenia patients, the brain energy supply is insufficient for certain reasons, such as mitochondrial dysfunction. Hence, body lipids and fatty acids are mobilized to compensate for the
brain energy shortage, resulting in elevated serum FFAs. Lipid peroxidation damages the membrane lipids, releasing free PUFAs into the plasma and further elevating plasma free PUFA levels.
Simultaneously, enhanced β-oxidation during the process of energy production of fatty acids increases ROS in the body, aggravating the oxidative stress in schizophrenia patients.14
Consequently, more free FFAs, such as PUFAs, are derived from the lipid peroxidation progress, further increasing the FFA levels in the blood of patients (Figure 3). Further research is
needed to verify this hypothesis. ADVERSE FFA PATTERNS FOR HEALTH Schizophrenia is a complex multi-pathogenic disease closely related to metabolic disorders. The morbidities of metabolic
syndrome, obesity, type II diabetes and cardiovascular disease are higher in subjects with schizophrenia than in healthy controls.26 Although no metabolic disorders were observed in either
SCZ or HC in our samples, the FFA pattern suggests a high risk of metabolic disorders in SCZ. Cardiovascular disease is the main cause of increased mortality in patients with
schizophrenia.27, 28 ω-3 and ω-6 PUFAs share the same desaturases and elongases. Many studies have indicated that ω-3 PUFAs play an important role in the nervous and cardiovascular
systems.29, 30 The ratio of ω-6/ω-3 PUFAs can be used as a health balance index, and the lower ratio the better, especially for cardiovascular disease.31 It is noteworthy that the ω-3 PUFAs
in the present study were less elevated (geometric mean of the FCs=1.16) than the ω-6 PUFAs (geometric mean of the FCs=1.46) in the discovery set. The geometric means of the FCs in the
validation set were 1.34 and 1.51, respectively. The higher ratio of ω-6/ω-3 in the schizophrenia group can be deduced from the results, which indicates the higher risk of cardiovascular
disease. The link between schizophrenia and diabetes has been known for over a century.32 The prevalence of diabetes in schizophrenia patients ranges from 10 to 15%, 2- to 3-fold higher than
in the general population.33 Antipsychotics increase metabolic risks, but antipsychotic-naïve patients also have a higher risk of developing diabetes. Impaired glucose tolerance,
hyperglycemia, and insulin resistance has been reported in first-episode drug-free schizophrenia patients.34, 35, 36, 37, 38 Although several factors including genetic susceptibility,
unhealthy lifestyles and neuroendocrine dysregulation are involved in the association between schizophrenia and diabetes, the etiology of the high comorbidity rate remains obscure.32
Numerous studies have reported that high plasma (or serum) FFAs are involved in the etiology of type II diabetes mellitus. Elevated plasma FFAs in humans result in reduced insulin
sensitivity throughout the entire body, increased insulin secretion and β-cell compensation.39 Glucose transport (GLUT-4) is inhibited by elevated plasma FFAs, and this inhibition is
followed by a reduction in the rate of glucose oxidation.40, 41 Among major human FFA, palmitic acid (C16:0) is particularly notable as a major culprit of type II diabetes.42, 43, 44, 45
Palmitate inhibits the insulin-stimulated phosphorylation of key insulin signaling molecules and facilitates their ubiquitination.46 We observed that 13 even-carbon FFAs were increased in
SCZ, including palmitic acid (C16:0). In SCZ serum, the concentrations of C16:0 were much higher, 276.41 μg/ml and 266.24 μg/ml in the discovery and validation sets, respectively, than in HC
serum, which exhibited values of 117.80 μg/ml and 115.48 μg/ml, respectively (Supplementary Table 4). The considerable difference in C16:0 between SCZ and HC may indicate a high risk of
