Play all audios:
ABSTRACT AIM: To investigate the effect of the axon guidance cue Slit2 on the density of blood vessels and permeability of the blood-brain barrier in mouse brain. METHODS: hSlit2 transgenic
mouse line was constructed, and the phenotypes of the mice were compared with wild-type mice in respect to the lateral ventricle (LV), ventricle pressure, and the choroids plexus. An _in
vivo_ Miles permeability assay and an amyloid-β permeability assay were used to assess the permeability of brain blood vessels. Brain vessel casting and intracerebral hemorrhage models were
built to investigate vessel density in the transgenic mice. An _in vitro_ permeability assay was used to test whether Slit2 could change the permeability and tight junctions of blood vessel
endothelial cells. RESULTS: Hydrocephalus occurred in some transgenic mice, and a significantly larger lateral ventricle area and significantly higher ventricle pressure were observed in the
transgenic mice. The transgenic mice displayed changed construction of the choroids plexus, which had more micro vessels, dilated vessels, gaps between epithelial cells and endothelial
cells than wild-type mice. Slit2 significantly increased brain vessel density and the permeability of brain vessels to large molecules. These blood vessels were more sensitive to cues that
induce brain hemorrhage. At the cellular level, Slit2 disturbed the integrity of tight junctions in blood vessel endothelial cells and improved the permeability of the endothelial cell
layer. Thus, it promoted the entry of amyloid-β peptides from the serum into the central nervous system, where they bound to neurons. CONCLUSION: Slit2 increases vessel density and
permeability in the brains of transgenic mice. Thus, Slit2 induces numerous changes in brain vessels and the barrier system. SIMILAR CONTENT BEING VIEWED BY OTHERS PS1 FAD MUTANTS DECREASE
EPHRINB2-REGULATED ANGIOGENIC FUNCTIONS, ISCHEMIA-INDUCED BRAIN NEOVASCULARIZATION AND NEURONAL SURVIVAL Article 15 June 2020 ENDOLYSOSOMAL DYSFUNCTION IN RADIAL GLIA PROGENITOR CELLS LEADS
TO DEFECTIVE CEREBRAL ANGIOGENESIS AND COMPROMISED BLOOD-BRAIN BARRIER INTEGRITY Article Open access 17 September 2024 VASCULAR ENDOTHELIUM DEPLOYS CAVEOLIN-1 TO REGULATE OLIGODENDROGENESIS
AFTER CHRONIC CEREBRAL ISCHEMIA IN MICE Article Open access 10 November 2022 INTRODUCTION Vasogenic brain edema, which is defined as the translocation of proteins and fluid from the vascular
space across the blood-brain barrier (BBB)1, is a major life-threatening complication of various injuries to the central nervous system (CNS)2, 3. Endothelial cells of the brain vasculature
form the BBB and maintain the homeostasis of the central nervous system (CNS). Pathological conditions such as brain tumors and head injuries increase the permeability of the brain
microvasculature and destroy the BBB4. Vascular endothelial growth factor (VEGF) is well known as the major inducer of angiogenesis, and it also increases the permeability of the
microvasculature and stimulates endothelial cell growth5, 6, 7, 8. Increased VEGF expression may cause vascular leakage in the CNS _in vivo_9. However, the underlying molecular and
pathogenic mechanisms behind edema and blood-brain vessel leakage are poorly understood. The Slit family of guidance cues interacts with the Roundabout (Robo) family of transmembrane
receptors in physiological and pathological processes requiring cell migration10, 11, 12, 13. During development of the nervous system, Slit-Robo signaling regulates the repulsion or
attraction of projecting axons and migrating neurons14, 15. Vascular endothelial cells secrete Slit2, which binds to Robo1 on leukocytes and acts as an endogenous inhibitor of leukocyte
chemotaxis16, 17, 18, 19, 20, 21. Additionally, Slit2 mediates directional migration of malignant cells22, 23, 24. We and others have previously reported that Slit proteins secreted by solid
tumors bind to Robo1, which is expressed on vascular and lymphatic endothelial cells, to stimulate angiogenesis and lymph angiogenesis25, 26, 27, 28, 29, 30, 31. Slit2 is expressed in the
CNS while Robo1 is expressed in blood vessel endothelial cells. However, whether expression of these proteins can change the permeability of blood vessels and whether abnormal expression can
