Play all audios:
ABSTRACT Few data are available on population genetic structure in nematode species, and little of the available data allows direct comparison of the genetic structures of species having
different life cycles. Here we use mtDNA sequence data to describe the genetic structure of a heterorhabditid nematode, and compare results to published data on other nematode species.
_Heterorhabditis marelatus_ is a parasite of soil-dwelling insects. Its life cycle and local ecology should result in small effective population sizes and restricted gene flow. As predicted,
_H. marelatus_ shows much lower mtDNA diversity within populations and over the species as a whole, and has a much more strongly subdivided population structure, than parasites of mobile
vertebrate hosts. From data such as these we can begin to generalize about the effects of life cycle variation on genetic structure in different nematode species. SIMILAR CONTENT BEING
VIEWED BY OTHERS THE PARASITIC NEMATODE _STRONGYLOIDES RATTI_ EXISTS PREDOMINANTLY AS POPULATIONS OF LONG-LIVED ASEXUAL LINEAGES Article Open access 13 October 2023 GLOBAL DETERMINANTS OF
INSECT MITOCHONDRIAL GENETIC DIVERSITY Article Open access 29 August 2023 GENETIC DIVERSITY AND POPULATION STRUCTURE OF THE ROCKPOOL SHRIMP _PALAEMON ELEGANS_ BASED ON MICROSATELLITES:
EVIDENCE FOR A CRYPTIC SPECIES AND DIFFERENTIATION ACROSS THE ATLANTIC–MEDITERRANEAN TRANSITION Article Open access 01 July 2020 INTRODUCTION We still know little about the population
genetic structure of most parasite species, the exceptions being mostly species of medical or agricultural importance (e.g. Lymbery et al., 1990; Tibayrenc et al., 1991; Day et al., 1992;
Anderson et al., 1995; Blouin et al., 1995; Dybdahl & Lively, 1996; Babiker & Walliker, 1997; Blair et al., 1997). This oversight is surprising because data on genetic structure are
necessary for understanding important evolutionary processes such as adaptation to host defences, host-race formation, speciation, and the evolution of resistance to drugs or vaccines.
Nematodes in particular are a grossly understudied taxon. Even though nematodes are one of the most species-rich, ecologically diverse and economically important taxa, we have information on
genetic structure for only a handful of nematode species, and almost all of these are human or agricultural parasites (recently reviewed in Anderson et al., 1998). Virtually nothing is
known of the genetic structure of any free-living nematode species (including _Caenorhabditis elegans_). Thus, more comparative studies on genetic structure in nematode species are clearly
needed. Indeed, Hughes et al. (1997) specifically called for more data on nematodes in their recent review of patterns of population differentiation in different taxa. Parasitic nematodes
display a wide variety of life cycles and life histories. For example, they parasitize almost all groups of plants and animals, and occur in virtually every marine, terrestrial and
freshwater habitat. Their breeding system can be obligately or facultatively amphimictic (two distinct sexes), parthenogenetic or hermaphroditic. They range from highly host-specific to
undiscriminating, and vary in the presence or absence of free-living stages and intermediate hosts. How this diversity of life cycles influences genetic structure in different nematode
species is unknown. We currently have too few comparative data from which to make any but the simplest predictions. What is needed are comparative studies of the genetic structure of
nematode species that differ in key features of the life cycle, using similar sampling designs and the same molecular markers. For example, using mtDNA sequence data Blouin et al. (1995)
showed that host mobility has a large effect on genetic structure in trichostrongylid species that parasitize different species of ruminants. We see the effect of differences in population
size in comparisons between trichostrongylids and _Ascaris_ species (Anderson et al., 1998). Both have similar life cycles (simple, one-host, obligately outcrossing, with a mobile vertebrate
host), but differ by orders of magnitude in population sizes, and correspondingly in levels of both mtDNA and nuclear intron diversity. In contrast, plant parasitic nematodes having a
predominantly parthenogenetic mode of reproduction show much lower overall mtDNA diversity than either _Ascaris_ or the trichostrongylids (_Meloidogyne_ spp.; Hugall et al., 1994). Here we
used mtDNA sequence data to describe the genetic structure of a species that parasitizes soil-dwelling insects. STUDY SPECIES AND PREDICTIONS ABOUT GENETIC STRUCTURE _Heterorhabditis
marelatus_ is in the family Heterorhabditidae, one of two main families of entomopathogenic nematodes (Gaugler & Kaya, 1990). Entomopathogenic nematodes are obligate parasites of
soil-dwelling insects. Infective juveniles (IJs) of these species actively seek insect hosts in soil. After penetrating a host, IJs release a symbiotic gut bacterium (_Photorhabdus_ spp.)