type II diabetes in the SCZ group. Obesity is closely correlated with diabetes. High levels of FFAs are a characteristic of obesity.47 A significant and positive correlation between delta-9
desaturase and markers of obesity has been reported.15 The estimated elevated delta-9 desaturase activity implies that schizophrenia patients are more likely to be obese. Thus, a vicious
cycle appears to occur: energy metabolic dysfunction in schizophrenia patients and oxidative stress cause elevated FFAs, which in turn, induce high risks of cardiovascular disease, diabetes
and other metabolic diseases. Drugs that can alleviate the vicious FFA pattern in schizophrenia patients would be beneficial and could be used as auxiliary treatment for schizophrenia. TFCS,
BCFAS AND OCFAS IN SERUM Trans-fatty acids and BCFAs cannot be synthesized in humans and are obtained from food. We did not observe differences in TFAs or BCFAs between SCZ and HC, implying
that there is no considerable difference in diet between the two groups. Twelve odd-numbered carbon fatty acids were detected in this study, and two of them, C17:1 and C19:1, were
significantly increased in patients. Odd-numbered carbon fatty acids have received little attention due to their indigestibility in humans and technique limitations. Odd-numbered carbon
fatty acids usually exist in bacteria, fungi, poriferans, plants and animals, with various types in different species. Bacteria usually contain odd-numbered fatty acids in general, most
often C15, C17 or C19.48 Many recent studies have suggested that microorganisms in the human body can affect the mental state. The relationship between gut microbiota and brain disease is
attracting increasing attention.49, 50 The differences in the odd-numbered carbon FFA contents in serum observed in this study may imply the disturbance of gut microbiota in schizophrenia
patients. To further investigate the potential association, research at the genome and metabolome levels is needed. STRENGTHS AND LIMITATIONS In this study, we tried to mediate medication
effects on our results by recruiting first-onset drug-naïve patients and patients without any antipsychotic drugs for at least 1 month. UPLC-QTOF-MS platform was employed to achieve a
comprehensive scan of serum free fatty acids of our samples. As many as 47 fatty acids were relatively quantified and 39 were absolutely quantified. This quantitative scale is impressive and
provides considerable information on FFA profiles. Alterations of FFA levels in schizophrenia reflect the abnormalities of energy metabolism in patients. Analysis of FFA patterns and
products-precursors ratios helped to draw our attention to the lipolysis, β-oxidation and desaturation process, and guide our follow-up researches on other molecular levels, such as related
proteins or genes. This study also has limitations. Generally speaking, there are two sources of free fatty acids in human body: dietary intake and lipolysis from triglyceride. Since no
survey has been conducted on detailed eating habits of participants, we can’t remove possible confounding effects of food intake when interpreting our results. However, whether schizophrenia
patients have dietary bias remains controversial. A series of literature on schizophrenic eating habits describes that patients have preference in unhealthy diet.51, 52, 53 Another work
discovers that individuals with schizophrenia and healthy controls seem to apply preference ratings to food in a similar manner.54 In addition, we have tried to mediate the impact of eating
habit on our results in the following ways: (1) The residence of our participants were limited to Wuhu, Anhui to minimize regional differences in diet structure. (2) BMIs were matched
between schizophrenia patients and healthy controls, and none of the participants had metabolic disorders. (3) Fasting blood was collected to avoid the disturbance of short-term food intake.