induce vessel leakage and edema remain to be determined. In this study, we constructed a transgenic mouse line that over-expresses human Slit2 and observed that hydrocephalous occurs in
some of these transgenic mice. The transgenic mice also had larger lateral ventricles and higher ventricle pressure than wild-type mice. Comparison of the choroids plexus, where
cerebrospinal fluid (CSF) is secreted, revealed that there was a change in the construction of the choroids plexus, with the transgenic mice having more microvessels, dilated vessels, gaps
between epithelial cells and endothelial cells. We also found that Slit2 could improve brain vessel density and promote the permeability of brain vessels to large molecules. These blood
vessels were also more sensitive to cues that induced brain hemorrhage. At the cellular level, Slit2 disturbed the integrity of tight junctions in blood vessel endothelial cells and
increased the permeability of the endothelial cell layer. The ability of Slit2 to increase the permeability of the BBB resulted in an increase in the transfer of amyloid-β peptides from the
serum to the CNS, where they bound to neurons. MATERIAL AND METHODS GENERATION OF HSLIT2 TRANSGENIC MICE AND DETECTION OF HSLIT2 OVER-EXPRESSION hSlit2 transgenic mice were generated
according to standard procedures. The transgene was constructed by cloning cDNA encoding full-length human Slit2 between the _Bam_H I and _Xbo_ I restriction sites of the MCS (multi clone
site) of pCEP4F. Genotypes were confirmed by Southern blot and PCR analysis. PCR screening of hSlit2 heterozygotes was performed on standard tail genomic DNA preparations using a pair of
primers specific for human Slit2 cDNA (forward: 5′-GGTGACGGATCCCATATCGCGGTAGAACTC-3′; reverse: 5′-GGACACCTCGAGCGTACAGCCGCACTTCAC-3′). PCR cycles were as follows: 95 °C, 4 min (1 cycle); 94
°C, 45 s; 55 °C, 45 s; and 72 °C, 1 min (63 cycles); and 72 °C, 10 min (2 cycles). PCR products were analyzed on 1% agarose gels. Slit2 homozygosity was confirmed by genetic methods based on
the principle that the progeny of Slit2 homozygotes mated to wild-type C57 mice should all be heterozygotes. The brains from C57 control littermate mice and hSlit2 transgenic mice from
founder #9 were snap frozen in liquid N2 and pulverized. The brain powder was homogenized in 1 mL RIPA lysis buffer [50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5%
deoxycholid acid, 0.1% sodium dodecyl sulfate (SDS), 5 mmol/L EDTA, 2 mmol/L phenylmethylsulfonyl fluoride (PMSF), 20 μg/mL aprotinin, 20 μg/mL leupeptin, 10 μg/mL pepstatin A, and 150
mmol/L benzamidine] in a Dounce tissue homogenizer. After homogenization, the samples were centrifuged at 12 000×_g_ for 10 min to remove tissue debris and boiled in SDS sample buffer for 5
min. They were then subjected to 7% SDS-PAGE electrophoresis, transferred to blotting membranes, probed with 1 μg/mL anti-Slit2 monoclonal antibody (5A5) and detected with the horseradish
peroxidase-conjugated goat anti-mIgG Ab using a chemiluminescent detection system. COMPARISON OF THE LV AREA AND THE CHOROID PLEXUS The mouse brains were fixed in 4% PFA (Sigma) and cut into
consecutive longitudinal sections. A photo was taken of every longitudinal sections from each brain with an Olympus MVX10. Then the area of the lateral ventricle was calculated by Image
Tool 3 (UTHSCSA), and the average area of consecutive sections from each mouse was calculated. The data represent the means for groups of six mice. For histological examination of the
choroid plexus, brains were isolated from age-matched C57 and Slit2 adult mice, cut into small coronal blocks, fixed in 4% formaldehyde, embedded in paraffin, and cut into 0.5-mm sections
following standard procedures. Tissue sections were counterstained with hematoxylin. The same regions of the choroid plexus in all mouse brains were photographed. ELECTRON MICROSCOPY The
choroid plexuses used for TEM were fixed in 2% glutaraldehyde and 1% sucrose in 0.1 mol/L cacodylate buffer for 3 h. The samples were washed in 0.1 mol/L cacodylate buffer and then
post-fixed in 1% osmium tetraoxide in 0.1 mol/L cacodylate buffer for 2 h, all at pH 7.0 and 25 °C. The choroid plexuses were embedded in epon after dehydration in a graded series of
ethanol. Epon was polymerized at 60 °C for 48 h. Serial sections of 80 nm were cut on a Leica Super Nova ultramicrotome with a diamond knife and collected on formvar-coated nickel grids.