that rapidly kills the host, usually within 24–48 h. Nematodes reproduce within the cadaver, and large numbers of IJs escape into the soil to seek additional hosts. _Heterorhabditis
marelatus_ occurs along the Pacific coast from the San Francisco Bay area (D. Strong, pers. comm.) to at least southern Washington (personal observation). Populations occur in sandy soils
under vegetation, usually behind the dunes of sandy beaches, and up to a few hundred metres inland. On the Pacific coast their habitat is subdivided into what is essentially a linear series
of habitat islands separated by stretches of rocky shoreline. Here we refer to the nematodes inhabiting a continuous stretch of suitable habitat (usually a discrete beach) as a population.
Like most nematodes, _Heterorhabditis_ have minimal powers of dispersal on their own. Gene flow on a regional scale will depend on the opportunities for nematodes to be transported either in
infected hosts, phoretically (by hitching a ride on nonparasitized hosts), or passively through the movement of wind and water. Infective juveniles are susceptible to UV light and to
desiccation, so they cannot be exposed to the air for long (Downes & Griffin, 1996; Strong et al., 1996). _Heterorhabditis_ are tolerant of salt water, so movement along shore by ocean
currents might occur in coastal species like _H. marelatus_ (Griffin et al., 1994). Transport in infected insects is possible, but heterorhabditids specialize on buried insects (as opposed
to insects walking on the soil surface), and hosts are killed rapidly following infection. Thus, the first prediction is that gene flow is very restricted on a regional scale. We also
predict that _H. marelatus_ populations will have small effective sizes, for two reasons. First, on a local scale (a few to tens of metres) the distribution of nematodes is very clumped and
patchy, and patches go extinct and are recolonized at high rates (Stuart & Gaugler, 1994; Strong et al., 1996). Secondly, it is likely that each patch consists of very closely related
individuals descended from one or a few maternal founders. An infective juvenile that enters a host must reproduce hermaphroditically. Its offspring then mature into separate males and
females who reproduce for one or more generations before producing infective juveniles that leave the host. A single infection can produce hundreds of thousands of IJs, and these tiny
nematodes cannot move far on their own. Thus, patches probably contain the descendants of one or a few maternal founders. This sort of metapopulation patch structure should result in very
small mitochondrial effective sizes within populations (McCauley, 1991; Caballero & Hill, 1992; Harrison & Hastings, 1996). Therefore, _H. marelatus_ should show lower overall
genetic diversity, and a more strongly subdivided genetic structure, than obligately outcrossing parasites of mobile vertebrates, such as the trichostrongylids. To test this hypothesis, we
used mtDNA sequence data to describe the genetic structure of _H. marelatus_ populations along the Pacific coast of California and Oregon, and compared these data to the data from
trichostrongylids (for which the same gene and sampling scheme were used, making the two datasets directly comparable). MATERIALS AND METHODS To describe population structure in _H.
marelatus_, we sequenced 474 bp of the 3′ end of the mitochondrial _ND4_ gene. _ND4_ codes for a membrane spanning polypeptide of the hydrophobic subunit of NADH dehydrogenase complex I, and
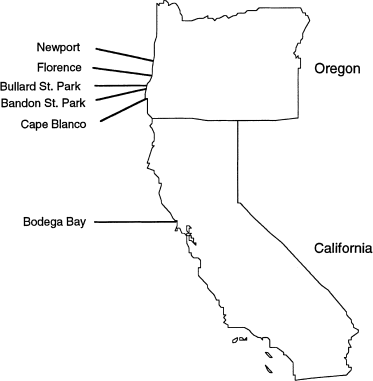
has been shown to be an excellent marker for population genetics studies in nematodes (Blouin et al., 1998). We sequenced each of nine or 10 individuals per population, in six populations
from coastal Oregon and California (Fig. 1). At each site we collected soil samples from an area spanning several hectares. We baited each soil sample with waxworms (_Galleria mellonella_),
and isolated a single first generation hermaphroditic nematode from infected hosts. To avoid sampling related individuals from the same patch of soil, we made sure that no samples were taken
any closer than several metres apart, and sequenced only one individual per soil sample. This region of the _ND4_ gene was used so that the results could be directly compared to those of
Blouin et al. (1995), who used the same gene and sample sizes to study the genetic structures of four species of trichostrongylid nematodes that parasitize ruminants in North America. Here