Another limitation in this study is that we estimated biochemical equilibrium status or metabolic enzyme activity by using indices based on product to precursor fatty acid ratios. Direct
measurement of the characteristics of related enzymes especially in lipolysis, desaturation and β-oxidation progress is necessary in the future. Metabonomics reflects a resultant status of
the body from a combined influence of genes and environment. Different genetic background in other populations may modify individual metabonomics and there is no systematic study of free
fatty acids in other population till now. Thus, genetic factors should be considered when exploring such signatures in other population. We used UPLC-QTOF-MS to characterize the serum fatty
acid pattern in schizophrenia patients. Forty-seven FFAs were detected and relatively quantitatively analyzed, and 39 of these FFAs were absolutely quantitated by establishing standard
curves. Sixteen of the 47 detected FFAs were significantly different in patients with schizophrenia and healthy controls. With the exception of the very long-chain fatty acid C24:0, these
significantly different FFAs were all increased in schizophrenia serum. Desaturation from SFAs to MUFAs and β-oxidation, particularly in the endoplasmic reticulum, were enhanced, as
estimated by the ratios of products to precursors. These results suggest upregulated lipolysis and β-oxidation in SCZ, presumably resulting from insufficient brain energy supply. Drugs that
can alleviate the vicious FFA pattern in schizophrenia patients would be beneficial and could be used as auxiliary treatment for schizophrenia. REFERENCES * Ross CA, Margolis RL, Reading SA,
Pletnikov M, Coyle JT . Neurobiology of schizophrenia. _Neuron_ 2006; 52: 139–153. Article CAS Google Scholar * Freedman R . Schizophrenia. _N Engl J Med_ 2003; 349: 1738–1749. Article
CAS Google Scholar * Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL _et al_. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and
oxidative stress. _Mol Psychiatry_ 2004; 9: 643. Article Google Scholar * Schwarz E, Prabakaran S, Whitfield P, Major H, Leweke FM, Koethe D _et al_. High throughput lipidomic profiling
of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. _J Proteome Res_ 2008; 7: 4266–4277. Article CAS Google
Scholar * Taha AY, Cheon Y, Ma K, Rapoport SI, Rao JS . Altered fatty acid concentrations in prefrontal cortex of schizophrenic patients. _J Psychiatr Res_ 2013; 47: 636–643. Article
Google Scholar * Misiak B, Stanczykiewicz B, Laczmanski L, Frydecka D . Lipid profile disturbances in antipsychotic-naive patients with first-episode non-affective psychosis: a systematic
review and meta-analysis. _Schizophr Res_ 2017 (e-pub ahead of print). * Solberg DK, Bentsen H, Refsum H, Andreassen OA . Association between serum lipids and membrane fatty acids and
clinical characteristics in patients with schizophrenia. _Acta Psychiatr Scand_ 2015; 132: 293–300. Article CAS Google Scholar * Yang J, Chen T, Sun L, Zhao Z, Qi X, Zhou K _et al_.
Potential metabolite markers of schizophrenia. _Mol Psychiatry_ 2013; 18: 67–78. Article CAS Google Scholar * Hodson L, Skeaff CM, Fielding BA . Fatty acid composition of adipose tissue
and blood in humans and its use as a biomarker of dietary intake. _Prog Lipid Res_ 2008; 47: 348–380. Article CAS Google Scholar * Ni Y, Zhao L, Yu H, Ma X, Bao Y, Rajani C _et al_.
Circulating Unsaturated Fatty Acids Delineate the Metabolic Status of Obese Individuals. _EBioMedicine_ 2015; 2: 1513–1522, (2352-3964 (Electronic)). Article Google Scholar * Trufelli H,
Famiglini G, Termopoli V, Cappiello A . Profiling of non-esterified fatty acids in human plasma using liquid chromatography-electron ionization mass spectrometry. _Anal Bioanal Chem_ 2011;
400: 2933–2941. Article CAS Google Scholar * Ni Y, Zhao L, Yu H, Ma X, Bao Y, Rajani C _et al_. Circulating Unsaturated Fatty Acids Delineate the Metabolic Status of Obese Individuals.
_EBioMedicine_ 2015; 2: 1513–1522. Article Google Scholar * Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I _et al_. Plasma and erythrocyte fatty acid patterns in patients
with recurrent depression: a matched case-control study. _PloS one_ 2010; 5: e10635. Article Google Scholar * Koerkamp MG, Rep M, Bussemaker HJ, Hardy GP, Mul A, Piekarska K _et al_.
