Play all audios:
ABSTRACT An abnormal female producing only female progeny was found in _Lymantria dispar_ in Hokkaido, Japan, in July 1996. Similarly, its progeny produced only females. Egg hatch rates were
near 50% in all-female matrilines. Therefore, a certain cytoplasmic factor was thought to kill males in eggs differentially, resulting in only female hosts. In the next generation, the
field population was estimated to contain 9.1% abnormal females. Severe inbreeding depression was also observed in egg hatch rates during confirmation of maternal inheritance. The cost of
inbreeding was estimated at 0.395, which is one of the highest in insects. Inbreeding avoidance by their host has been cited as one of the advantages of a male-killing factor, but we suggest
that this is not applicable in this moth. SIMILAR CONTENT BEING VIEWED BY OTHERS POLYANDRY AND SPERM COMPETITION IN TWO TRAUMATICALLY INSEMINATING SPECIES OF STREPSIPTERA (INSECTA) Article
Open access 07 May 2024 BIAS IN SEX RATIOS AND POLYANDRY RATE IN REPRODUCTION OF _LEPTINOTARSA DECEMLINEATA_ Article Open access 14 December 2022 WHY PUT ALL YOUR EGGS IN ONE BASKET?
EVOLUTIONARY PERSPECTIVES ON THE ORIGINS OF MONOGENIC REPRODUCTION Article Open access 16 June 2023 INTRODUCTION Richard Goldschmidt, in the 1920s, used local Japanese races of the gypsy
moth _Lymantria dispar_ L. for his famous studies of sex determinants (Goldschmidt, 1934, 1940). He concluded that crosses between different sex-races resulted in abnormal sex conditions.
For example, a female of a very weak race crossed to a strong male produced only sons. Inversely, the backcross, i.e. F1 females of the cross between ‘strong female’ and ‘very weak male’
mated with ‘very weak males’, produced all-female broods including both normal females and females by sex reversal (genetic males) (Goldschmidt, 1934). He characterized the Hokkaido
population as a ‘very weak race’. In the same location, we have accidentally found an abnormal female producing only female progeny. Goldschmidt (1934) also stated that those females
produced by sex reversal rarely survived. Thus, almost all of the remaining females may be expected to produce progeny of a normal sex ratio. On the other hand, it is well known today that
maternally inherited cytoplasmic factors kill male embryos to increase female progeny, i.e. hosts. The aim of this paper is to provide the results of maternal inheritance of all-female
broods in crosses of progeny of the abnormal female _L. dispar_. Male-killing factors, transmitted only through the female line, may bestow an advantage on females carrying the factor by
reducing the number of brothers and thereby minimizing inbreeding (Werren, 1987). There are only a few experiments showing inbreeding depression in species with male-killing factors (Hurst
& Majerus, 1993). In confirmation of the maternal inheritance, we also found high inbreeding depression in a single generation of brother–sister matings. We will discuss whether
inbreeding avoidance by their host is an advantage of the male-killing factor in _L._ _dispar_ with an estimation of inbreeding and non-mating in a field population. Although the effects of
inbreeding have attracted great attention under both laboratory and field conditions (Pray & Goodnight, 1995; Saccheri et al., 1998), these effects have been investigated very little
among invertebrates, except for _Drosophila_ and _Tribolium_ (Roff, 1998). In species that are usually outcrossing, comparing only full-sibling lines with outbreeding lines reveals some
important traits of a population, such as the cost of inbreeding and the magnitude of genetic load (Ralls et al., 1988). The effect of inbreeding varies between different life-stage periods.