the geographical scale over which we sampled _H. marelatus_ populations (Oregon and northern California) is about the same as that over which populations of two of the trichostrongylids were
sampled (south-eastern U.S. for _Mazamastrongylus odocoilei_ and _Haemonchus placei_), and is smaller than the scale over which populations of the other two were sampled (entire U.S. for
_Haemonchus contortus_ and _Teladorsagia circumcincta_). Individual nematodes were crushed with a pestle in 20 μL of a 5% chelex solution, and incubated overnight at 55°C. Four μL of the
supernatant was used to amplify the _ND4_ region in a 25-μL PCR reaction (1.5 mM MgCl, 0.3 μM primers, GIBCO Taq and buffer) using a Perkin Elmer 9600 thermocycler (94°C denature for 3 min,
then 35 cycles of 94°C for 45 s, 54°C for 1 min, 72°C for 1.5 min, then a 7-min extension at 72°C). The PCR product was then run on a 1% agarose gel, isolated using a Supelco GenElute spin
column, and sequenced on an ABI 377 automated sequencer using the PCR primers as sequencing primers. Primers used were: forward (mb5): 5′-GGC TGG CTT ATT ATT AAA ATT AG-3′ reverse (mb9):
5′-CAA AGA ATA ATA AAA AGA TAC CAA-3′. RESULTS _Heterorhabditis marelatus_ shows strong differentiation among populations and low genetic diversity, both within populations and in the
species as a whole (Table 1). We found only four distinct haplotypes (labelled A, B, B′ and C) out of 58 sequences in the entire sample (Figs 2 and Figs. 3), and at most two haplotypes in
any population (Table 1). This diversity was strongly structured, with 86% of the total sequence diversity (_N_ST; Lynch & Crease, 1990) and 78% of the haplotype diversity (_F_ST)
distributed among populations (Table 1). Even the two closest populations (Bandon, OR, and Bullard, OR, 8 km apart; Fig. 1) had no haplotypes in common, and the only private allele (Slatkin,
1985) in the sample had a frequency of 8/10 in its population. Note also that this most geographically restricted allele (allele B′) also appears to be the most recently derived of the four
haplotypes (Fig. 3a), and that it occurs in a population with its parent allele (allele B; Table 1). This pattern is exactly what one expects under restricted gene flow, because the
geographical range of a haplotype should be strongly correlated with its age (Templeton et al., 1995). Finally, the distribution of pairwise sequence differences in _H. marelatus_ clearly
differs from that expected in a single population under drift–mutation equilibrium, there being too few haplotypes, given the distances among them (Fig. 3b; Tajima’s _D_=3.12, _P_ < 0.01;
Tajima, 1989). Assuming neutrality, this pattern is again consistent with historical subdivision of the species into isolated units. DISCUSSION DRIFT AND GENE FLOW IN _H. MARELATUS_ The
above results are all consistent with the small effective population sizes and low rates of gene flow predicted by the life cycle of _H. marelatus_. That there are too few haplotypes given
the large genetic distances between them is interesting. A selective sweep cannot be ruled out, but rapid drift within populations, combined with occasional long-distance gene flow, could
produce the same pattern. That two common alleles (A and B) are widespread throughout the species’ range, whereas even adjacent populations can be fixed for different alleles, is consistent
with this scenario. Perhaps migration occurs in an isolation-by-distance fashion on land, and occasionally over long distances via ocean currents. More intensive sampling of populations
throughout the range of the species might well reveal more haplotypes, but the overall pattern of strongly restricted gene flow on a local scale, with widespread common alleles, is unlikely
to change. PRACTICAL APPLICATIONS _Heterorhabditis_ spp. are extensively studied for their biocontrol abilities, and there is great interest in finding new strains that differ in characters
such as host-seeking behaviour, environmental tolerance and ability to control different pests (Bedding et al., 1983; Kung & Gaugler, 1991; Gaugler et al., 1997). Their symbiotic
bacteria are equal partners in killing insects, and trait variation in the bacteria may be as or more important than in the nematodes. For example, the toxins produced by _Photorhabdus_ spp.
are some of the most potent insect killers known, rivalling the well-known _Bacillus thuringensis_ toxin, and different species and strains of _Photorhabdus_ carry different toxins (Bowen
et al., 1998). Consequently, there is also a major impetus to search for and characterize new strains of symbiotic bacteria, particularly those adapted to unusual hosts or habitats.
Nevertheless, surprisingly little work has been carried out on the basic ecology and genetics of _Heterorhabditis_ or their bacteria in nature (Gaugler & Kaya, 1990; Strong et al., 1996;
Gaugler et al., 1997). Ours are the first data on genetic structure in a heterorhabditid, and there has been no population genetic work on _Photorhabdus_. Because the symbiotic bacteria can
presumably only disperse in association with their nematode, their population genetic structure should mirror that of the nematode. As part of an unrelated study we recently sequenced 616
bp of the bacterial 16S gene of bacterial isolates from each of five nematodes from Florence, OR, and from five nematodes from Newport, OR (≈80 km apart; Fig. 1) (unpubl. data). Isolates
from the two populations were fixed for different 16S rRNA haplotypes. Although these data are anecdotal, they suggest that the nematode and their symbiotic bacterium may both have
population genetic structures that promote genetic drift and the opportunity for local adaptation over short distances. Thus it may be fruitful to search for useful new strains of nematode
and bacteria (i.e. those adapted to unique hosts or environmental conditions) over very small geographical scales. COMPARISON WITH OTHER NEMATODE SPECIES Table 2 confirms the prediction that
_H. marelatus_ has lower overall diversity (both species-wide and within individual populations) and a more strongly subdivided genetic structure than the trichostrongylids. Only four
unique haplotypes were found in 58 sequences from _H. marelatus_, whereas samples of 40 trichostrongylid sequences yielded 31–39 unique haplotypes. Within populations the haplotype and
nucleotide diversity is almost an order of magnitude greater in the trichostrongylids than in _H. marelatus_, and a much greater proportion of the total sequence diversity is distributed
among populations in _H. marelatus_. Because the geographical scale over which _H. marelatus_ populations were sampled is smaller than that over which some of the trichostrongylids were
sampled, the higher _F_ST in _H. marelatus_ is even more striking. Within each of the four trichostrongylid species Tajima’s _D_ is not significantly different from zero, indicating tree
topologies that are not significantly different from that expected under neutrality in a single population (see figs 2,3,4,5 in Blouin et al., 1995, for haplotype trees). So in these species
we do not see the signature of historical subdivision into isolated populations that is apparent in the tree of _H. marelatus_ haplotypes. In the trichostrongylids vs. _H. marelatus_ we see
two extremes in a spectrum of genetic structures. Trichostrongylids show levels of mtDNA variation that are greater than those typically seen in other taxa, and the species that infect
livestock show exceptionally high rates of gene flow over vast geographical areas (Blouin et al., 1995; also M. Blouin, S. Richter and E. Hoberg, unpubl. data on _Teladorsagia circumcincta_
from Iceland vs. North America; C. Constant, unpubl. data on _Ostertagia ostertagi_ from Australia vs. North America). In contrast, _H. marelatus_ shows very low variation within populations
and in the species as a whole, and very restricted gene flow on a small scale. In these respects the genetic structure of _H. marelatus_ may be more similar to that of parthenogens such as
_Meloidogyne_ spp. than to that of outcrossing parasites of vertebrates. For example, only six unique mtDNA haplotypes were found in 48 _Meloidogyne_ individuals sampled from throughout the
eastern half of Australia (data from RFLP of entire mtDNA; Hugall et al., 1994). Individual _Meloidogyne_ samples were spread over a wide geographical area in that study, so we cannot
directly compare levels of within- and between-population diversity in _Meloidogyne_ spp. to that in _H. marelatus_ or the trichostrongylids. However, a testable prediction is that the
distribution of mtDNA diversity within and among populations in _Meloidogyne_ will be most similar to that in _Heterorhabditis._ Here we designed a study to compare the genetic structures of
two groups of nematodes, by using the same molecular marker and similar sampling schemes. Obviously more comparative data such as these are needed before we can generalize about the effects
of life cycle on genetic structure in nematodes. In particular, we need data on species in ‘natural’ habitats (i.e. species that are not human associates). To our knowledge, there are no
data on genetic structure in any free-living species, and of the parasitic species, only three are not parasites of humans or their domesticated plants or livestock (these include the
present data on _H. marelatus_, mtDNA data on _Mazamastrongylus odocoilei_, which is a parasite of deer [Blouin et al., 1995], and allozyme data on Anisakid nematodes of fish and cetaceans
[e.g. Paggi et al., 1991; Nascetti et al., 1993;]). Clearly this is a wide-open field of study that deserves more attention. REFERENCES * Anderson, T. J. C., Romero-Abal, M. E. and Jaenike,
J. (1995). Mitochondrial DNA and _Ascaris_ microepidemiology: the composition of parasite populations from individual hosts, families and villages. _Parasitology_, 110: 221–229. Article
PubMed Google Scholar * Anderson, T. J. C., Blouin, M. S. and Beech, R. N. (1998). Population biology of parasitic nematodes: applications of genetic markers. _Adv Parasitol_, 41: 219–283.
Article CAS PubMed Google Scholar * Babiker, H. A. and Walliker, D. (1997). Current views on the population structure of _Plasmodium falciparum_: implications for control. _Parasitol
Today_, 13: 262–267. Article CAS PubMed Google Scholar * Bedding, R. A., Molyneux, A. S. and Akhurst, R. J. (1983). _Heterorhabditis_ spp., _Neoaplectana_ spp. and _Steinernema
kraussei_: interspecific and intraspecific differences in infectivity for insects. _Exp Parasitol_, 55: 249–257. Article CAS PubMed Google Scholar * Blair, D., Agatsuma, T., Watanobe,
T., Okamoto, M. and Ito, A. (1997). Geographical genetic structure within the human lung fluke, _Paragonimus westermani_ detected from DNA sequences. _Parasitology_, 115: 411–417. Article
CAS PubMed Google Scholar * Blouin, M. S., Yowell, C. A., Courtney, C. H. and Dame, J. B. (1995). Host movement and the genetic structure of populations of parasitic nematodes.
_Genetics_, 141: 1007–1014. CAS PubMed PubMed Central Google Scholar * Blouin, M. S., Yowell, C. A., Courtney, C. H. and Dame, J. B. (1998). Substitution bias, rapid saturation, and the
use of mtDNA for nematode systematics. _Mol Biol Evol_, 15: 1719–1727. Article CAS PubMed Google Scholar * Bowen, D., Rocheleau, T. A., Blackburn, M., Andreev, O., Golubeva, E., Bhartia,
R. and Ffrench-Constant, R. H. (1998). Insecticidal toxins from the bacterium _Photorhabdus luminescens_. _Science_, 280: 2129–2132. Article CAS PubMed Google Scholar * Caballero, A.
and Hill, W. G. (1992). Effective size of nonrandom mating populations. _Genetics_, 130: 909–916. CAS PubMed PubMed Central Google Scholar * Day, K. P., Koella, J. C., Nee, S., Gupta, S.
and Read, A. F. (1992). Population genetics and dynamics of _Plasmodium falciparum_: an ecological view. _Parasitology_, 104: S35–S52. Article PubMed Google Scholar * Downes, M. J. and
Griffin, C. T. (1996). Dispersal behaviour and transmission strategies of the entomopathogenic nematodes _Heterorhabditis_ and _Steinernema_. _Biocontrol Sci Technol_, 6: 347–356. Article
Google Scholar * Dybdahl, M. F. and Lively, C. M. (1996). The geography of coevolution: comparative population structures for a snail and its trematode parasites. _Evolution_, 50:
2264–2275. Article PubMed Google Scholar * Gaugler, R. and Kaya, H. K. (1990) _Entomopathogenic Nematodes in Biological Control_. CRC Press, Boca Raton, FL. Google Scholar * Gaugler, R.,
Lewis, E. and Stuart, R. J. (1997). Ecology in the service of biological control: the case of entomopathogenic nematodes. _Oecologia_, 109: 483–489. Article CAS PubMed Google Scholar *
Griffin, C. T., Finnegan, M. M. and Downes, M. J. (1994). Environmental tolerances and the dispersal of _Heterorhabditis_– survival and infectivity of European _Heterorhabditis_ following
prolonged immersion in seawater. _Fund Appl Nematol_, 17: 415–421. Google Scholar * Harrison, S. and Hastings, A. (1996). Genetic and evolutionary consequences of metapopulation structure.
_Trends Ecol Evol_, 11: 180–183. Article CAS PubMed Google Scholar * Hugall, A., Moritz, C., Stanton, J. and Wolstenholme, D. R. (1994). Low but strongly structured mitochondrial DNA
diversity in root knot nematodes (Meloidogyne). _Genetics_, 136: 903–912. CAS PubMed PubMed Central Google Scholar * Hughes, J. B., Daily, G. C. and Ehrlich, P. R. (1997). Population
diversity: its extent and extinction. _Science_, 278: 689–692. Article CAS PubMed Google Scholar * Kung, S. -P. and Gaugler, R. (1991). Effects of soil temperature, moisture, and
relative humidity on entomopathogenic nematode persistence. _J Invert Pathol_, 57: 242–249. Article Google Scholar * Lymbery, A. J., Thompson, R. C. A. and Hobbs, R. P. (1990). Genetic
diversity and genetic differentiation in _Echinococcus granulosus_ (Batsch, 1786) from domestic and sylvatic hosts on the mainland of Australia. _Parasitology_, 101: 283–289. Article PubMed
Google Scholar * Lynch, M. and Crease, T. J. (1990). The analysis of population survey data on DNA sequence variation. _Mol Biol Evol_, 7: 377–394. CAS PubMed Google Scholar *
Mccauley, D. E. (1991). Genetic consequences of local population extinction and recolonization. _Trends Ecol Evol_, 6: 5–8. Article CAS PubMed Google Scholar * Nascetti, G., Cianchi, R.,
Mattiucci, S., D’amelio, S., Orecchia, P., Paggi, L. _et al_ (1993). Three sibling species within _Contracaecum osculatum_ (Nematoda, Ascaridida, Ascaridoidea) from the Atlantic
Arctic–Boreal region: reproductive isolation and host preferences. _Int J Parasitol_, 23: 105–120. Article CAS PubMed Google Scholar * Paggi, L., Nascetti, G., Cianchi, R., Orecchia, P.,
Mattiucci, S., D’amelio, S. _et al_ (1991). Genetic evidence for three species within _Pseudoterranova decipiens_ (Nematoda, Ascaridida, Ascaridoidea) in the North Atlantic and Norwegian
and Barents Seas. _Int J Parasitol_, 21: 195–212. Article CAS PubMed Google Scholar * Slatkin, M. (1985). Rare alleles as indicators of gene flow. _Evolution_, 39: 53–65. Article PubMed
Google Scholar * Strong, D. R., Kaya, H. K. and Maron, J. L. (1996). Entomopathogenic nematodes: natural enemies of root-feeding caterpillars on bush lupine. _Oecologia_, 108: 167–173.
Article CAS PubMed Google Scholar * Stuart, R. J. and Gaugler, R. (1994). Patchiness in populations of entomopathogenic nematodes. _J Invert Pathol_, 64: 39–45. Article Google Scholar
* Tajima, F. (1989). Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. _Genetics_, 123: 585–595. CAS PubMed PubMed Central Google Scholar * Templeton,
A. R., Routman, E. and Phillips, C. A. (1995). Separating population structure from population history: a cladistic analysis of the geographical distribution of mitochondrial DNA haplotypes
in the tiger salamander, _Ambystoma tigrinum_. _Genetics_, 140: 767–782. CAS PubMed PubMed Central Google Scholar * Tibayrenc, M., Kjellberg, F., Arnaud, J., Oury, B.,
Frédériquebrenière, S., Dardé, M. -L. and Ayala, F. J. (1991). Are eukaryotic microorganisms clonal or sexual? A population genetics vantage. _Proc Natl Acad Sci USA_, 88: 5129–5133. Article
CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS Thanks to D. Strong, J. Johnston, G. Poinar and D. Anderson for help collecting samples, and to A. Rabe,
K. Monsen and A. Giese for comments on an earlier draft. This work was supported by the OSU Agricultural Research Foundation and by U.S. Department of Agriculture CSREES 96–34354–3072
through the Oregon State University Center for Gene Research and Biotechnology. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Zoology, Oregon State University, Corvallis,
97331, OR, USA Michael S Blouin * Department of Entomology, Oregon State University, Corvallis, 97331, OR, USA Jie Liu & Ralph E Berry Authors * Michael S Blouin View author publications
You can also search for this author inPubMed Google Scholar * Jie Liu View author publications You can also search for this author inPubMed Google Scholar * Ralph E Berry View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Michael S Blouin. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Blouin, M., Liu, J. & Berry, R. Life cycle variation and the genetic structure of nematode populations. _Heredity_ 83, 253–259 (1999).
https://doi.org/10.1038/sj.hdy.6885420 Download citation * Received: 01 September 1998 * Accepted: 02 March 1999 * Published: 01 September 1999 * Issue Date: 01 September 1999 * DOI:
https://doi.org/10.1038/sj.hdy.6885420 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * effective size * gene flow * _Heterorhabditis_ *
mitochondrial DNA * _ND4_