Dissection of transient oxidative stress response in Saccharomyces cerevisiae by using DNA microarrays. _Mol Biol Cell_ 2002; 13: 2783–2794. Article CAS Google Scholar * Warensjo E,
Ohrvall M, Vessby B . Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. _Nutr Metab Cardiovasc Dis_ 2006; 16:
128–136. Article Google Scholar * Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F _et al_. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity
after 3 d of high-carbohydrate feeding. _Am J Clin Nutr_ 2008; 87: 817–823. Article CAS Google Scholar * Riserus U, Tan GD, Fielding BA, Neville MJ, Currie J, Savage DB _et al_.
Rosiglitazone increases indexes of stearoyl-CoA desaturase activity in humans: link to insulin sensitization and the role of dominant-negative mutation in peroxisome proliferator-activated
receptor-gamma. _Diabetes_ 2005; 54: 1379–1384. Article CAS Google Scholar * Salim S . Oxidative stress and psychological disorders. _Curr Neuropharmacol_ 2014; 12: 140–147. Article CAS
Google Scholar * Yao JK, Leonard S, Reddy R . Altered glutathione redox state in schizophrenia. _Dis Markers_ 2006; 22: 83–93. Article CAS Google Scholar * Pedrini M, Massuda R, Fries
GR, de Bittencourt Pasquali MA, Schnorr CE, Moreira JC _et al_. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and
late stages of chronicity. _J Psychiatr Res_ 2012; 46: 819–824. Article Google Scholar * Yao JK, Reddy R, McElhinny LG, van Kammen DP . Reduced status of plasma total antioxidant capacity
in schizophrenia. _Schizophr Res_ 1998; 32: 1–8. Article CAS Google Scholar * Tsaluchidu S, Cocchi M, Tonello L, Puri BK . Fatty acids and oxidative stress in psychiatric disorders. _BMC
Psychiatry_ 2008; 8 (Suppl 1): S5. Article Google Scholar * Adibhatla RM, Hatcher JF . Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic
opportunities. _Antioxid Redox Signal_ 2010; 12: 125–169. Article CAS Google Scholar * Bentsen H, Solberg DK, Refsum H, Gran JM, Bohmer T, Torjesen PA _et al_. Bimodal distribution of
polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. _Biol Psychiatry_ 2011; 70: 97–105. Article CAS Google Scholar * Hoen WP, Lijmer JG, Duran M,
Wanders RJ, van Beveren NJ, de Haan L . Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. _Psychiatry Res_ 2013; 207: 1–12. Article
CAS Google Scholar * Subashini R, Deepa M, Padmavati R, Thara R, Mohan V . Prevalence of diabetes, obesity, and metabolic syndrome in subjects with and without schizophrenia (CURES-104).
_J Postgrad Med_ 2011; 57: 272–277. Article CAS Google Scholar * Tandon R, Nasrallah HA, Keshavan MS . Schizophrenia, "just the facts" 4. Clinical features and
conceptualization. _Schizophr Res_ 2009; 110: 1–23. Article Google Scholar * Azad MC, Shoesmith WD, Al Mamun M, Abdullah AF, Naing DK, Phanindranath M _et al_. Cardiovascular diseases
among patients with schizophrenia. _Asian J Psychiatr_ 2016; 19: 28–36. Article Google Scholar * Crupi R, Marino A, Cuzzocrea S . n-3 fatty acids: role in neurogenesis and neuroplasticity.