Saccheri et al. (1996) examined egg hatch rate, fecundity and some other traits affected by inbreeding in the butterfly, _Bicyclus anynana_. Among them, egg hatching shows the effect of
inbreeding most clearly, but fecundity is also somewhat depressed. We compare egg hatch rate and fecundity among full-sibling and outbreeding lines, and the all-female brood. Abnormal
females producing only female progeny are known in many insect orders: Diptera, Hymenoptera, Coleoptera, Hemiptera and Lepidoptera (Hurst & Pomiankowski, 1991; Hurst, 1991). To our
knowledge, this is the first report of high inbreeding depression in a lepidopteran species also exhibiting all-female broods. MATERIALS AND METHODS Overwintering eggs of _L. dispar_ hatch
in May in Hokkaido. Larvae feed on leaves of Japanese larch _Larix_ _leptolepis_, Japanese birch _Betula_ _platyphylla_ and many other broad-leaved trees. They pupate in July and emerge
between July and September. Because females deposit all eggs _en masse_ on tree trunks, we can recognize properties of the original female by rearing eggs from individual masses. We
collected eight egg masses in a birch forest in Bibai, Hokkaido, in February 1996. The egg masses were kept individually in plastic boxes in a cold room at 0°C until late May. Then, about
100 eggs from each egg mass were put in an incubator at 20°C. After hatching, larvae and unhatched eggs were counted to give egg hatch rates. Larvae were reared to pupation on cut larch
foliage. We obtained the sex ratio among pupae for each original egg mass. In July 1996, we crossed adults from the eight egg masses in various combinations. Within a day after adult
emergence, we put a female and a male into a Petri dish that was 8.5 cm in diameter and 8.5 cm high. The inner walls of the Petri dish were covered with filter paper. In October 1996, each
egg mass deposited on the paper was placed in a vinyl bag and kept in the cold room. We obtained 31, 40 and 49 egg masses of crosses with abnormal females, brother–sister matings and
outbreeding respectively. Six non-mated females from the egg mass of the abnormal female were put into Petri dishes individually to ascertain whether producing all females was caused by
parthenogenesis. In May 1997, the eggs in each of the 120 crosses were counted to give female fecundity. There were two egg categories, infertile reddish eggs and brown embryonated eggs
containing larvae. Egg embryonation begins soon after oviposition, and larvae are fully formed inside the egg in about a month. We reared 101–240 eggs of each cross to obtain egg hatch
rates, except for two inbreeding and one outbreeding egg masses, which contained only infertile eggs. We reared larvae of 19, 5 and 13 egg masses of crosses with abnormal females,
brother–sister matings and outbreeding respectively. All reared larvae were fed foliage of cut larch. The sex ratio of the progeny of each cross was obtained in the adults in July 1997.
During August and September 1996, vinyl flagging tape was fastened to every tree trunk bearing egg masses in the birch forest (0.2 ha in area) from which we collected the egg mass of the
abnormal female in the previous generation. Marks were put on the tape to indicate egg mass positions, and above-ground heights were recorded. In October 1996, we collected about 80 eggs in
each egg mass deposited below 4.8 m. Because the number of eggs contained in each egg mass is highly correlated with the product of length times width of the egg mass (Higashiura &
Kamijo, 1978), the length and width of each egg mass were recorded at the time of egg collection. All eggs were kept as an individual egg mass in the cold room. About 30 eggs in each egg
mass were placed in the incubator (20°C) in February 1997. After hatching, eggs and hatched larvae were counted to give egg hatch rates. In May 1997, using the same method as above, we
reared all seven egg batches whose hatch rates had been less than 70%, and another seven egg batches whose hatch rates had been more than 70%, until adults emerged in July 1997. In this way,
fecundity, fertility, egg hatch rate and sex ratio were estimated for the field population. Fisher’s randomization method with 5000 replications was used to test the null hypothesis that
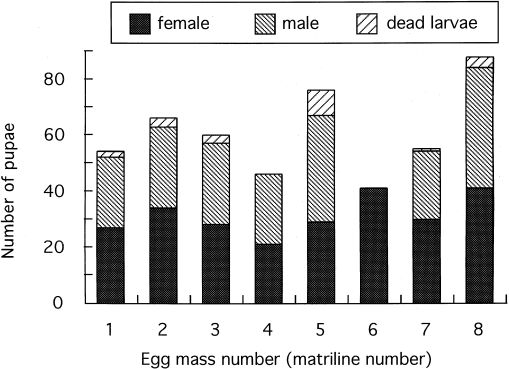
the sex ratio was 1:1 (Manly, 1997). RESULTS All 41 reared larvae from one of the eight egg masses became female pupae (Fig. 1). The sex ratio was significantly different from 1:1 (_P_ = 0).