Sections were contrasted with uranylacetate and stained with 1% toluidine blue. The sample grids were observed using a Hitachi 600 TEM. _IN VIVO_ MILES PERMEABILITY ASSAY AND AMYLOID-Β
PERMEABILITY ASSAY To investigate the effects of over-expression of hSlit2 in the brain on vascular permeability, a Miles assay was performed. Mice received an iv injection of sterile 0.5%
Evans blue dye (200 μL) via the tail vein. Mice were killed 20 min after the injections by cervical dislocation after anesthesia. The brains (weighted) were cut into small pieces and
incubated in 500 mL of formamide at 37 °C for 48 h to extract the Evans blue dye. The absorbance of the extracts was read at 630 nm in a spectrophotometer (Beckman DU 640). For the amyloid-β
permeability assay, mice received an iv injection of 6.9 μmol/L FITC-Aβ (100 μL) via the tail vein. Mice were killed 48 h after the injections by cervical dislocation after anesthesia. The
brains of the mice were prepared on crystal slides, and the nuclei were stained with DAPI. LV PRESSURE ASSAY Mice (12 weeks of age) were anesthetized by ip injection of sodium pentobarbital
(70 mg/kg). A small hole 0.5 mm posterior and 1.0 mm lateral of the bregma was drilled to perforate the skull. A pressure transducer linked to a monitor was injected into the brain at a
depth of 2.3 mm unilaterally. Then the LV pressure was read on a computer that was linked to the monitor (Powerlab 4/30). The pressure was measured as mmH2O. IMMUNOHISTOCHEMICAL STAINING
Antibodies against vWF (Antibody Diagnostica Inc; a 1:200 dilution for paraffin-embedding), Slit2 (5 μg/mL for paraffin-embedding sections), and Robo1 (20 μg/mL for paraffin-embedding
sections) were used for immunohistochemical staining as described previously. BRAIN VESSEL CASTING After systemic heparinization with 750 IU/kg intravenous heparin, the common carotid
arteries were cannulated and perfused with approximately 100 mL of 27 °C saline, followed by a 2.5% buffered glutaraldehyde solution (Sigma) at pH 7.4. The casts were made by perfusion of
the arteries with 100 mL of Mercox (SPI, West Chester, PA, USA) diluted with 20% methyl methacrylate monomers (Aldrich Chemical, Milwaukee, WI, USA). After complete polymerization, the
brains were harvested and macerated in 5% potassium hydroxide, followed by drying and mounting for scanning electron microscopy. The microvascular corrosion casts were imaged after being
coated with gold with a Hitachi S-450 scanning electron microscope. INTRACEREBRAL HEMORRHAGE (ICH) MODEL Mice (12 weeks of age) were prepared for surgery and anesthetized by ip injection of
sodium pentobarbital (70 mg/kg)32. A small hole 1.0 mm posterior and 3.0 mm lateral of the bregma was drilled to perforate the skull. A 1-μL Hamilton syringe was used to deliver 500 nL of
collagenase/saline (150 U/μL) to the caudate/putamen at a depth of 4.0 mm unilaterally. After the injection of collagenase/saline (∼30 s), the needle remained in place for another 2 min to
prevent reflux of fluid. Then the scalp skin was closed using 4.0 nylon sutures. Twenty-four hours later, the mice were perfused with PBS, the brains were harvested, and 14-μm sections were
prepared using a cryostat and mounted on glass slides. An Olympus BX100 upright systems microscope with a digital camera was used to capture images. The hemorrhage volume was measured using
the Stereologer software system. _IN VITRO_ PERMEABILITY ASSAY We coated transwell inserts (Corning, 48-well, 3-mm pore) with collagen and seeded HUVEC cells at a density of 30 000 cells per
well. Cells were then cultured for another 24 h. One hour later, Slit2, VEGF, R5, and FITC-DEXTRAN (25 mg/mL, Sigma) were added to the top of the inserts. The absorbance of the solution in
each well was measured at 492 nm (_n_=6 wells each). STATISTICAL ANALYSIS Statistical significance was determined by Student's _t_-test. _P_-values of 0.05 and 0.01 were considered
statistically significant and very significant, respectively. RESULTS GENERATION OF HSLIT2 TRANSGENIC MICE AND OVER-EXPRESSION OF HSLIT2 IN MOUSE BRAINS To study the function of Slit2 in the
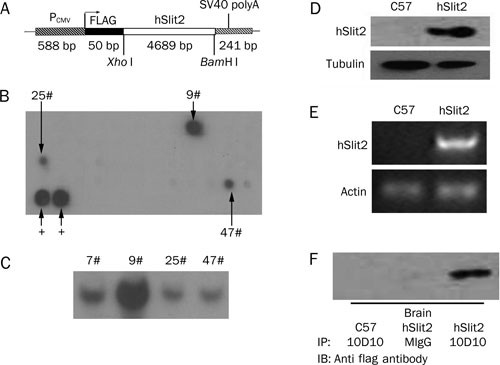
whole blood vessel system, we constructed a Slit2 over-expressing mouse transgenic plasmid, for which a schematic display is shown in Figure 1A. The full-length human Slit2 cDNA, which is
4689 bases, was inserted between the _Bam_H I/_Xbo_ I restriction sites of the MCS of the pCEP4F vector, which has a pCMV promoter and an N-terminal flag-tag. The plasmid was linearized with
restriction enzymes, injected into the pronuclei of fertilized C57×CBA F1 oocytes and transplanted into the mother mouse. The offspring mice were analyzed by dot blot (Figure 1B). Mouse
lines 9, 25, and 47 were shown to express human Slit2. The presence of the human Slit2 transgene was also confirmed by Southern blot analysis (Figure 1C). Among the three transgenic strains,
we found that strain 9 had the strongest signal in dot blot and Southern blot assays, so we focused our research on strain 9. We observed that the transgenic mice expressed more Slit2 in
the brain compared with C57 mice at the transcriptional and translational levels (Figure 1D, 1E). We used the Slit2 antibody 10D10 to immune-precipitate the mouse brain lysate and detected
the precipitate with anti-flag antibody, and we found that flag-Slit2 was expressed in the transgenic mouse brain (Figure 1F). THE PHENOTYPE OF SLIT2 MICE AND COMPARISON OF THE LV AREA Most
of the transgenic mice initially appeared normal (Figure 2A), but about 5% of the transgenic mice had an intumescent head (Figure 2B). This phenotype appeared at the age of 4–6 weeks in all
three of the transgenic mouse lines, line 9, line 25, and line 47. Mice with an intumescent head died within 2 weeks, at the age of 6–8 weeks. Anatomical analysis of these mice revealed high
levels of encephalopathic edema. In mice where this brain phenotype was observed, the brain edema was serious and the brain tissue was destroyed to such a degree that the structure of the
brain could not be recognized. Thus, we focused our research on the transgenic mice that did not have this phenotype. Brain edema is linked to the CSF system, including the production,
circulation and absorption of CSF. We therefore examined brain structures related to the CSF system. In the brain, CSF is produced in the lateral ventricle, so we compared the lateral
ventricle of transgenic mice and non-transgenic mice. We found that the lateral ventricle area was larger in the brains of transgenic mice (Figure 2C, 2D) than in control mice (Figure 2E,
2F). For this study, the mice were all 8 weeks old, and the same result was seen in male and female mice. Figure 2G shows a schematic display of the mouse brain. The arrow indicates the
lateral ventricle. We calculated the entire area of the LV in slides created from the brains of each mouse with Image Tool 3 (UTHSCSA) and found that the LV area was larger in transgenic
mice (_n_=6, _P_<0.01) (Figure 2H). LV pressure was also detected in the lateral ventricles of the mouse brains. In transgenic mice, the pressure was 128 mmH2O, and in control mice, it
was 81 mmH2O (Figure 2I) (_n_=7, _P_<0.01). Thus, the LV pressure was higher in the brains of transgenic mice compared with control mice. CHANGES IN THE STRUCTURE AND FUNCTION OF THE
CHOROID PLEXUS In mouse brains, the choroid plexus produces CSF. The structure of the choroids plexus was obviously different between transgenic and control mice. In control mice, it was a
tight, mono-cell layer covered with microvessels (Figure 3A); however, in transgenic mice, there were gaps between the epithelial cell layer and the vessels (Figure 3B, 3C), the vessels were
enlarged and had gaps, and the epithelial cells were crenated (Figure 3C). The mice used in these experiments were all 10 weeks old. Examination under an electron microscope revealed gaps
between the epithelial tight junctions in transgenic mice (Figure 3E); such gaps were not observed in control mice (Figure 3D). ZO-1 is an important component of cell-cell tight junctions
and can be used as a marker for the completeness and density of tight junctions. We used fluorescence immunostaining to detect ZO-1 in the choroid plexus. In control mice, ZO-1 had an equal
and continuous distribution (Figure 3F), while in transgenic mice, the distribution of ZO-1 was disturbed (Figure 3G) and the tight junctions were destroyed. MORE VESSELS ARE PRESENT IN THE
CHOROID PLEXUS AND THE BRAIN OF TRANSGENIC MICE Staining of slides of the brain cortex with anti-CD31 antibody showed that more micro-blood vessels were present in the brains of hSlit2
transgenic mice (Figure 4B) compared with C57 mice (Figure 4A). The gray density was 1.7% in C57 mice and 4.6% in hSlit2 transgenic mice (_P_<0.01, Figure 4C). We also stained the brain
cortex slides with anti-vWF antibody and found that hSlit2 transgenic mice had an increased number of vessels (Figure 4E) compared with C57 mice (Figure 4D). Calculating the numbers of
vessels present on the slides revealed that C57 mice had about 200 micro-vessels/mm2, while hSlit2 transgenic mice had about 450 micro-vessels/mm2 (Figure 4F), and most of these brain
micro-vessels consisted of only one endothelial cell. Furthermore, we performed brain vessel casting, which also showed that hSlit2 transgenic mice had more brain vessels (Figure 4H)
compared with C57 mice (Figure 4G). SLIT2 CHANGES THE PERMEABILITY OF CELL-CELL ADHESIONS To evaluate whether Slit2 improves the permeability of blood vessels, we performed a Miles assay on
hSlit2 transgenic mice. An increased amount of Evans blue dye was detected in the brain tissue of transgenic mice compared with C57 mice (Figure 5A), which indicates that the blood vessels
in the brains of transgenic mice are more permeable, allowing the entry of Evans blue dye into the tissue. Previous research has indicated that hyperplastic vessels are incomplete, more
permeable, and more sensitive to destructive mechanism compared with normal vessels. Thus, we induced intracerebral hemorrhaging in the transgenic mice. This was accomplished by injecting
collagenase into the mouse brains, which destroys blood vessels in the brain and causes intracerebral hemorrhaging. Examination of brain slides from mice in which intracerebral hemorrhaging
was induced showed that transgenic mice have a larger hemorrhage area compared with the control mice (Figure 5C, 5D). Next, we measured the hemorrhage volume using the Stereologer software
system and found that the hemorrhage volume also was increased in the transgenic mice (Figure 5B). These results indicate that the blood vessels in the brains of the transgenic mice are more
permeable and much more sensitive to collagenase, which destroys the vessel structure. Thus, Slit2 may not only promote angiogenesis but could also increase the permeability of blood vessel
endothelial cells, which are the most important component of blood vessels. VE-cadherin is a member of the cadherin family, which is expressed specifically in endothelial cells and plays
important roles in endothelial cell cell-cell adhesion. Immunofluorescent detection of HUVECs using an anti-VE-Cadherin antibody revealed that, without incubation with Slit2, VE-Cadherin
expression was equal, continuous and mostly localized to the conjunction sites of cell-cell adhesions (Figure 5E), while HUVECs incubated with the Slit2 protein had a disturbed distribution
and lacked continuous expression of VE-Cadherin (Figure 5F). To verify that Slit2 can affect the permeability of blood vessels, we performed an _in vitro_ permeability assay. We coated
transwell inserts (Corning, 48-well, 3-mm pore) with collagen and seeded HUVECs at a density of 30 000 cells per well. Once these cells formed a monolayer, we added Slit2 protein and other
stimulating factors to the upper well. FITC-dextran was added 1 h later, and fluorescence was detected in the bottom wells. Our results show that VEGF-A can improve the permeability of
HUVECs and Slit2 by 1.5 fold and 2 fold, respectively, and that a blocking antibody against the Slit2-Robo signal, R5, can block the effect of Slit2 on cell permeability, returning it to
basal levels (Figure 5G). THE PRESENCE OF AMYLOID-Β PEPTIDE IN THE BRAINS OF SLIT2 MICE Amyloid-β 40 peptides were detected on slides of the transgenic mouse brains (Figure 6A) but not
slides of C57 mouse brains (Figure 6D). In the cortex and hippocampus, amyloid-β 42 was detected in granual and pyramid cells in transgenic mice (Figure 6B, 6C) but not in C57 mice of the
same age (Figure 6E, 6F). Furthermore, we injected FITC-Amyloid-β40 into the circulation by ip to see if the circulating peptides could enter the CNS. Florescence was observed on brain
cortex slides prepared from the transgenic mice 1 h (Figure 6G), 24 h (Figure 6H), and 48 h (Figure 6I) after injection. The florescence was higher with increasing time, but even 48 h after
injection, no florescence was detected on brain cortex slides prepared from C57 mice (Figure 6J, 6K). HSLIT2 TRANSGENIC MICE HAVE NORMAL AQUEDUCT AND SUBARACHNOID SPACE, AND SLIT2
OVER-EXPRESSION DOES NOT ALTER VEGF EXPRESSION LEVELS To determine the course of the enlarged lateral ventricles observed in Slit2 transgenic mice, we compared the aqueduct and the
subarachnoid space of transgenic and C57 mice. However, there were no obvious differences in these two areas between transgenic and C57 mice. The aqueduct was smooth and clear, and the
aqueduct tube had the same inside diameter and did not contain any clogs (Figure 7A–7D). The SAS also had a complete and clear structure with no clogs or signs of collapse in the transgenic
mice (Figure 7E–7H). We also found that Slit2 over-expression did not alter VEGF expression at either the protein or mRNA level (Figure 7I, 7J). These results show that the structures linked
to circulation and absorption of CSF were complete and normal in the transgenic mice and that the enlargement of the lateral ventricle is caused by the abnormal production of CSF in the
choroid plexus. On the other hand, the normal circulation and absorption of CSF could compensate for the abnormal production of cerebrospinal fluid, which could explain why edema was only
observed in a small percentage of the transgenic mice. DISCUSSION The brain barriers, including the blood-brain barrier, the blood-CSF barrier and the ventricular wall, provide a stable
micro-environment for the proper functioning of the central nervous system. At the bases of the barrier structures are the junction structures, such as adherence junctions and tight
junctions between endothelial cells, epithelial cells and pericytes33. These junction structures are dynamic structures that consist of transmembrane proteins, cytoplasmic accessory proteins
and scaffold proteins. Under different physiological and pathological conditions, changes occur in the expression, distribution, modification and interaction of these proteins34. These
changes are regulated by several cell signaling pathways, and to date, the calcium channel pathway, the phosphorylation signaling pathway and the G-protein signaling pathway have been shown
to change the expression and distribution of junction proteins, further affecting the function of barrier structure35. The brain blood vessel system is the structural basis of the
blood-brain barrier, and the junctions between vessel endothelial cells have the most important effect on the permeability of the BBB. Many molecules, such as small chemical molecules,
signaling proteins and inflammatory factors, can change the permeability of blood vessels. VEGF is an important angiogenic cue, and some reports have shown that VEGF improves the
permeability of blood vessels and the BBB. VEGF binds to its receptor on blood vessel endothelial cells and triggers signaling pathways in the cell cytoplasm. This, in turn, alters the
expression, phosphorylation and distribution of VE-cadherin and thus changes the permeability of blood vessels by disturbing the junction structures36. However, barrier structures are very
complex and have many components. Therefore, whether other cues that induce angiogenesis have some effect on the permeability of the blood-brain barrier should be investigated. The guidance
cue Slit2 has been reported to regulate a number of physiological processes, mostly in the central nervous system, by controlling cell migration. Previous research from our lab indicated
that Slit2 promotes tumor angiogenesis in a manner similar to VEGF25. In this paper, we found that Slit2 improved blood vessel density in the brain and promoted the permeability of brain
blood vessels to large molecules. In addition, these blood vessels were more sensitive to cues that can induce brain hemorrhage. At the cellular level, Slit2 disturbed the integrity of blood
vessel endothelial cell tight junctions and improved the permeability of the endothelial cell layer, thus promoting the entry of amyloid-β peptides from the serum into the central nervous
system, where they bind to neurons. We also found that hydrocephalous occurred in some of the hSlit2 transgenic mice. In addition, we observed a larger lateral ventricle area and higher
ventricle pressure in the transgenic mice. A comparison of the choroids plexus, where CSF is secreted, revealed that transgenic mice have changes in the structure of the choroids plexus,
including more micro-vessels, dilated vessels, and gaps between epithelial cells and endothelial cells. Thus, Slit2 could bind to its receptor Robo1 on endothelial cells and affect the
junction through signaling in the cytoplasm. It has been reported that Slit2 modifies the activity of cytoplasmic GTP enzymes, which affect tight junction structures by regulating adherence
proteins and cell scaffold proteins37. However, the signaling pathway by which Slit2 affects cell-cell adhesion structures requires further investigation. Because adherent structures consist
of a large number of proteins, it is unclear which proteins are affected by Slit2. For example, which changes in protein expression or function are the direct results of Slit2 signaling,
and which protein changes are the result of subsequent disturbances of junction structures? These are all important questions that need to be answered to understand the molecular mechanism
by which Slit2 alters the permeability of barriers. In the brains of transgenic mice of 6-week-old, we observed binding of the amyloid-β peptide to neurons. This suggested that Slit2
promotes the entry of amyloid-β peptides from the serum into the central nervous system, where they then bind to neurons. The binding of amyloid-β peptides to neurons is a phenotype of the
early stage of Alzheimer's disease. However, whether Slit2 is over-expressed in patients with Alzheimer's disease and the relationship between Slit2 and Alzheimer's disease
need to be further investigated and will be the focus of future work in our lab. Slit2 improves the permeability of the blood-brain barrier and thus may have some medical application for the
delivery of drugs to the central nervous system, a problem that has puzzled many researchers because drugs targeting the central nervous system often cannot penetrate the blood-brain
barrier at sufficient therapeutic doses38. In our study, we found that Slit2 promotes the entry of a fluorescent-labeled peptide into the central nervous system. This result suggests that
Slit2 may promote the penetration of large-molecule drugs from the peripheral circulation into the central nervous system by increasing the permeability of the blood-brain barrier. Whether
manipulation of Slit2 levels or activity can be applied to the field of central nervous system drug delivery requires further investigation, which we plan on pursuing in the future. AUTHOR
CONTRIBUTION Hai-xiong HAN and Jian-guo GENG designed the research. Hai-xiong HAN performed the research, analyzed the data, and wrote the paper. REFERENCES * Hackett PH . High altitude
cerebral edema and acute mountain sickness: a pathophysiology update. _Adv Exp Med Biol_ 1999; 474: 23–45. Article CAS PubMed Google Scholar * Murakami K, Kondo T, Yang G, Chen SF,
Morita-Fujimura Y, Chan PH . Cold injury in mice: a model to study mechanisms of brain edema and neuronal apoptosis. _Prog Neurobiol_ 1999; 57: 289–99. Article CAS PubMed Google Scholar
* Pilitsis JG, Rengachary SS . Complications of head injury. _Neurol Res_ 2001; 23: 227–36. Article CAS PubMed Google Scholar * Wang W, Dentler WL, Borchardt RT . VEGF increases BMEC
monolayer permeability by affecting occludin expression and tight junction assembly. _Am J Physiol Heart Circ Physiol_ 2001; 280: H434–40. Article CAS PubMed Google Scholar * Ferrara N .
The role of vascular endothelial growth factors in pathological angiogenesis. _Breast Cancer Res Treat_ 1995; 36: 127–37. Article CAS PubMed Google Scholar * Senger DR, Van de Water L,
Brown LF, Nagy JA, Yeo KT, Yeo TK, _et al_. Vascular permeability factor (VPF, VEGF) in tumor biology. _Cancer Metastasis Rev_ 1993; 12: 303–24. Article CAS PubMed Google Scholar *
Stephan CC, Brock TA . Vascular endothelial growth factor, a multifunctional polypeptide. _P R Health Sci J_ 1996; 15: 169–78. CAS PubMed Google Scholar * Thomas KA . Vascular endothelial
growth factor, a potent and selective angiogenic agent. _J Biol Chem_ 1996; 271: 603–6. Article CAS PubMed Google Scholar * Schoch HJ, Fischer S, Marti HH . Hypoxia-induced vascular
endothelial growth factor expression causes vascular leakage in the brain. _Brain_ 2002; 125: 2549–57. Article PubMed Google Scholar * Kidd T, Brose K, Mitchell KJ, Fetter RD,
Tessier-Lavigne M, Goodman CS, _et al_. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. _Cell_ 1998; 92:
205–15. Article CAS PubMed Google Scholar * Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, _et al_. Slit proteins bind Robo receptors and have an evolutionarily conserved
role in repulsive axon guidance. _Cell_ 1999; 96: 795–806. Article CAS PubMed Google Scholar * Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, _et al_. Vertebrate Slit, a secreted ligand
or the transmembrane protein Roundabout, is a repellent or olfactory bulb axons. _Cell_ 1999; 96: 807–18. Article CAS PubMed Google Scholar * Plachez C, Andrews W, Liapi A, Knoell B,
Drescher U, Mankoo B, _et al_. Slit1 and Slit2 cooperate o prevent premature midline crossing of retinal axons in the mouse visual system. _Neuron_ 2002; 33: 219–32. Article Google Scholar
* Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS . Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. _Cell_ 2000; 101: 703–15.
Article CAS PubMed Google Scholar * Dickson BJ, Gilestro GF . Regulation of commissural axon path finding by Slit and its Robo receptors. _Annu Rev Cell Dev Biol_ 2006; 22: 651–75.
Article CAS PubMed Google Scholar * Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, _et al_. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors.
_Nature_ 2001; 410: 948–52. Article CAS PubMed PubMed Central Google Scholar * Guan H, Zu G, Xie Y, Tang H, Jonhson M, Xu X, _et al_. Neuronal repellent Slit2 inhibits dendritic cell
migration and the development of immune responses. _J Immunol_ 2003; 171: 6519–26. Article CAS PubMed Google Scholar * Kanellis J, Garcia GE, Li P, Parra G, Wilson CB, Rao Y, _et al_.
Modulation of inflammation by Slit protein _in vivo_ in experimental crescentic glomerulonephritis. _Am J Pathol_ 2004; 165: 341–52. Article CAS PubMed PubMed Central Google Scholar *
Prasad A, Qamri Z, Wu J, Ganju RK . Slit2/Robo1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. _J Leukoc Biol_ 2007; 82: 465–76. Article CAS PubMed Google Scholar * Altay T,
McLaughlin B, Wu JY, Park TS, Gidday JM . Slit modulates cerebrovascular inflammation and mediates neuroprotection against global cerebral ischemia. _Exp Neurol_ 2007; 207: 186–94. Article
CAS PubMed PubMed Central Google Scholar * Tole S, Mukovozov IM, Huang YW, Magalhaes MA, Yan M, Crow MR, _et al_. The axonal repellent, Slit2, inhibits directional migration of
circulating neutrophils. _J Leukoc Biol_ 2009; 86: 1403–15. Article CAS PubMed Google Scholar * Schmid BC, Rezniczek GA, Fabjani G, Yoneda T, Leodolter S, Zeillinger R . The neuronal
guidance cue Slit2 induces targeted migration and may play a role in brain metastasis of breast cancer cells. _Breast Cancer Res Treat_ 2007; 106: 333–42. Article PubMed Google Scholar *
Mertsch S, Schmitz N, Jeibmann A, Geng JG, Paulus W, Senner V . Slit2 involvement in glioma cell migration is mediated by Robo1 receptor. _J Neurooncol_ 2008; 87: 1–7. Article CAS PubMed
Google Scholar * Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, Wu JY . Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. _Proc Natl
Acad Sci U S A_ 2009; 106: 14530–5. Article CAS PubMed PubMed Central Google Scholar * Wang B, Xiao Y, Ding BB, Zhang N, Yuan X, Gui L, _et al_. Induction of tumor angiogenesis by
Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. _Cancer Cell_ 2003; 4: 19–29. Article PubMed Google Scholar * Wang LJ, Zhao Y, Han B, Ma YG, Zhang J, Yang
DM, _et al_. Targeting Slit–Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. _Cancer Sci_ 2008; 99: 510–7. Article CAS PubMed Google
Scholar * Urbich C, Rössig L, Kaluza D, Potente M, Boeckel JN, Knau A, _et al_. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial
cells. _Blood_ 2009; 113: 5669–79. Article CAS PubMed Google Scholar * Shen F, Liu X, Geng JG, Guo SW . Increased immunoreactivity to Slit/Robo1 in ovarian endometriomas. _Am J Pathol_
2009; 175: 479–88. Article CAS PubMed PubMed Central Google Scholar * Zhang B, Dietrich UM, Geng JG, Bicknell R, Esko JD, Wang L . Repulsive axon guidance molecule Slit3 is a novel
angiogenic factor. _Blood_ 2009; 114: 4300–9. Article CAS PubMed PubMed Central Google Scholar * Ma S, Liu X, Geng JG, Guo SW . Increased SLIT immunoreactivity as a biomarker for
recurrence in endometrial carcinoma. _Am J Obstet Gynecol_ 2010 202; 68.e1–68.e11. Article Google Scholar * Yang XM, Han HX, Sui F, Dai YM, Chen M, Geng JG . Slit-Robo signaling mediates
lymphangiogenesis and promotes tumor lymphatic metastasis. _Biochem Biophys Res Commun_ 2010; 396: 571–7. Article CAS PubMed PubMed Central Google Scholar * Xu F, Previti ML, Nieman MT,
Davis J, Schmaier AH, Van Nostrand WE . AbetaPP/APLP2 family of Kunitz serine proteinase inhibitors regulate cerebral thrombosis. _J Neurosci_ 2009; 29: 5666–70. Article CAS PubMed
PubMed Central Google Scholar * Engelhardt B, Sorokin L . The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. _Semin Immunopathol_ 2009; 31: 497–511.
Article PubMed Google Scholar * Pottiez G, Flahaut C, Cecchelli R, Karamanos Y . Understanding the blood-brain barrier using gene and protein expression profiling technologies. _Brain Res
Rev_ 2009; 62: 83–98. Article CAS PubMed Google Scholar * Terry S, Nie M, Matter K, Balda MS . Rho signaling and tight junction functions. _Physiology (Bethesda)_ 2010; 25: 16–26. CAS
Google Scholar * Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR . VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. _Proc Natl Acad Sci U S A_ 2009;
106: 1977–82. Article CAS PubMed PubMed Central Google Scholar * Liu D, Hou J, Hu X, Wang X, Xiao Y, Mou Y, _et al_. Neuronal chemorepellent Slit2 inhibits vascular smooth muscle cell
migration by suppressing small GTPase Rac1 activation. _Circ Res_ 2006; 98; 480–9. Article CAS PubMed Google Scholar * Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J, _et al_.
Strategy for effective brain drug delivery. _Eur J Pharm Sci_ 2010; 40: 385–403. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr Biao WANG for the
construction of the pCMV-hSlit2 expression plasmid. This work was supported by grants from the National Natural Science Foundation of China (30811120438, 30721065, 30700409, and 30630036),
the Ministry of Science and Technology of China (2007CB914501, 2007CB947102, 2009ZX09103-685, and 2010CB529700), Shanghai Municipal Commission for Science and Technology (08JC1421400), and
the National Institute of Health (RO1AI064743 and RO1CA126897). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratory of Molecular Cell Biology, Institute of Biochemistry and Cell
Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, 200031, China Hai-xiong Han & Jian-guo Geng Authors * Hai-xiong Han View author publications
You can also search for this author inPubMed Google Scholar * Jian-guo Geng View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to Jian-guo Geng. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Han, Hx., Geng, Jg. Over-expression of Slit2 induces vessel formation
and changes blood vessel permeability in mouse brain. _Acta Pharmacol Sin_ 32, 1327–1336 (2011). https://doi.org/10.1038/aps.2011.106 Download citation * Received: 31 March 2011 * Accepted:
29 June 2011 * Published: 10 October 2011 * Issue Date: November 2011 * DOI: https://doi.org/10.1038/aps.2011.106 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative KEYWORDS * Slit2 * blood-brain barrier * permeability * vessel endothelial cell * tight junction