_Curr Med Chem_ 2013; 20: 2953–2963. Article CAS Google Scholar * Zugno AI, Chipindo HL, Volpato AM, Budni J, Steckert AV, de Oliveira MB _et al_. Omega-3 prevents behavior response and
brain oxidative damage in the ketamine model of schizophrenia. _Neuroscience_ 2014; 259: 223–231. Article CAS Google Scholar * Harris WS, Poston WC, Haddock CK . Tissue n-3 and n-6 fatty
acids and risk for coronary heart disease events. _Atherosclerosis_ 2007; 193: 1–10. Article CAS Google Scholar * Annamalai A, Tek C . An overview of diabetes management in schizophrenia
patients: office based strategies for primary care practitioners and endocrinologists. _Int J Endocrinol_ 2015; 2015: 969182. Article Google Scholar * Schreurs M DEH, Vancampfort V, D,
VANW R . Metabolic syndrome in people with schizophrenia: a review. _World Psychiatry_ 2009; 8: 15–22. Article Google Scholar * Fernandez-Egea E, Bernardo M, Donner T, Conget I, Parellada
E, Justicia A _et al_. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. _Br J Psychiatry_ 2009; 194: 434–438. Article Google Scholar * Fernandez-Egea E,
Bernardo M, Parellada E, Justicia A, Garcia-Rizo C, Esmatjes E _et al_. Glucose abnormalities in the siblings of people with schizophrenia. _Schizophr Res_ 2008; 103: 110–113. Article
Google Scholar * Guest PC, Wang L, Harris LW, Burling K, Levin Y, Ernst A _et al_. Increased levels of circulating insulin-related peptides in first-onset, antipsychotic naive schizophrenia
patients. _Mol Psychiatry_ 2010; 15: 118–119. Article CAS Google Scholar * Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH . Impaired glucose tolerance in first-episode drug-naive
patients with schizophrenia. _Diabet Med_ 2007; 24: 481–485. Article CAS Google Scholar * Ryan MC, Collins P, Thakore JH . Impaired fasting glucose tolerance in first-episode, drug-naive
patients with schizophrenia. _Am J Psychiatry_ 2003; 160: 284–289. Article Google Scholar * Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF . Acute enhancement of
insulin secretion by FFA in humans is lost with prolonged FFA elevation. _Am J Physiol_ 1999; 276 (6 Pt 1): E1055–E1066. CAS PubMed Google Scholar * Roden M, Price TB, Perseghin G,
Petersen KF, Rothman DL, Cline GW _et al_. Mechanism of free fatty acid-induced insulin resistance in humans. _J Clin Invest_ 1996; 97: 2859–2865. Article CAS Google Scholar * Tremblay F,
Lavigne C, Jacques H, Marette A . Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and
atypical protein kinase C (zeta/lambda) activities. _Diabetes_ 2001; 50: 1901–1910. Article CAS Google Scholar * Mordier S, Iynedjian PB . Activation of mammalian target of rapamycin
complex 1 and insulin resistance induced by palmitate in hepatocytes. _Biochem Biophys Res Commun_ 2007; 362: 206–211. Article CAS Google Scholar * Patel PS, Sharp SJ, Jansen E, Luben RN,
Khaw KT, Wareham NJ _et al_. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a
pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. _Am J Clin Nutr_ 2010; 92: 1214–1222. Article CAS Google Scholar * Gustavo
Vazquez-Jimenez J, Chavez-Reyes J, Romero-Garcia T, Zarain-Herzberg A, Valdes-Flores J, Manuel Galindo-Rosales J _et al_. Palmitic acid but not palmitoleic acid induces insulin resistance in
a human endothelial cell line by decreasing SERCA pump expression. _Cell Signal_ 2015; 28: 53–59. Article Google Scholar * Hirabara SM, Curi R, Maechler P . Saturated fatty acid-induced
insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. _J Cell Physiol_ 2010; 222: 187–194. Article CAS Google Scholar * Ishii M, Maeda A, Tani S,
Akagawa M . Palmitate induces insulin resistance in human HepG2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. _Arch Biochem Biophys_
2015; 566: 26–35. Article CAS Google Scholar * Koutsari C, Jensen MD . Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity. _J Lipid Res_ 2006;
47: 1643–1650. Article CAS Google Scholar * Rezanka T, Sigler K . Odd-numbered very-long-chain fatty acids from the microbial, animal and plant kingdoms. _Prog Lipid Res_ 2009; 48:
206–238. Article CAS Google Scholar * Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF . Microbiota and neurodevelopmental windows: implications for brain disorders.