The sex ratios in the other matriline were not different from 1:1 (_P_ > 0.2). The percentage of eggs hatching in this one egg mass was only 57%, whereas in all others, it exceeded 97%.
Progenies from the abnormal female, designated fe-6, were all females (Table 1). The sex ratios of the progenies were statistically different from 1:1 (_P_ < 0.001). Sib-matings did not
skew the sex ratio (_P_=0.091 in no. 55 and _P_ > 0.5 in all others; Table 1). The sex ratios of outbreeding pairs, except for fe-6, were not different from 1:1 (_P_ > 0.1), although
65% of the progeny of no. 88 became females (_P_=0.013). Six fe-6 non-mated females deposited only infertile eggs. All pairs of mean egg hatch rates were significantly different from each
other among the three types of progenies (ANOVA for unbalanced data using the arcsine transformation: _F_2, 114=146.14, _P_ < 0.0001; SAS Institute, 1990) (Tukey’s studentized range test
using the arcsine transformation: MSE=0.0602, _P_ < 0.05; SAS Institute, 1990) (Fig. 2). The mean egg hatch rate of all-female matrilines (0.475 ± 0.018: mean ± 95% CL) was lower than
that of inbreeding pairs (0.580 ± 0.075) and that of outbreeding pairs, except for fe-6 (0.959 ± 0.018). Egg hatch rates of all-female matrilines were distributed in a narrow range around
0.5 (Fig. 2a). Egg hatch rate of 38 pairs in one generation of brother–sister matings were scattered between 0.117 and 1.00 (Fig. 2c). Only seven sib-matings in five matrilines had more than
80% egg hatching. There were no particular inbreeding lines escaping the depression, because the mean hatch rates were not different among inbreeding lines (ANOVA for unbalanced data using
the arcsine transformation: _F_6, 31=0.61, _P_ > 0.7). Almost all of the outbreeding pairs had more than 90% egg hatch rates (Fig. 2b). Unfertilized reddish eggs were usually less than
10% of total eggs and packed at the end of each individual egg mass, i.e. deposited last. On the other hand, unhatched brown eggs were distributed randomly throughout egg masses of
all-female broods. Dead larvae were fully formed inside unhatched eggs of these broods. Hatching larvae were never observed eating unhatched eggs. Fecundity was also different among the
three types of pairs (ANOVA for unbalanced data: _F_2, 117=6.55, _P_ < 0.002) (Fig. 3). The mean fecundity of the progenies of the abnormal female (601 ± 42 eggs) was the smallest and
significantly different from the other two pairs (Tukey’s studentized range test: MSE=21492.97, _P_ < 0.05). The difference in mean fecundity, however, was not significant between
inbreeding (699 ± 55 eggs) and outbreeding pairs (719 ± 41 eggs) (_P_ > 0.05). After hatching, the viability to adults was 90 ± 3%, 82 ± 15% and 91 ± 4% in all-female matrilines,
inbreeding and outbreeding pairs respectively. The difference was not significant (ANOVA for unbalanced data using the arcsine transformation: _F_2, 34=1.99, _P_ > 0.15) (Table 1). We
collected eggs from 68 out of 82 egg masses in the 0.2 ha birch forest. A trimodal distribution was observed in egg hatch rates in the field population (Fig. 4). The first mode was at zero
hatch rate for the two egg batches containing only infertile eggs. After hatching in June 1997, we collected all eggs of those two egg masses and ascertained that they contained only
infertile eggs. The second mode consisted of eggs of all-female broods according to our rearing results (Table 2). The last mode was probably for normal sex ratio broods, in which two of the
eight broods (nos f13 and f46) had heterogeneous sex ratios and produced an excess of males or females respectively (_P_=0.0184 and _P_=0.00740) (Table 2). Egg masses higher than 4.8 m were
not collected or analysed. But the height distributions were not different among egg masses of unfertilized, all-female broods and normal sex ratio broods below 4.8 m (ANOVA for unbalanced
data: _F_2, 65=0.60, _P_ > 0.5). Therefore, we estimated the frequencies of unfertilized and all-female broods from that egg mass collection, or 2/68=2.9% non-mating females, and
6/66=9.1% abnormal females in this field population. The egg mass sizes were not different among the three types of broods in the field (ANOVA for unbalanced data: _F_2, 65=0.12, _P >_
0.8). DISCUSSION ABNORMAL FEMALES PRODUCING ONLY FEMALE PROGENY The Hokkaido population of _Lymantria_ _dispar_ is referred to as a ‘very weak’ sex-race (Goldschmidt, 1934, 1940). Therefore,
it is possible to produce all-female broods by the transport of ‘strong females’, which live in Aomori Prefecture adjacent to Hokkaido, according to the theory of Goldschmidt (1934). But he
never observed females by sex reversal, because such females rarely survived. If we had collected such an all-female brood in one of the eight egg masses, surviving females would have been
normal genetic females, and both males and females would have appeared at a normal sex ratio. The entire progeny reared from the abnormal female, however, produced only female adults,
irrespective of the mating male. Six fe-6 non-mated females deposited only infertile eggs, suggesting that the phenomenon is not parthenogenesis. Egg hatch rates of abnormal females were
distributed in a narrow range around 0.5. In our study, a maternally inherited cytoplasmic factor differentially killed male embryos. Abnormal females producing only female progeny are found
in many insect orders. To the 12 recorded species of Lepidoptera (Ishihara, 1994), _L. dispar_ should now be added. Although a causative agent has not actually been identified in any of
these lepidopteran species, Jiggins et al. (1998) first found evidence of a male-killing bacterium as a causative agent of female-biased sex ratios in _Acraea_ _encedon_, indicating ‘cure’
of the trait by antibiotic treatment. Further studies are needed to identify the causative agent of all-female broods in _L. dispar_. All-female broods of _L. dispar_ have not been detected
in North America (Myers et al., 1998). In the next generation, the percentage of all-female broods was 9.1% in the same birch forest, because six out of 66 egg masses produced only female
progeny. The egg hatch rates were more than 65% in normal sex ratio broods in our field collection. Therefore, the frequency of all-female broods can be estimated by counting the frequency
of egg batches whose egg hatch rates are between 30% and 60%. The mean fecundity of all-female matrilines was significantly lower under laboratory conditions, but not lower in the field,
than that of outbreeding normal sex ratio broods. To persist in a population, all-female broods need to produce more females than normal sex ratio broods. This condition leads the population
to contain too many females and into extinction without dispersing all female broods widely or recovering to normal sex ratio (Ishihara, 1992, 1994). Recovery was not observed in _L.
dispar_. We need further studies to reveal the mechanisms of persistence of this condition in the field population. INBREEDING DEPRESSION Severe inbreeding depression was found in egg hatch
rates under laboratory conditions in _L. dispar_. The extent of the depression varied widely irrespective of line. Also, some broods escaped this depression. But fecundity and viability
after hatching were not different between inbreeding and outbreeding pairs. Saccheri et al. (1996) have also pointed out and discussed that the inbreeding depression in fecundity is weaker
than that in hatch rate. There were no inbreeding broods in the field population. Therefore, the inbreeding coefficient (_F_) was thought to be zero in the broods of pairs from different
original egg masses. According to Ralls et al. (1988), we can estimate the cost of inbreeding (_i_) in a single generation of brother–sister matings (the inbreeding coefficient, _F_=0.25)
from egg hatch rate as follows: where IN and OUT stand for the mean egg hatch rate of inbreeding (_F_=0.25) and outbreeding (_F_=0) pairs respectively. The mean hatch rates were 0.580 (38
egg masses) and 0.959 (48 egg masses) for inbreeding and outbreeding pairs respectively. Therefore, the cost of inbreeding from egg hatch rate is estimated at 0.395 (95% confidence interval
of 0.304–0.483). The cost of inbreeding at _F_=0.25 may also be represented as follows (Ralls et al., 1988): where _B_ is a measure of the rate at which survival decreases with increased
inbreeding. Then _B_ may be estimated using _i_, if the Morton et al. (1956) model is applied. and _B_ for that population of _L. dispar_ is 2.01. Thus, _B_ and _i_ are among the largest in
insects investigated hitherto (Table 3). The number of lethal equivalents per zygote or individual is usually twice the value of _B_ (Morton et al., 1956; Ralls et al., 1988; but see
Dobzhansky et al., 1963). Thus, each individual _L. dispar_ in that population is estimated to have the equivalent of four lethal recessive genes in heterozygous conditions. Recently,
inbreeding depression has been found substantially to affect the fitness components, such as egg-hatching rate, in natural populations (Keller, 1998; Saccheri et al., 1998). But the effect
of inbreeding has been investigated only in subdivided populations and in limited genera. The estimation of the cost of inbreeding is not painstaking and reveals the variation in the
magnitude of genetic load in natural populations (Keller, 1998; Saccheri et al., 1998). In comparison with other insect orders, Table 3 shows that inbreeding depression is generally severe
in Lepidoptera. In _Acraea encedon_, however, the egg-hatching rates of sib-matings were not lower than those of normal matrilines (Jiggins et al., 1998). After the removal of lethal genes,
fitness recovered rapidly in bottlenecked populations (Saccheri et al., 1996). The population of _L. dispar_ in North America was founded by limited numbers of individuals (Liebhold et al.,
1989). However, Rossiter (1987) found some genetic variation in the population. The genetic load of such bottlenecked populations is most interesting. ADVANTAGE OF KILLING MALES Although the
evidence is scarce, inbreeding avoidance by their host has been cited as one of the advantages of a male-killing factor (Werren, 1987). Hurst et al. (1997) stated that the prerequisite
condition for this advantage to occur was some level of inbreeding and suffering some level of inbreeding depression. However, there are only a few experiments showing inbreeding depression
in species with a male-killing factor (Hurst & Majerus, 1993; Hurst et al., 1997). A second advantage may be that, by killing males, increased resources become available to sibling
females. This advantage is easily applicable to insects living on limited resources, such as hymenopteran endoparasitoids or bark beetles (Scolytidae) (Hurst & Majerus, 1993).
Furthermore, dead male eggs provide added resources through egg cannibalism to their sisters bearing clonal cytoplasmic factors; this is known in Coccinellidae and the butterfly _Acraea
encedon_ (Hurst et al., 1997; Jiggins et al., 1998). These two advantages are in early male-killing types, in which cytoplasmic factors kill males either during embryogenesis or during the
first instar (Hurst, 1991). The third advantage is that the microbe from dead males is transferred horizontally and in late male-killing, in which the factor kills males during the fourth
instar or later (Hurst, 1991). In _L. dispar_, male death is probably in developed larvae in egg shells. Therefore, the former two advantages are relevant. However, we conclude that, for _L.
dispar_, these hypotheses are not applicable because hatching larvae of _L. dispar_ disperse widely and then feed among larvae from many other broods. A small percentage of females were
unable to mate with males and deposited only infertile eggs in the field. Therefore, the males were not in excess in the population. Hurst et al. (1996) estimated the rate of inbreeding in
the field and pointed out that inbreeding avoidance is not advantageous in _Adalia bipunctata_ because of the rare occurrence of inbreeding. Evidence of inbreeding was also not found in the
field in _L. dispar_. Moreover, resource advantage may not be adopted in male-killing in _L. dispar_, as we have never seen larvae feeding on unhatched eggs. A new hypothesis may be needed
to explain the evolutionary advantage of male-killing in _L. dispar_. REFERENCES * Bryant, E. H., Mccommas, S. A. and Combs, L. M. (1986). The effect of an experimental bottleneck upon
quantitative genetic variation in the housefly. _Genetics_, 114: 1191–1211. CAS PubMed PubMed Central Google Scholar * Di Mare, R. A. and Araújo, A. M. (1986). A first survey of
inbreeding effects in _Heliconius erato phyllis_ (Lepidoptera; Nymphalidae). _Rev Brasil Genet_, 9: 11–20. Google Scholar * Dobzhansky, T., Spassky, B. and Tidwell, T. (1963). Genetics of
natural populations. XXXII. Inbreeding and the mutational and balanced genetic loads in natural populations of _Drosophila pseudoobscura_. _Genetics_, 48: 361–373. CAS PubMed PubMed
Central Google Scholar * Fernández, A., Toro, M. A. and López-Fanjul, C. (1995). The effect of inbreeding on the redistribution of genetic variance of fecundity and viability in _Tribolium
castaneum_. _Heredity_, 75: 376–381. Article Google Scholar * García, N., López-Fanjul, C. and García-Dorado, A. (1994). The genetics of viability in _Drosophila melanogaster_: effects of
inbreeding and artificial selection. _Evolution_, 48: 1277–1285. PubMed Google Scholar * Goldschmidt, R. (1934). Lymantria. _Bibl Genet_, 11: 1–185. Google Scholar * Goldschmidt, R.
(1940) _The Material Basis of Evolution_. Yale University Press, New Haven, CT (reprinted by Pageant, New Jersey,1960). Google Scholar * Haag, K. L. and Araújo, A. M. (1994). Inbreeding,
genetic load and morphometric variation in natural populations of _Dryas iulia_ (Lepidoptera, Nymphalidae). _Rev Brasil Genet_, 17: 35–39. Google Scholar * Higashiura, Y. and Kamijo, K.
(1978). Mortality factors during the declining phase of a gypsy moth outbreak in a larch plantation in Hokkaido, Japan. _Bull Hokkaido For Exp Stn_, 15: 9–16. Google Scholar * Hurst, G. D.
D., Hurst, L. D. and Majerus, M. E. N. (1997). Cytoplasmic sex-ratio distorters. In: O’Neill, S. L. Hoffman, A. A. and Werren, J. H. (eds) _Influential Passengers: Inherited Microorganisms
and Arthropod Reproduction_. pp. 125–154. Oxford University Press, Oxford. Google Scholar * Hurst, G. D. D. and Majerus, M. E. N. (1993). Why do maternally inherited microorganisms kill
males? _Heredity_, 71: 81–95. Article Google Scholar * Hurst, G. D. D., Sloggett, J. J. and Majerus, M. E. N. (1996). Estimation of the rate of inbreeding in a natural population of
_Adalia bipunctata_ (Coleoptera: Coccinellidae) using a phenotypic indicator. _Eur J Entomol_, 93: 145–150. Google Scholar * Hurst, L. D. (1991). The incidences and evolution of cytoplasmic
male killers. _Proc R Soc B_, 244: 91–99. Article Google Scholar * Hurst, L. D. and Pomiankowski, A. (1991). Causes of sex ratio bias may account for unisexual sterility in hybrids: a new
explanation of Haldane’s rule and related phenomena. _Genetics_, 128: 841–858. CAS PubMed PubMed Central Google Scholar * Ishihara, M. (1992). Persistence of females that produce only
female progeny in Lepidoptera. _Res Popul Ecol_, 34: 331–347. Article Google Scholar * Ishihara, M. (1994). Persistence of abnormal females that produce only female progeny with occasional
recovery to normal females in Lepidoptera. _Res Popul Ecol_, 36: 261–269. Article Google Scholar * Jiggins, F. M., Hurst, G. D. D. and Majerus, M. E. N. (1998). Sex ratio distortion in
_Acraea encedon_ (Lepidoptera: Nymphalidae) is caused by a male-killing bacterium. _Heredity_, 81: 87–91. Article Google Scholar * Keller, L. F. (1998). Inbreeding and its fitness effects
in an insular population of song sparrows (_Melospiza melodia_). _Evolution_, 52: 240–250. PubMed Google Scholar * Liebhold, A., Mastro, V. and Schaefer, P. W. (1989). Learning from the
legacy of Léopold Trouvelot. _Bull Entomol Soc Am_, 35: 20–22. Google Scholar * Manly, B. F. J. (1997) _Randomization Bootstrap and Monte Carlo Methods in Biology_, 2nd edn. Chapman &
Hall, London. Google Scholar * Morton, N. E., Crow, J. F. and Muller, H. J. (1956). An estimate of the mutational damage in man from data on consanguineous marriages. _Proc Natl Acad Sci
USA_, 42: 855–863. Article CAS PubMed PubMed Central Google Scholar * Myers, J. H., Boettner, G. and Elkinton, J. (1998). Maternal effects in gypsy moth: only sex ratio varies with
population density. _Ecology_, 79: 305–314. Article Google Scholar * Pray, L. A. and Goodnight, C. J. (1995). Genetic variation in inbreeding depression in the red flour beetle _Tribolium
castaneum_. _Evolution_, 49: 176–188. Article PubMed Google Scholar * Ralls, K., Ballou, J. D. and Templeton, A. (1988). Estimates of lethal equivalents and the cost of inbreeding in
mammals. _Conserv Biol_, 2: 185–193. Article Google Scholar * Roff, D. A. (1998). Effects of inbreeding on morphological and life history traits of the sand cricket, _Gryllus firmus_.
_Heredity_, 81: 28–37. Article Google Scholar * Rossiter, M. C. (1987). Genetic and phenotypic variation in diet breadth in a generalist herbivore. _Evol Ecol_, 1: 272–282. Article Google
Scholar * Saccheri, I. J., Brakefield, P. M. and Nichols, R. A. (1996). Severe inbreeding depression and rapid fitness rebound in the butterfly _Bicyclus anynana_ (Satyridae). _Evolution_,
50: 2000–2013. Article PubMed Google Scholar * Saccheri, I., Kuussaari, M., Kankare, M., Vikman, P., Fortelius, W. and Hanski, I. (1998). Inbreeding and extinction in a butterfly
metapopulation. _Nature_, 392: 491–494. Article CAS Google Scholar * SAS INSTITUTE. (1990) _SAS/STAT User’s Guide_ _Version 6_ 4th edn. SAS Institute Inc., Cary, NC. * Werren, J. H.
(1987). The coevolution of autosomal and cytoplasmic sex ratio factors. _J Theor Biol_, 124: 317–334. Article Google Scholar Download references ACKNOWLEDGEMENTS We thank Sandy Liebhold,
without whose request to rear _L. dispar_ larvae for a pupal predation study, this research would not have begun. We are very grateful to Michiko, Kyousuke and Takuro Higashiura for their
help in rearing larvae. We are also grateful to Kihachiro Kikuzawa for his review of an early draft. We thank anonymous referees for their helpful comments to improve this manuscript. AUTHOR
INFORMATION Author notes * Yasutomo Higashiura: After October 1999: Hokkaido Forestry Research Institute, Koshunai, Bibai, Hokkaido 079-0198, Japan, E-mail: [email protected] AUTHORS AND
AFFILIATIONS * Hokkaido Forestry Research Institute, Bibai, Hokkaido, 079-0198, Japan Yasutomo Higashiura * Biological Laboratory, Hyogo College of Medicine, Nishinomiya, Hyogo, 663-8501,
Japan Michio Ishihara * USDA, Agricultural Research Service, Beneficial Insects Introduction Research Laboratory, 501 South Chapel Street, Newark, DE 19713-3814, USA Paul W Schaefer Authors
* Yasutomo Higashiura View author publications You can also search for this author inPubMed Google Scholar * Michio Ishihara View author publications You can also search for this author
inPubMed Google Scholar * Paul W Schaefer View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Yasutomo Higashiura.
RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Higashiura, Y., Ishihara, M. & Schaefer, P. Sex ratio distortion and severe inbreeding depression in
the gypsy moth _Lymantria dispar_ L. in Hokkaido, Japan. _Heredity_ 83, 290–297 (1999). https://doi.org/10.1038/sj.hdy.6885590 Download citation * Received: 15 December 1998 * Accepted: 17
April 1999 * Published: 01 September 1999 * Issue Date: 01 September 1999 * DOI: https://doi.org/10.1038/sj.hdy.6885590 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative KEYWORDS * cost of inbreeding * inbreeding depression * Lepidoptera * _Lymantria dispar_ * male-killing * sex ratio