Play all audios:
ABSTRACT Resveratrol (3,5,4′-trihydroxy-_trans_-stilbene) is a well-known polyphenol that is present in grapes, peanuts, pine seeds, and several other plants. Resveratrol exerts deleterious
effects on various types of human cancer cells. Here, we analyzed the cell death-inducing mechanisms of resveratrol-006 (Res-006), a novel resveratrol derivative in human liver cancer cells
_in vitro_. Res-006 was more effectively suppressed the viability of HepG2 human hepatoma cells than resveratrol (the IC50 values were 67.2 and 354.8 μmol/L, respectively). Co-treatment with
the ER stress regulator 4-phenylbutyrate (0.5 mmol/L) or the ROS inhibitor N-acetyl-_L_-cysteine (NAC, 1 mmol/L) significantly attenuated Res-006-induced HepG2 cell death, suggesting that
pro-apoptotic ER stress and/or ROS may govern the Res-006-induced HepG2 cell death. We further revealed that treatment of HepG2 cells with Res-006 (65 μmol/L) immediately elicited the
dysregulation of mitochondrial dynamics and the accumulation of mitochondrial ROS. It also collapsed the mitochondrial membrane potential and further induced ER stress and cell death. These
events, except for the change in mitochondrial morphology, were prevented by the exposure of the HepG2 cells to the mitochondrial ROS scavenger, Mito-TEMPO (300–1000 μmol/L). The results
suggest that Res-006 may kill HepG2 cells through cell death pathways, including the ER stress initiated by mitochondrial ROS accumulation. The cell death induced by this novel resveratrol
derivative involves crosstalk between the mitochondria and ER stress mechanisms. SIMILAR CONTENT BEING VIEWED BY OTHERS CHALCOMORACIN PROMOTES APOPTOSIS AND ENDOPLASMIC RETICULUM STRESS IN
HEPATOCELLULAR CARCINOMA CELLS Article 09 May 2024 NUPR1 INHIBITOR ZZW-115 INDUCES FERROPTOSIS IN A MITOCHONDRIA-DEPENDENT MANNER Article Open access 01 October 2021 COROSOLIC ACID
SENSITIZES FERROPTOSIS BY UPREGULATING HERPUD1 IN LIVER CANCER CELLS Article Open access 29 August 2022 INTRODUCTION Resveratrol (3,5,4′-trihydroxy-_trans_-stilbene; Res) is a nonflavonoid
polyphenol present in grapes, peanuts, berries, and the constituents of several other plants1. It possesses a wide range of beneficial effects, including anti-cancer, anti-aging,
anti-atherogenic, anti-inflammatory and anti-oxidant activities1,2,3. However, its utilization and development in products have been hampered by its poor solubility, poor chemical stability,
and low bioavailability4,5. Many attempts have sought to improve the bioactivity and bioavailability of Res6,7. For example, trimethylated Res is up to 100-fold more cytotoxic than
unamended Res in cancer cell lines due to the depletion of the intracellular pool of polyamines and altered microtubule polymerization8. Mitochondria are essential in cellular energy
metabolism and are now recognized as being central in apoptotic cell death. Stresses, including growth-factor withdrawal, DNA damage, and exposure to certain chemotherapeutic agents,
activate mitochondria-mediated intrinsic apoptosis pathways9. During intrinsic apoptosis signaling, mitochondrial outer membrane permeabilization (MOMP) leads to the release of pro-apoptotic
proteins (cytochrome _c_, Smac/DIABLO, Omi/HtrA2, AIF and endonuclease G) contained in the intermembrane space9. These pro-apoptotic proteins trigger the execution of cell death by
promoting the caspase activation cascade or by acting as caspase-independent death effectors9. Several recent reports suggested that Res and its derivatives trigger apoptosis through the
intrinsic mitochondrial-dependent pathway, which is associated with mitochondrial dysfunctions, such as mitochondrial membrane potential (MMP) collapse and reactive oxygen species (ROS)
production10,11,12. The endoplasmic reticulum (ER) is a principal cellular compartment for the biosynthesis, folding and modification of membrane and secretory proteins, production of lipids
and sterols, and calcium storage and gated release in eukaryotic cells. The pathological, environmental, or physiological stimuli that interfere with ER functions cause ER stress. To cope
with the ER stress conditions, the ER activates a set of signal transduction pathways, collectively termed the unfolded protein response (UPR), to assist with protein folding and secretion
and to facilitate the degradation of misfolded proteins in the ER lumen13. In mammals, the UPR is mediated by three basic signal transducers: inositol-requiring 1α (IRE1α), PERK
(double-strand RNA-activated protein kinase-like ER kinase), and ATF6α (activating transcription factor 6α)13,14. During ER stress, the cytoplasmic nuclease domain of activated IRE1α
processes the mRNA encoding the XBP-1 (X-box-binding protein 1) transcription factor to generate mature _Xbp1_ mRNA (_Xbp1s_)15. The activated PERK phosphorylates the α subunit of eukaryotic
translation initiation factor 2 (eIF2α) at Ser51 to attenuate global translation16 and increase the translation of mRNAs, such as those encoding the ATF4 transcription factor17. Upon
activation, ATF6α translocates from the ER to the Golgi complex, where it is cleaved by the S1P and S2P proteases to release a cytosolic fragment (ATF6αΔC) that migrates to the nucleus to
activate transcription18,19. For ER stress adaptation, XBP1s and ATF6αΔC by themselves or together induce many UPR genes to enhance ER protein folding, trafficking, secretion, and
ER-associated protein degradation (ERAD)20,21. ATF4 induces the expression of several genes involved in amino acid biosynthesis and transport, anti-oxidative stress, and ER protein folding
and secretion22. However, if the ER stress is too strong and persistent to re-establish ER homeostasis, the ER preferentially elicits several cell-death signaling pathways, including three
UPR pathway-mediated apoptosis pathways, over time23,24. Under chronic ER stress, IRE1α recruits the tumor necrosis factor receptor-associated factor 2 (TRAF2) and apoptosis
signal-regulating kinase 1 (ASK1) and then causes the activation of c-Jun N-terminal kinase (JNK), which is implicated in apoptosis25,26. In addition, prolonged IRE1α-mediated activation of
the regulated IRE1-dependent decay (RIDD) pathway may promote apoptosis by degrading mRNAs encoding essential ER-translocating proteins27 and microRNAs repressing the translation of
pro-apoptotic _caspase-2_ mRNA28. Although the PERK-eIF2α phosphorylation-ATF4 and ATF6α pathways conduct important adaptive mechanisms to relieve the ER stress, ATF4 and ATF6αΔC converge on
the promoter of the gene encoding C/EBP homologous protein (CHOP)21,29, an important pro-apoptotic transcription factor of ER stress-mediated cell death23, which controls the expression of
the pro-apoptotic (_Bim_)30 and anti-apoptotic (_Bcl-2_) genes31. Furthermore, CHOP expression increases the expression of several pro-apoptotic genes, such as ER oxidoreductase 1-α (ERO1α),
growth arrest and DNA damage 34 (GADD34), tribbles-related protein3 (Trb3) and death receptor 5 (Dr5)23. A growing body of data indicates that Res and Res derivatives can induce
pro-apoptotic ER stress in cancer cells by disrupting the N-linked glycosylation of proteins or by increasing intracellular calcium levels32,33,34. Here, we sought to detect the cell death
effects of a new Res derivative in a human liver cancer cell line and to determine the underlying mechanism of its activity, which involves apoptosis associated with mitochondrial
dysfunctions and ER stress. MATERIALS AND METHODS REAGENTS AND ANTIBODIES Resveratrol (Res), N-acetyl-_L_-cysteine (NAC), 4-phenylbutyric acid (4-PBA), BAPTA-AM, TEMPOL, Mito-TEMPO, and
Hoechst 33258 were purchased from Sigma-Aldrich (St Louis, MO, USA). Tunicamycin and thapsigargin were purchased from EMD Millipore (Billerica, MA, USA). MitoTracker Red and JC-1 were
purchased from Molecular Probes (Eugene, OR, USA). Resveratrol-005 (Res-005) and resveratrol-006 (Res-006, Korea Patent #1016328390000) were synthesized by Prof Hyoungsu KIM (see
Supplementary information for details). A Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technology (Rockville, MD, USA). The antibodies, including anti-BAK, anti-BAX,
anti-Bcl-2, anti-Bcl-xL, anti-cleaved caspase-3, anti-CHOP, anti-IRE1α, anti-JNK, anti-p-JNK, anti-PARP, and anti-PERK, were purchased from Cell Signaling Technology (Danvers, MA, USA).
Anti-β-actin and horseradish peroxidase-conjugated anti-Flag were purchased from Sigma-Aldrich (St Louis, MO, USA). In addition, the following antibodies were used: anti-eIF2α from Santa
Cruz Biotechnology (Dallas, TX, USA), anti-BiP from BD Bioscience (San Jose, CA, USA), anti-KDEL from Assay Designs (Farmingdale, NY, USA), anti-p-IRE1α from Novus Biologicals (Littleton,
CO, USA), anti-MTCO1 and anti-SDHA from Abcam (Cambridge, UK), and anti-p-eIF2α from Invitrogen (Carlsbad, CA, USA). The secondary peroxidase-conjugated antibodies were purchased from Thermo
Fisher Scientific (Waltham, MA, USA) or Jackson ImmunoResearch (West Grove, PA, USA). CELL CULTURE HepG2 human hepatocellular carcinoma cancer cells were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA). The Huh-7 cells were provided by Dr Sung Key JANG (Department of Life Sciences, Pohang University of Science and Technology (POSTECH), Korea)35.
Both the HepG2 and Huh-7 cells were cultured in DMEM (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (ATCC) and 5% penicillin-streptomycin (Gibco). The THLE-2 cells
(ATCC) originated from human primary normal liver cells and were provided by Dr Jong-heon KIM (Cancer Cell and Molecular Biology Branch, National Cancer Center, Korea). THLE-2 cells were
plated on culture plates precoated with a solution containing 0.01 mg/mL fibronectin (Thermo Scientific, Waltham, MA, USA), 0.03 mg/mL bovine collagen type I (Thermo Scientific), and 0.01
mg/mL bovine serum albumin (Sigma-Aldrich) dissolved in bronchial epithelial basal medium (BEBM, Lonza, Basel, Switzerland). The THLE-2 cells were cultured in BEBM supplemented with 5 ng/mL
EGF (R&D Systems, Minneapolis, MN, USA), 70 ng/mL phosphoethanolamine (Sigma-Aldrich), 10% fetal bovine serum (ATCC, Manassas, VA, USA), and BEGM SingleQuots (Lonza) with no
gentamicin/amphotericin (GA) and epinephrine. The cells were grown in a 5% CO2 incubator at 37 °C. CELL VIABILITY ASSAYS The cell viability assay was performed using a CCK-8 kit (Dojindo
Molecular Technologies) according to the manufacturer's instructions. Briefly, the cells were plated in 96-well plates and grown overnight. The next day, the cells were treated with the
indicated chemicals for 24 h, and then, the cells were treated with the CCK-8 solution for 3 h. Absorbance was measured at 450 nm using a microplate reader (Molecular Devices, Sunnyvale,
CA, USA). TRANSFECTION HepG2 cells (1×105) were plated in 60-mm dishes or collagen-coated 35-mm coverglass bottom dishes and cultured overnight. The next day, the flag-tagged
ATF6α-expressing plasmid (pCMV-Flag3x-ATF6α, a kind gift from Ron Prywes, Addgene plasmid #11975) or mitochondria-targeted EYFP-expressing plasmid (pEYFP-Mito, Clontech, Mountain View, CA,
USA) was transfected into HepG2 cells using the Fugene6 transfection reagent (Promega, Madison, WI, USA) for 48 h. The cells were treated with the chemicals (Tm, Tg, or Res-006) for the
indicated time. WESTERN BLOT ANALYSIS Cells were lysed with NP40 lysis buffer (1% Nonidet P-40, 0.05% sodium dodecyl sulfate, 50 mmol/L Tris-Cl pH 7.5, 150 mmol/L NaCl, 0.5 mmol/L sodium
vanadate, 100 mmol/L sodium fluoride, and 50 mmol/L β-glycerophosphate) supplemented with Halt protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA). The homogenates were
centrifuged at 12 000×_g_ for 15 min at 4 °C, and the supernatants were collected. The protein concentration was determined using a BCA protein assay kit (Bio-Rad, Hercules, CA, USA).
Protein samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride or nitrocellulose membranes (GE Healthcare Life
Sciences, Marlborough, MA, USA). The membranes were blocked for 3 h at room temperature with 5% skim milk in Tris-buffered saline Tween buffer (0.1% Tween 20, 20 mmol/L Tris-HCl, pH 7.5, and
150 mmol/L NaCl). The membranes were then incubated with the indicated primary antibodies overnight at 4 °C and then with the horseradish peroxidase-conjugated secondary antibody.
Membrane-bound antibodies were detected by enhanced chemiluminescence (ECL) (Thermo Scientific). FLOW CYTOMETRY ANALYSIS OF APOPTOSIS OR THE CELLULAR ROS LEVEL HepG2 cells (2×105) were
plated in 6-well plates and cultured overnight. The cells were treated with the indicated chemicals. After treatment, the cells were harvested and then washed twice with cold-phosphate
buffered saline (PBS). Next, the cells were double-stained with annexin V and 7-aminoactinomycin D (7AAD) (BD Pharmingen, Franklin Lakes, NJ, USA) in binding buffer for 15 min. Finally, the
cells were analyzed by flow cytometry using a FACSCanto II apparatus (BD Biosciences, Franklin Lakes, NJ, USA). FlowJo software (Ashland, OR, USA) was used for the analysis. HepG2 cells
(2×105) were plated in 6-well plates and cultured overnight. The next day, the cells were treated with the indicated chemicals. After treatment, the cells were stained with dihydroethidium
(15 μmol/L, Sigma-Aldrich) in culture medium for 30 min. The cells were harvested and analyzed by flow cytometry as described above. IMMUNOFLUORESCENCE HepG2 cells (2×105) were plated in
6-well plates coated with collagen 0.01% in PBS and cultured overnight. The next day, the cells were treated with the indicated chemicals, fixed with 4% paraformaldehyde in PBS for 15 min,
and permeabilized with 0.2% Triton X-100 in PBS for 2 min. The cells were blocked with 1% bovine serum albumin in PBS for 30 min and incubated with the indicated primary antibody overnight
at 4 °C. The cells were further incubated with a fluorescein isothiocyanate-conjugated secondary antibodies at room temperature for 1 h. The nuclei were stained with Hoechst 33258
(Sigma-Aldrich). Finally, the cells were observed by confocal laser microscopy using an FV1200-OSR microscope (Olympus, Tokyo, Japan). MITOCHONDRIAL MEMBRANE POTENTIAL (MMP) ANALYSIS THLE-2
and HepG2 cells (2×105) were plated in collagen-coated 35 mm coverglass bottom dishes (SPL, Pocheon-si, Gyeonggi-do, Korea) and cultured overnight. The next day, the cells were treated with
the indicated chemicals. After treatment, the cells were stained with MitoTracker Red (200 nmol/L) or JC-1 (2.5 μmol/L) and Hoechst 33258 (4 μg/mL) in culture medium for 1 h. Fluorescence
images of living cells were obtained using an FV1200-OSR confocal laser microscope. TIME-LAPSE CONFOCAL MICROSCOPY Mitochondria-targeted EYFP-expressing HepG2 cells were treated with mock or
Res-006 for 20 min, and then the mitochondria were imaged for 4 min and 30 s using an FV1200-OSR confocal laser microscope (Olympus). Frames were taken every 30 s. The microscopic field was
63.4 μm×63.4 μm. SEMI-QUANTITATIVE PCR AND QRT-PCR Total RNA was prepared from the HepG2 cells treated with the indicated chemicals using an RNeasy Plus Mini Kit (Qiagen, Venlo,
Netherlands). The cDNA was prepared with a High Capacity cDNA RT Kit (Ambion, Life Technologies, Waltham, MA, USA) for semi-quantitative PCR using standard methods or for qRT-PCR normalized
to the levels of β-actin as previously described36. The primers for the semi-quantitative PCR analysis were as follows: forward primer for _Xbp1_ mRNA splicing analysis,
5′-CCGCAGCAGGTGCAGG-3′ and reverse primer 5′-GGGGCTTGGTATATATGTGG-3′; forward primer for _Gapdh_ mRNA, 5′-ACATCAAGAAGGTGGTGAAG-3′ and reverse primer 5′-CTGTTGCTGTAGCCAAATTC-3′. The primers
for the qRT-PCR analysis are as follows: _β-actin_ forward primer 5′-TCCCCCAACTTGAGATGTATGAAG-3′ and _β-actin_ reverse primer 5′-AACTGGTCTCAAGTCAGTGTACAGG-3′; _Xbp1s_ forward primer
5′-CCGCAGCAGGTGCAGG-3′ and _Xbp1s_ reverse primer 5′-GAGTCAATACCGCCAGAATCCA-3′; _Xbp1t_ forward primer 5′- GCAAGCGACAGCGCCT-3′ and _Xbp1t_ reverse primer 5′- TTTTCAGTTTCCTCCTCAGCG-3′;
_ERdj4_ forward primer 5′- GGAAGGAGGAGCGCTAGGTC-3′ and _ERdj4_ reverse primer 5′-ATCCTGCACCCTCCGACTAC-3′; _Chop_ forward primer 5′-ATGGCAGCTGAGTCATTGCCTTTC-3′ and _Chop_ reverse primer
5′-AGAAGCAGGGTCAAGAGTGGTGAA-3′; and _Grp78_ (_BiP_) forward primer 5′-GCCTGTATTTCTAGACCTGCC-3′ and _Grp78_ (_BiP_) reverse primer 5′-TTCATCTTGCCAGCCAGTTG-3′. STATISTICAL ANALYSIS All data
are represented as the mean±SEM of three or four independent experiments. The data were analyzed using GraphPad Prism 5 (GraphPad Software, Inc, La Jolla, CA, USA). Unpaired 2-tailed
Student's _t_-tests were performed to determine the statistical significance for paired samples. _P_<0.05 was considered significant. RESULTS RES DERIVATIVE
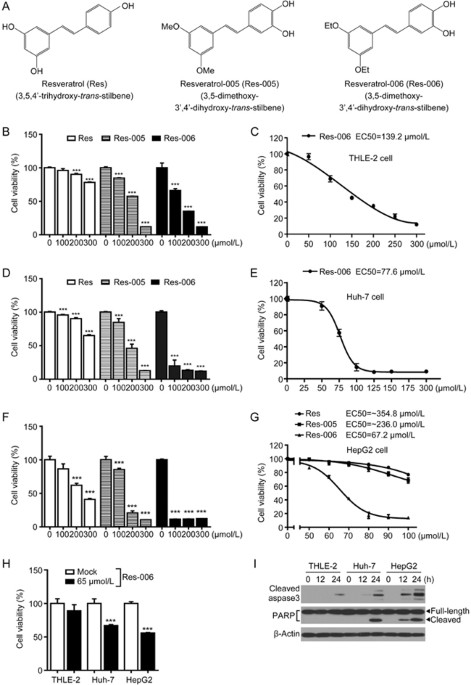
3,5-DIETHOXY-3′,4′-DIHYDROXY-TRANS-STILBENE DISPLAYS ER STRESS AND/OR OXIDATIVE STRESS-MEDIATED CYTOTOXICITY IN HEPG2 CELLS To investigate the cytotoxic effect of Res and its derivatives
(Figure 1A), one normal human liver cell line, THLE-2, and two human hepatoma cell lines, Huh-7 and HepG2, were treated with various concentrations of the compounds (Figure 1B-1H). The
dehydrogenase-based cell viability assay revealed that the Res derivative designated Res-006 had stronger cellular toxicity than Res and Res-005 in all cell lines used (Figure 1B, 1D, 1F,
and 1G). The IC50 value (67.2 μmol/L) of Res-006 in HepG2 cells was approximately 2-fold lower than that (139.2 μmol/L) in THLE-2 cells (Figure 1C, 1E, and 1G). The cytotoxic effect of 65
μmol/L Res-006 against HepG2 cells was higher than that against THLE-2 and Huh-7 cells (Figure 1H). In addition, the time-dependent expression of apoptotic marker proteins (cleaved caspase-3
and PARP) in lysates of Res-006-treated HepG2 cells was higher compared with that of Res-006-treated THLE-2 and Huh-7 cells (Figure 1I). Therefore, subsequent experiments were focused on
the cytotoxic effects of Res-6 against HepG2 cells. To investigate whether Res-006 can induce apoptosis in HepG2 cells, we examined the apoptotic effect of Res-006 by flow cytometry and
Western blot analyses. In the mock-treated group, <6.0% of cells underwent apoptosis (Q2+Q4 in Figure 2A). In contrast, in cells treated with 65 μmol/L Res-006, 26.1% of cells underwent
apoptosis (Figure 2A). Moreover, both caspase-3 and PARP were significantly cleaved and activated by Res-006 (Figure 2B). The results indicated that Res-006 can significantly induce
apoptosis in HepG2 cells. Several recent reports suggested that Res and its derivatives can elicit ER stress-mediated cell death32 by increasing the level of cellular calcium by inhibiting
sarco/endoplasmic reticulum Ca2+ATPase (SERCA)34 or disrupting the _N_-linked glycosylation of proteins33. In addition, oxidative stress induced by the drugs may be responsible for cell
death12,37. To verify whether ER stress and/or oxidative stress are associated with Res-006-mediated HepG2 cell death, the ER stress regulator 4-phenylbutyrate (PBA) and/or the ROS inhibitor
N-acetyl-_L_-cysteine (NAC) were applied along with Res-006. Inhibition of either ER stress or ROS antagonized the cell death activity of Res-006 toward HepG2 cells (Figure 2C). Moreover,
compared with the treatment with drug alone, co-treatment with 4-PBA and NAC further increased cell viability (Figure 2C), indicating that pro-apoptotic ER stress and/or ROS may govern the
cell death induced by Res-006 in HepG2 cells. However, treatment with the Ca2+ chelator BAPTA/AM did not restore the viability of Res-006-treated HepG2 cells (Figure 2D), suggesting that an
increase in intracellular Ca2+ levels may not relate with Res-006-mediated cell death. Next, to determine the effect of Res-006 on the N-linked glycosylation of the proteins, we monitored
the N-linked glycosylation of ATF6α, an N-linked glycosylated ER membrane protein18 in HepG2 cells. Tunicamycin (Tm), an established inhibitor of N-linked glycosylation38, was used to
generate a positive control. The 3xFlag-tagged human ATF6α proteins were transiently overexpressed in HepG2 cells. The deglycosylation-induced mobility change of 3xFlag-ATF6α was examined by
Western blot analysis with tunicamycin, the SERCA inhibitor thapsigargin (Tg), or Res-006-treated HepG2 cells. As expected, the 3xFlag-ATF6α in the Tm-treated cell lysates migrated faster
compared with the mock or Tg-treated cell lysates (Figure 2E), indicating that the protein was deglycosylated by tunicamycin treatment. However, there were no fast migrating bands observed
in Res-006-treated lysates until 24 h (Figure 2E), suggesting that the Res-006 may not elicit ER stress through the disruption of the N-linked glycosylation of the proteins. Taken together,
these results suggest that Res-006 induces ER stress- and/or ROS-mediated cell death, which is not associated with acute calcium mobilization or N-linked glycosylation inhibition. RES-006
DYSREGULATES MITOCHONDRIAL DYNAMICS AND DISRUPTS MMP IN HEPG2 CELLS It is possible that mitochondrial dysfunction can induce ER stress37,39 and vice versa24,40,41. To explore this, we first
used mitochondria-targeted enhanced yellow fluorescent protein (Mito-EYFP) to monitor the morphological changes of mitochondria during Res-006 treatment in HepG2 cells (Figure 3A). Following
the addition of Res-006, the mitochondrial morphology changed from interconnected filaments to large spheres within 60 min, whereas the morphology was barely changed in the mock-treated
cells (Figure 3A). Because mitochondrial morphology is determined by a dynamic equilibrium between organelle fusion and fission, we imaged mitochondria for 4.5 min in mock-treated and
Res-006-treated cells (Figure 3B and Supplementary Movie S1 and S2). In the mock-treated cells, the mitochondria were highly mobile, and several fusion and fission events were observed
during the recordings (Supplementary Movie S1). However, in the Res-006-treated cells, many mitochondria had already become ovoid or spherical, possibly due to fusion before the time-lapse
experiments. Later on, the fusion or fission events were rarely observed. One fusion event was observed, which generated a large spherical mitochondrion (arrows and ovals in Figure 3B and
Supplementary Movie S2). In addition to reduced fusion and fission, the time-lapse videos revealed striking defects in the mobility of the mitochondria in Res-006-treated HepG2 cells. These
results suggest that Res-006 alters mitochondrial dynamics, which can lead to a mitochondrial morphology change. Next, we tested whether Res-006 treatment could disrupt MMP, which is an
important parameter of mitochondrial function. MitoTracker Red, a lipophilic cationic dye that is sensitive to the MMP, was used to stain Res-006-treated HepG2 cells. The number of stained
mitochondria and their fluorescence intensities were gradually reduced in Res-006-treated cells until 24 h; however, they were not changed in mock-treated cells (Figure 3C and 3E) and
Res-006-treated THLE-2 cells (Supplementary Figure S1). However, the levels of the mitochondrial DNA-encoded protein MTCO1 and a nuclear-encoded protein SDHA remained unchanged in
Res-006-treated HepG2 cells until 24 h (Figure 3D). In addition, immunofluorescence analysis of MTCO1 showed that the fluorescence intensity of mitochondria labeled with the anti-MTCO1
antibody did not decrease in Res-006-treated cells for 24 h compared with the mock-treated cells; whereas the fluorescence intensity of MMP-dependent MitoTracker Red was significantly
decreased by the drug treatment (Figure 3E). The observations suggested that Res-006 treatment disrupts mitochondrial function but does not cause the removal of malfunctioning mitochondria
from the cells. RES-006-INDUCED CELL DEATH IS PREVENTED BY SUPEROXIDE SCAVENGER Mitochondria are an important source of ROS within most mammalian cells42,43. In addition, cellular redox
homeostasis is interconnected with MMP42,44,45. The level of accumulated ROS was measured by flow cytometry following staining of the cells with the superoxide indicator dihydroethidium
(DHE). The ROS levels in cells treated for 2 h with Res-006 was significantly higher (approximately 2.3 fold) than in the mock-treated cells (Figure 4A). From these results, we hypothesized
that in Res-006 treated cells, the ROS accumulation leads to mitochondrial dysfunction (Figure 3A-3E) and cell death (Figure 2B), which can be ameliorated by its modulation. To verify this
hypothesis, HepG2 cells were co-treated with the superoxide dismutase mimetic TEMPOL (Tem) and Res-006. The TEMPOL dose-dependently increased the viability of Res-006-treated cell
populations up to approximately 80% (Figure 4B, 4D, and 4E). Given that Res-006 led to mitochondrial dysfunctions (Figure 3A-3C and 3E) associated with mitochondrial ROS generation, we next
used a mitochondria-targeted TEMPOL (Mito-TEMPO, Mito) to specifically reduce the mitochondrial ROS levels. Intriguingly, the Mito-TEMPO treatments completely prevented cell death induced by
Res-006 at concentrations over 300 μmol/L (Figures 4C-4E). The flow cytometry of cells stained with annexin V and 7AAD clearly showed that the Mito-TEMPO treatment nearly abrogated
Res-006-induced cell death (Figure 4F). In addition, the Mito-TEMPO treatment markedly inhibited caspase-3 activation and PARP cleavage induced by Res-006, although TEMPOL also showed
significant inhibitory effects against the events (Figure 4G). Taken together, these data indicate that Res-006 induces cell death, which can be prevented by the removal of mitochondrial
ROS. MITOCHONDRIA-TARGETED SUPEROXIDE SCAVENGER PREVENTS RES-006-MEDIATED MMP DISRUPTION To explore the efficiency of the inhibition of ROS accumulation by two superoxide scavengers in
Res-006-treated HepG2 cells, flow cytometry was used to examine dihydroethidium (DHE)-stained cells. Both superoxide scavengers significantly inhibited ROS accumulation induced by Res-006
treatment. Notably, the ROS levels in the Mito-TEMPO treated cells were as low as those in the mock-treated cells (Figure 5A). Next, to verify whether the increased viability due to ROS
scavenging could be correlated with the recovery of MMP in Res-006-treated HepG2 cells, we used JC-1, a cationic MMP probe that accumulates in energized mitochondria. The cells that were
exposed to Res-006 for 2 h displayed greatly reduced red J-aggregate fluorescence and significantly increased cytoplasmic green monomer fluorescence compared with the mock-treated cells
(Figure 5B), indicating that Res-006 treatment causes the rapid collapse of MMP, which may have preceded apoptotic cell death (Figure 2B). However, both superoxide scavengers significantly
restored red J-aggregate fluorescence, suggesting that ROS scavenging can prevent the MMP loss in Res-006-treated HepG2 cells (Figure 5B). In addition, the TEMPOL and Mito-TEMPO treatments
greatly restored both the number and fluorescence intensities of the MitoTracker Red-stained mitochondria in Res-006-treated cells (Figure 5C); however, they did not prevent Res-006-mediated
mitochondrial fragmentation and swelling, indicating that the mitochondrial morphological changes may not be related with the Res-006-mediated ROS accumulation and cell death. RES-006
INDUCES ER STRESS RESPONSES Treatment with 4-phenylbutyric acid, an ER stress inhibitor, ameliorated Res-006-mediated cellular toxicity (Figure 2C). Thus, we investigated whether Res-006
could operate as an ER stress inducer. ROS-006 treatment induced the quick activation of all three UPR sensors (IRE1α, PERK, and ATF6α) in HepG2 cells13,14 (Figure 6A-6D). First, IRE1α
phosphorylation46 and its mediated downstream events, including increases in JNK phosphorylation25, _Xbp1_ mRNA splicing47, and XBP1s-dependent _Erdj4_ mRNA expression48, occurred in
Res-006-treated cells (Figure 6A and 6D). Second, Res-006 treatment induced the activation of the PERK/eIF2α-dependent pathway, including marked and persistent PERK phosphorylation, eIF2α
phosphorylation at 3 h (Figure 6B), and increases in _Chop_ transcripts (Figure 6D)29,49 and CHOP proteins (Figure 6B)50. Third, the activation of the last UPR sensor ATF6α was determined by
the observation of the S1P and S2P protease-mediated cleavage fragment (3XFlag-ATF6αΔC) generated19 from Flag-tagged ATF6α protein exogenously expressed in Res-006-treated HepG2 cells
(Figure 6C). As expected, the level of the cleavage product (3XFlag-ATF6αΔC) was increased in the HepG2 cells treated with the ER stress inducer, dithiothreitol (DTT) (Figure 6C)18,19.
Similarly, the Flag-tagged cleavage products increased over time in the Res-006-treated HepG2 cells. Furthermore, consistent with the ATF6α activation, the Res-006 treatment significantly
increased the expression of an ATF6α downstream target gene _Grp78_ (Figure 6C and 6D)20,21. Taken together, these results clearly suggest that Res-006 is a strong ER stress inducer that can
activate all three UPR pathways in HepG2 cells. RES-006 INDUCES MITOCHONDRIAL ROS-MEDIATED ER STRESS Since the removal of mitochondrial ROS prevented Res-006-mediated cell death, we
questioned whether mitochondrial ROS scavenging can reduce ER stress responses in Res-006-treated HepG2 cells. Mitochondria-targeted Mito-TEMPO treatment significantly prevented IRE1α and
JNK phosphorylation (Figure 7A). It strongly inhibited IRE1α-mediated _Xbp1_ mRNA splicing (Figure 7B) and subsequent expression of _Erdj4_, an XBP1s target gene (Figure 7C) in Res-006
treated cells. In addition, the Mito-TEMPO treatment robustly inhibited PERK phosphorylation (Figure 7B) and strongly suppressed pro-apoptotic CHOP expression (Figure 7A and 7C). Lastly, it
significantly reduced the expression of _Grp78_, an ATF6α downstream target gene (Figure 7C), suggesting that ATF6α activation was blocked by ROS scavenger treatment in Res-006-treated HepG2
cells. Although non-mitochondria-targeted TEMPOL could substantially suppress ER stress responses induced by Res-006 treatment, it was not as strong as Mito-TEMPO (Figure 7A-7C).
Collectively, these results strongly support the view that mitochondrial ROS that accumulate during Res-006 treatment induces ER stress responses that can induce cell death. DISCUSSION
Currently, the chemical derivatization of Res produced two novel cytotoxic drugs with improved cell death activity compared with Res. Res-006 produced mitochondrial dysfunction and ER
stress, which triggers the death of HepG2 cells. However, partial restoration of the mitochondrial dysfunctions via ROS scavengers, especially a mitochondria-targeted ROS scavenger
(Mito-TEMPO) robustly prevented ER stress and cell death. We conclude that the pro-oxidant activity of Res-006 is critical in inducing ER stress and cell death. The Res-006 treatment quickly
induced ROS accumulation in advance of cell death (compare Figure 4A with Figure 2B), suggesting that ROS accumulation triggered cell death. If so, what is the source of ROS in
Res-006-treated HepG2 cells? Treatment with the mitochondria-targeted ROS scavenger, Mito-TEMPO, robustly blocked ROS accumulation and restored MMP in Res-006-treated cells (Figure 5A and
5B), indicating that mitochondria are the main source of ROS. In addition, Res-006 treatment immediately caused a change in mitochondrial morphology, which led to large spherical
mitochondria (Figure 3A and 3B and Supplementary Movie S1 and S2). The changes may have been caused by changes in mitochondrial fusion and/or fission and its movement, suggesting that the
chemical alters mitochondrial dynamics. Mitochondrial morphology and dynamics are interlinked with cellular and mitochondrial redox homeostasis51. Cells deficient in mitochondrial fusion
proteins (Mfn1, Mfn2, or Opa1) display a fragmented mitochondrial morphology and increased ROS levels and then die. Conversely, chemical or genetic inhibitions of mitochondrial fission
proteins (Drp1 or Fis) induce mitochondrial elongation and reduce ROS production51,52. Thus, mitochondrial fragmentation allows increases in ROS production. However, in our experimental
conditions, treatment with the mitochondrial fission inhibitor, Mdivi-1, did not prevent Res-006-induced cell death, but rather inversely increased it (data not shown), suggesting that
Res-006-mediated ROS production and cell death may not be related with increased mitochondrial fission. The time-lapse imaging experiments provided evidence that Res-006 can induce
mitochondrial fusion at early time points, which rendered the mitochondria as large and spherical in HepG2 cells (Figure 3A and 3B, and Supplementary Movie S1 and S2). Therefore, the
morphological changes of mitochondria may not be triggered by the inhibition of mitochondrial fusion proteins (Mfn1, Mfn2, or Opa1) in Res-006-treated HepG2 cells. In addition, the removal
of ROS could not prevent the morphological changes of the mitochondria induced by the Res-006 treatment (Figure 5C). Thus, these data strongly suggest that ROS accumulation is not
responsible for the morphological change. Inversely, it is also possible that the morphological change of mitochondria is not related with ROS accumulation and cell death in Res-006-treated
HepG2 cells. The exact mechanism and role of the morphological change remain to be clarified. Although several reports suggested that Res and its derivatives target multiple intracellular
components (such as tumor suppressors p53 and Rb and apoptosis and survival regulators, Bax, Bak, AKT, Bcl-2, and Bcl-xL; see reference review3), including mitochondrial proteins11,53, which
can induce mitochondrial ROS and mitochondria-mediated apoptosis pathways (Figure 7D), there is increasing evidence that Res and Res derivatives can induce ER stress and mediate cell death
in several cancer cell types by disrupting the N-linked glycosylation of proteins or by increasing the level of intracellular Ca2+54. In this study, the novel Res derivative, Res-006, also
elicited ER stress. However, the ER stress inhibitor, 4-phenylbutyric acid, partially blocked Res-006-mediated ER stress (data not shown) and cell death (Figure 2C), whereas a
mitochondria-targeted ROS scavenger robustly inhibited both ER stress and cell death (Figures 4C-4G and 7A-7C), indicating that the ER stress partially contributes to Res-006-mediated cell
death and occurs downstream of mitochondria-ROS accumulation. Furthermore, Res-006 may not be a direct ER stress inducer inhibiting ER-mediated N-linked glycosylation or increasing
intracellular calcium level because the results in Figure 2D and 2E showed that the drug-mediated cell death was not linked with the general conditions that ER stress induced. Until now,
there have been no reports that ROS accumulation caused by Res or Res derivatives can elicit ER stress, but the role of other drug-mediated mitochondrial ROS in ER stress induction has been
demonstrated in a variety of cell types39,55. Drug-mediated ROS generation precedes UPR induction and is efficiently blocked by ROS scavengers55. In terms of mechanisms, the drugs trigger
mitochondrial ROS production through the regulation of gene expression or enzyme activity of mitochondrial ROS-producing proteins, such as NADPH oxidase 4 or cytochrome _c_ reductase
(complex III), respectively39. Although the precise mechanism of how mitochondrial ROS induce ER stress requires further investigation, it is proposed that oxidative protein damage and/or
its mediated ER calcium release may trigger ER stress39,55. However, Res-006-mediated mitochondrial ROS may not induce ER stress through the disruption of ER calcium homeostasis because
treatment with the intracellular calcium chelator BAPTA/AM could not inhibit Res-006-mediated cell death (Figure 2D). This issue requires further studies. In conclusion, our results
demonstrate that cell death in human hepatoma HepG2 cells induced by the novel Res derivative, Res-006, is mediated by the activation of ER stress and the dysfunction of mitochondria that
require ROS generation. The proposed cell death pathway induced by Res-006 is depicted in Figure 7D. The death involves cross-talk between the mitochondria and ER stress mechanisms. Our
study provides a rationale for the development of a new resveratrol derivative as chemotherapeutic agent targeting both mitochondria and the ER. AUTHOR CONTRIBUTION Jae-woo PARK, Woo-gyun
CHOI, Hyoungsu KIM, Hong-pyo KIM, and Sung-hoon BACK designed the research; Jae-woo PARK, Woo-gyun CHOI, Su-wol CHUNG, and Phil-jun LEE performed the experiments; Byung-sam KIM, Hun-taeg
CHUNG, Sungchan CHO, Jong-heon KIM and Byoung-heon KANG contributed to the acquisition of the data; Jae-woo PARK, Woo-gyun CHOI, Phil-jun LEE, Hyoungsu KIM, Hong-pyo KIM, and Sung-hoon BACK
analyzed and interpreted the data; Sung-hoon BACK wrote the draft manuscript, which was subsequently edited by all the authors, all of whom have read and approved the final manuscript.
SUPPLEMENTARY INFORMATION The supplementary information on the website of Acta Pharmacologica Sinica provides the confocal time-lapse movies of EYFP-labeled mitochondria in Mock- or
Res-006-treated HepG2 cells, the MitoTracker Red-labeled mitochondria images of Res-006-treated THLE-2 and HepG2 cells, and the synthesis methods for Res-005 and Res-006. REFERENCES * Baur
JA, Sinclair DA . Therapeutic potential of resveratrol: the _in vivo_ evidence. _Nat Rev Drug Discov_ 2006; 5: 493–506. Article CAS PubMed Google Scholar * Ramprasath VR, Jones PJ .
Anti-atherogenic effects of resveratrol. _Eur J Clin Nutr_ 2010; 64: 660–8. Article CAS PubMed Google Scholar * Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL . Multiple molecular
targets of resveratrol: Anti-carcinogenic mechanisms. _Arch Biochem Biophys_ 2009; 486: 95–102. Article CAS PubMed PubMed Central Google Scholar * Zupancic S, Lavric Z, Kristl J .
Stability and solubility of _trans_-resveratrol are strongly influenced by pH and temperature. _Eur J Pharm Biopharm_ 2015; 93: 196–204. Article CAS PubMed Google Scholar * Walle T,
Hsieh F, DeLegge MH, Oatis JE Jr, Walle UK . High absorption but very low bioavailability of oral resveratrol in humans. _Drug Metab Dispos_ 2004; 32: 1377–82. Article CAS PubMed Google
Scholar * Rivera H, Shibayama M, Tsutsumi V, Perez-Alvarez V, Muriel P . Resveratrol and trimethylated resveratrol protect from acute liver damage induced by CCl4 in the rat. _J Appl
Toxicol_ 2008; 28: 147–55. Article CAS PubMed Google Scholar * Tabata Y, Takano K, Ito T, Iinuma M, Yoshimoto T, Miura H, _et al_. Vaticanol B, a resveratrol tetramer, regulates
endoplasmic reticulum stress and inflammation. _Am J Physiol Cell Physiol_ 2007; 293: C411–8. Article CAS PubMed Google Scholar * Schneider Y, Chabert P, Stutzmann J, Coelho D,
Fougerousse A, Gosse F, _et al_. Resveratrol analog (Z)-3,5,4'-trimethoxystilbene is a potent anti-mitotic drug inhibiting tubulin polymerization. _Int J Cancer_ 2003; 107: 189–96.
Article CAS PubMed Google Scholar * Kroemer G, Galluzzi L, Brenner C . Mitochondrial membrane permeabilization in cell death. _Physiol Rev_ 2007; 87: 99–163. Article CAS PubMed Google
Scholar * Lin X, Wu G, Huo WQ, Zhang Y, Jin FS . Resveratrol induces apoptosis associated with mitochondrial dysfunction in bladder carcinoma cells. _Int J Urol_ 2012; 19: 757–64. Article
CAS PubMed Google Scholar * Sassi N, Mattarei A, Azzolini M, Szabo I, Paradisi C, Zoratti M, _et al_. Cytotoxicity of mitochondria-targeted resveratrol derivatives: interactions with
respiratory chain complexes and ATP synthase. _Biochim Biophys Acta_ 2014; 1837: 1781–9. Article CAS PubMed Google Scholar * Mukherjee N, Parida PK, Santra A, Ghosh T, Dutta A, Jana K,
_et al_. Oxidative stress plays major role in mediating apoptosis in filarial nematode _Setaria cervi_ in the presence of _trans_-stilbene derivatives. _Free Radic Biol Med_ 2016; 93:
130–44. Article CAS PubMed Google Scholar * Ron D, Walter P . Signal integration in the endoplasmic reticulum unfolded protein response. _Nat Rev Mol Cell Biol_ 2007; 8: 519–29. Article
CAS PubMed Google Scholar * Back SH, Kaufman RJ . Endoplasmic reticulum stress and type 2 diabetes. _Annu Rev Biochem_ 2012; 81: 767–93. Article CAS PubMed PubMed Central Google
Scholar * Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K . XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor.
_Cell_ 2001; 107: 881–91. Article CAS PubMed Google Scholar * Wek RC, Cavener DR . Translational control and the unfolded protein response. _Antioxid Redox Signal_ 2007; 9: 2357–71.
Article CAS PubMed Google Scholar * Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, _et al_. Regulated translation initiation controls stress-induced gene expression in
mammalian cells. _Mol Cell_ 2000; 6: 1099–108. Article CAS PubMed Google Scholar * Haze K, Yoshida H, Yanagi H, Yura T, Mori K . Mammalian transcription factor ATF6 is synthesized as a
transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. _Mol Biol Cell_ 1999; 10: 3787–99. Article CAS PubMed PubMed Central Google Scholar *
Shen J, Prywes R . Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. _J Biol Chem_ 2004; 279:
43046–51. Article CAS PubMed Google Scholar * Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, _et al_. Transcriptional induction of mammalian ER quality control proteins is
mediated by single or combined action of ATF6alpha and XBP1. _Dev Cell_ 2007; 13: 365–76. Article CAS PubMed Google Scholar * Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T,
Wang J, _et al_. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. _Dev Cell_ 2007; 13: 351–64. Article CAS PubMed Google Scholar *
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, _et al_. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. _Mol Cell_ 2003; 11: 619–33.
Article CAS PubMed Google Scholar * Tabas I, Ron D . Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. _Nat Cell Biol_ 2011; 13: 184–90. Article CAS
PubMed PubMed Central Google Scholar * Kim I, Xu W, Reed JC . Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. _Nat Rev Drug Discov_ 2008; 7:
1013–30. Article CAS PubMed Google Scholar * Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, _et al_. Coupling of stress in the ER to activation of JNK protein kinases by
transmembrane protein kinase IRE1. _Science_ 2000; 287: 664–6. Article CAS PubMed Google Scholar * Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, _et al_. ASK1 is
essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. _Genes Dev_ 2002; 16: 1345–55. Article CAS PubMed PubMed Central
Google Scholar * Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, _et al_. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell
fates. _Cell_ 2009; 138: 562–75. Article CAS PubMed PubMed Central Google Scholar * Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, _et al_. IRE1alpha cleaves select microRNAs
during ER stress to derepress translation of proapoptotic Caspase-2. _Science_ 2012; 338: 818–22. Article CAS PubMed PubMed Central Google Scholar * Ma Y, Brewer JW, Diehl JA,
Hendershot LM . Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. _J Mol Biol_ 2002; 318: 1351–65. Article CAS PubMed
Google Scholar * Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, _et al_. ER stress triggers apoptosis by activating BH3-only protein Bim. _Cell_ 2007; 129:
1337–49. Article CAS PubMed Google Scholar * McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ . Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2
and perturbing the cellular redox state. _Mol Cell Biol_ 2001; 21: 1249–59. Article CAS PubMed PubMed Central Google Scholar * Park JW, Woo KJ, Lee JT, Lim JH, Lee TJ, Kim SH, _et al_.
Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. _Oncol Rep_ 2007; 18: 1269–73. CAS PubMed Google Scholar * Gwak H, Kim S, Dhanasekaran DN,
Song YS . Resveratrol triggers ER stress-mediated apoptosis by disrupting N-linked glycosylation of proteins in ovarian cancer cells. _Cancer Lett_ 2016; 371: 347–53. Article CAS PubMed
Google Scholar * Fan XX, Yao XJ, Xu SW, Wong VK, He JX, Ding J, _et al_. (Z)3,4,5,4′-_trans_-tetramethoxystilbene, a new analogue of resveratrol, inhibits gefitinb-resistant non-small cell
lung cancer via selectively elevating intracellular calcium level. _Sci Rep_ 2015; 5: 16348. Article CAS PubMed PubMed Central Google Scholar * Jee MH, Hong KY, Park JH, Lee JS, Kim HS,
Lee SH, _et al_. New mechanism of hepatic fibrogenesis: Hepatitis C virus infection induces transforming growth factor beta1 production through glucose-regulated protein 94. _J Virol_ 2015;
90: 3044–55. Article PubMed Google Scholar * Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, _et al_. Translation attenuation through eIF2alpha phosphorylation prevents oxidative
stress and maintains the differentiated state in beta cells. _Cell Metab_ 2009; 10: 13–26. Article CAS PubMed PubMed Central Google Scholar * Gu S, Chen C, Jiang X, Zhang Z .
ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction underlie apoptosis induced by resveratrol and arsenic trioxide in A549 cells. _Chem Biol Interact_ 2016; 245: 100–9.
Article CAS PubMed Google Scholar * Olden K, Pratt RM, Jaworski C, Yamada KM . Evidence for role of glycoprotein carbohydrates in membrane transport: specific inhibition by tunicamycin.
_Proc Natl Acad Sci U S A_ 1979; 76: 791–5. Article CAS PubMed PubMed Central Google Scholar * Zhou L, Jiang L, Xu M, Liu Q, Gao N, Li P, _et al_. Miltirone exhibits antileukemic
activity by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction pathways. _Sci Rep_ 2016; 6: 20585. Article CAS PubMed PubMed Central Google Scholar * Boya P, Cohen
I, Zamzami N, Vieira HL, Kroemer G . Endoplasmic reticulum stress-induced cell death requires mitochondrial membrane permeabilization. _Cell Death Differ_ 2002; 9: 465–7. Article CAS
PubMed Google Scholar * Win S, Than TA, Fernandez-Checa JC, Kaplowitz N . JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. _Cell
Death Dis_ 2014; 5: e989. Article CAS PubMed PubMed Central Google Scholar * Orrenius S, Gogvadze V, Zhivotovsky B . Mitochondrial oxidative stress: implications for cell death. _Annu
Rev Pharmacol Toxicol_ 2007; 47: 143–83. Article CAS PubMed Google Scholar * Murphy MP . How mitochondria produce reactive oxygen species. _Biochem J_ 2009; 417: 1–13. Article CAS
PubMed Google Scholar * Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, Ramachandrarao SP, _et al_. Selective role for superoxide in InsP3 receptor-mediated mitochondrial
dysfunction and endothelial apoptosis. _J Cell Biol_ 2005; 170: 1079–90. Article CAS PubMed PubMed Central Google Scholar * Galloway CA, Yoon Y . Perspectives on: SGP symposium on
mitochondrial physiology and medicine: what comes first, misshape or dysfunction? The view from metabolic excess. _J Gen Physiol_ 2012; 139: 455–63. Article CAS PubMed PubMed Central
Google Scholar * Tirasophon W, Welihinda AA, Kaufman RJ . A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein
kinase/endoribonuclease (Ire1p) in mammalian cells. _Genes Dev_ 1998; 12: 1812–24. Article CAS PubMed PubMed Central Google Scholar * Calfon M, Zeng H, Urano F, Till JH, Hubbard SR,
Harding HP, _et al_. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. _Nature_ 2002; 415: 92–6. Article CAS PubMed Google Scholar * Lee AH,
Iwakoshi NN, Glimcher LH . XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. _Mol Cell Biol_ 2003; 23: 7448–59. Article CAS
PubMed PubMed Central Google Scholar * Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ . Complexes containing activating transcription factor
(ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response.
_Biochem J_ 1999; 339: 135–41. CAS PubMed PubMed Central Google Scholar * Palam LR, Baird TD, Wek RC . Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF
to enhance CHOP translation. _J Biol Chem_ 2011; 286: 10939–49. Article CAS PubMed PubMed Central Google Scholar * Willems PH, Rossignol R, Dieteren CE, Murphy MP, Koopman WJ . Redox
homeostasis and mitochondrial dynamics. _Cell Metab_ 2015; 22: 207–18. Article CAS PubMed Google Scholar * Picard M, Shirihai OS, Gentil BJ, Burelle Y . Mitochondrial morphology
transitions and functions: implications for retrograde signaling? _Am J Physiol Regul Integr Comp Physiol_ 2013; 304: R393–406. Article CAS PubMed PubMed Central Google Scholar *
Gledhill JR, Montgomery MG, Leslie AG, Walker JE . Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. _Proc Natl Acad Sci U S A_ 2007; 104: 13632–7. Article
CAS PubMed PubMed Central Google Scholar * Holtz WA, Turetzky JM, Jong YJ, O'Malley KL . Oxidative stress-triggered unfolded protein response is upstream of intrinsic cell death
evoked by parkinsonian mimetics. _J Neurochem_ 2006; 99: 54–69. Article CAS PubMed Google Scholar * Kim SH, Kim KY, Yu SN, Seo YK, Chun SS, Yu HS, _et al_. Silibinin induces
mitochondrial NOX4-mediated endoplasmic reticulum stress response and its subsequent apoptosis. _BMC Cancer_ 2016; 16: 452. Article PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS This work was supported by the Basic Science Research Program (2011-0011433 and 2014R1A1A4A01004329), the Bio & Medical Technology Development Program
(2012M3A9C3050632), and the Priority Research Centers Program (2014R1A6A1030318) of the National Research Foundation of Korea (NRF) funded by the Korean government. AUTHOR INFORMATION Author
notes * Jae-woo Park and Woo-gyun Choi: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * School of Biological Sciences, University of Ulsan, Ulsan, 44610, Korea
Jae-woo Park, Woo-gyun Choi, Su-wol Chung, Byung-sam Kim, Hun-taeg Chung & Sung-hoon Back * School of Pharmacy, Ajou University, Suwon, 16499, Korea Phil-jun Lee, Hyoungsu Kim &
Hong-pyo Kim * Targeted Medicine Research Center, Korea Research Institute of Bioscience and Biotechnology, Chungbuk, 28116, Cheongwon Sungchan Cho * Department of Biomolecular Science,
University of Science and Technology, Daejeon, 34554, Korea Sungchan Cho * and Department of System Cancer Science, Cancer Cell and Molecular Biology Branch, Research Institute, Graduate
School of Cancer Science and Policy, National Cancer Center, Goyang, 10408, Korea Jong-heon Kim * School of Biological Sciences, Ulsan National Institute of Science and Technology (UNIST),
Ulsan, 44919, Korea Byoung-heon Kang Authors * Jae-woo Park View author publications You can also search for this author inPubMed Google Scholar * Woo-gyun Choi View author publications You
can also search for this author inPubMed Google Scholar * Phil-jun Lee View author publications You can also search for this author inPubMed Google Scholar * Su-wol Chung View author
publications You can also search for this author inPubMed Google Scholar * Byung-sam Kim View author publications You can also search for this author inPubMed Google Scholar * Hun-taeg Chung
View author publications You can also search for this author inPubMed Google Scholar * Sungchan Cho View author publications You can also search for this author inPubMed Google Scholar *
Jong-heon Kim View author publications You can also search for this author inPubMed Google Scholar * Byoung-heon Kang View author publications You can also search for this author inPubMed
Google Scholar * Hyoungsu Kim View author publications You can also search for this author inPubMed Google Scholar * Hong-pyo Kim View author publications You can also search for this author
inPubMed Google Scholar * Sung-hoon Back View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Sung-hoon Back.
ADDITIONAL INFORMATION Supplementary information is available on the website of Acta Pharmacologica Sinica. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE S1 Mitochondria labeling with
MMP-dependent MitoTracker Red in Res-006-treated THLE-2 and HepG2 cells (DOC 1679 kb) SUPPLEMENTARY METHODS Synthesis of Resveratrol-005 (DOC 253 kb) SUPPLEMENTARY MOVIE S1 a mock-treated
HepG2 cell. (AVI 3471 kb) SUPPLEMENTARY MOVIE S2 a Res-006-treated HepG2 cell (AVI 679 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG.
3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 POWERPOINT SLIDE FOR FIG. 6 POWERPOINT SLIDE FOR FIG. 7 POWERPOINT SLIDE FOR FIG. 8 RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Park, Jw., Choi, Wg., Lee, Pj. _et al._ The novel resveratrol derivative 3,5-diethoxy-3′,4′-dihydroxy-_trans_-stilbene induces mitochondrial
ROS-mediated ER stress and cell death in human hepatoma cells _in vitro_. _Acta Pharmacol Sin_ 38, 1486–1500 (2017). https://doi.org/10.1038/aps.2017.106 Download citation * Received: 17
January 2017 * Accepted: 19 May 2017 * Published: 10 August 2017 * Issue Date: November 2017 * DOI: https://doi.org/10.1038/aps.2017.106 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative KEYWORDS * resveratrol * resveratrol-006 * HepG2 human hepatoma cells * mitochondria * ROS * endoplasmic reticulum stress * 4-phenylbutyrate * NAC *
Mito-TEMPO * apoptosis