_Trends Mol Med_ 2014; 20: 509–518. Article Google Scholar * Clarke G, O'Mahony SM, Dinan TG, Cryan JF . Priming for health: gut microbiota acquired in early life regulates
physiology, brain and behaviour. _Acta Paediatr_ 2014; 103: 812–819. Article CAS Google Scholar * Elman I, Borsook D, Lukas SE . Food intake and reward mechanisms in patients with
schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. _Neuropsychopharmacology_ 2006; 31: 2091–2120. Article CAS Google Scholar
* Heald A, Pendlebury J, Anderson S, Narayan V, Guy M, Gibson M _et al_. Lifestyle factors and the metabolic syndrome in Schizophrenia: a cross-sectional study. _Ann Gen Psychiatry_ 2017
16: 12. Article Google Scholar * Hahn LA, Galletly CA, Foley DL, Mackinnon A, Watts GF, Castle DJ _et al_. Inadequate fruit and vegetable intake in people with psychosis. _Aust N Z J
Psychiatry_ 2014; 48: 1025–1035, (1440-1614 (Electronic)). Article Google Scholar * Folley BS, Park S . Relative food preference and hedonic judgments in schizophrenia. _Psychiatry Res_
2010; 175: 33–37. Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (81271486, 81421061), Ministry of
Science and Technology of China (2016YFC1306802, 2016YFC1306900), the Program for NSFC International (Regional) Cooperation and Exchange (81361120389), Grants of Shanghai Brain-Intelligence
Project from STCSM(16JC1420500). AUTHOR INFORMATION Author notes * X Yang and L Sun: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Bio-X Institutes, Key
Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders (Ministry of Education), Key Laboratory of Translational Psychiatry, Shanghai Mental Health Center, Shanghai Jiao
Tong University, Shanghai, China X Yang, L Sun, X Hu, Y Qing, J Jiang, C Yang, J Zhang, L He & C Wan * Center for Translational Medicine and Shanghai Key Laboratory of Diabetes Mellitus,
Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China A Zhao, J Liu & W Jia * Department of Anatomy, Discipline of Neuroscience, Histology and
Embryology, Collaborative Innovation Center for Brain Science, Shanghai Jiao Tong University School of Medicine, Shanghai, China T Xu * The Fourth People’s Hospital of Wuhu, Wuhu, China P
Wang * Collaborative Innovation Center of Genetics and Development, Shanghai, China C Wan Authors * X Yang View author publications You can also search for this author inPubMed Google
Scholar * L Sun View author publications You can also search for this author inPubMed Google Scholar * A Zhao View author publications You can also search for this author inPubMed Google
Scholar * X Hu View author publications You can also search for this author inPubMed Google Scholar * Y Qing View author publications You can also search for this author inPubMed Google
Scholar * J Jiang View author publications You can also search for this author inPubMed Google Scholar * C Yang View author publications You can also search for this author inPubMed Google
Scholar * T Xu View author publications You can also search for this author inPubMed Google Scholar * P Wang View author publications You can also search for this author inPubMed Google
Scholar * J Liu View author publications You can also search for this author inPubMed Google Scholar * J Zhang View author publications You can also search for this author inPubMed Google
Scholar * L He View author publications You can also search for this author inPubMed Google Scholar * W Jia View author publications You can also search for this author inPubMed Google
Scholar * C Wan View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to W Jia or C Wan. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the _Translational Psychiatry_ website SUPPLEMENTARY
INFORMATION SUPPLEMENTARY MATERIAL (DOCX 467 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included
under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, X., Sun, L., Zhao, A. _et al._ Serum fatty acid patterns in patients
with schizophrenia: a targeted metabonomics study. _Transl Psychiatry_ 7, e1176 (2017). https://doi.org/10.1038/tp.2017.152 Download citation * Received: 27 January 2017 * Revised: 25 April
2017 * Accepted: 07 June 2017 * Published: 25 July 2017 * Issue Date: July 2017 * DOI: https://doi.org/10.1038/tp.2017.152 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative