Play all audios:
ABSTRACT Oxygen minimum zones (OMZs) contain the largest pools of oceanic methane but its origin and fate are poorly understood. High-resolution (<15 m) water column profiles revealed a
300 m thick layer of elevated methane (20–105 nM) in the anoxic core of the largest OMZ, the Eastern Tropical North Pacific. Sediment core incubations identified a clear benthic methane
source where the OMZ meets the continental shelf, between 350 and 650 m, with the flux reflecting the concentration of methane in the overlying anoxic water. Further incubations
characterised a methanogenic potential in the presence of both porewater sulphate and nitrate of up to 88 nmol g−1day−1 in the sediment surface layer. In these methane-producing sediments,
the majority (85%) of methyl coenzyme M reductase alpha subunit (_mcrA_) gene sequences clustered with Methanosarcinaceae (⩾96% similarity to _Methanococcoides_ sp.), a family capable of
performing non-competitive methanogenesis. Incubations with 13C-CH4 showed potential for both aerobic and anaerobic methane oxidation in the waters within and above the OMZ. Both aerobic and
anaerobic methane oxidation is corroborated by the presence of particulate methane monooxygenase (_pmoA_) gene sequences, related to type I methanotrophs and the lineage of _Candidatus_
Methylomirabilis oxyfera, known to perform nitrite-dependent anaerobic methane oxidation (N-DAMO), respectively. SIMILAR CONTENT BEING VIEWED BY OTHERS THERMOCHEMICAL OXIDATION OF METHANE BY
MANGANESE OXIDES IN HYDROTHERMAL SEDIMENTS Article Open access 24 June 2023 SEAWATER SULPHATE HERITAGE GOVERNED EARLY LATE MIOCENE METHANE CONSUMPTION IN THE LONG-LIVED LAKE PANNON Article
Open access 13 June 2023 METHANE LEAKAGE THROUGH THE SULFATE–METHANE TRANSITION ZONE OF THE BALTIC SEABED Article 06 December 2024 INTRODUCTION Methane is the most abundant hydrocarbon in
the atmosphere and a potent greenhouse gas, which has contributed ~20% to the Earth’s warming since pre-industrial times (Lashof and Ahuja, 1990; Reeburgh, 2007; Kirschke et al., 2013). The
marine environment encompasses large reservoirs of methane (Zhang et al., 2011; Pack et al., 2015), particularly in oxygen minimum zones (OMZs). Here, oxygen is consumed faster than it is
resupplied, resulting in a layer of hypoxic waters surrounding a functionally anoxic core (Thamdrup et al., 2012), where methane accumulates (Wright et al., 2012). Under a warming climate,
the dissolution of oxygen in seawater will decrease, whereas its consumption through respiration will likely increase (Vázquez-Domínguez et al., 2007) and thermal stratification could become
more intense. Together, these biotic and abiotic changes will thicken OMZs moving these large methane pools closer to the zone of atmospheric exchange (Stramma et al., 2008; Keeling et al.,
2010; Helm et al., 2011). Marine methanogenesis, which produces 0.7–1.4Tg each year (Krüger et al., 2005), forms an essential link in the carbon cycle, preventing the long-term burial of
carbon in the sediments by mineralising it and returning it to the water in gaseous form (Ferry and Lessner, 2008). The thermodynamics of organic matter oxidation dictate that sulphate
reduction and methanogenesis should be mutually exclusive reactions. Although the clear spatial partitioning of these two microbial processes has been widely observed in marine sediments
(Martens and Berner, 1977; Whiticar, 2002; Reeburgh, 2007), non-competitive methanogenesis can co-occur with other anaerobic processes (Valentine, 2011). Non-competitive methanogenisis
disproportionates methylated substrates (for example, methyl amine, methane thiols and methanol) to yield methane and carbon dioxide, and since its discovery in the early 1980s (Oremland et
al., 1982), it has been found to occur in all major oceans (D’Hondt et al., 2002; Mitterer, 2010; Valentine, 2011). However, relatively little scientific attention has focused on microbial
methanogenesis compared with that around gas hydrates and cold seeps (Shakhova et al., 2005; Valentine, 2011; Boetius and Wenzhofer, 2013). The majority of methane produced in marine
sediments is thought to be oxidised anaerobically, limiting its flux to the overlying water (Knittel and Boetius, 2009). Any methane leaking into the water column may still be oxidised, by
pelagic aerobic or anaerobic bacteria, which form a final barrier preventing its escape to the atmosphere (Reeburgh et al., 1991; Blumenberg et al., 2007; Kessler et al., 2011; Heintz et
al., 2012). We focused on locating the origin of methane in the Eastern Tropical North Pacific (ETNP), between 70 and 720 km off the Guatemalan coast. The ETNP OMZ is both the world’s
largest OMZ (Paulmier and Ruiz-Pino, 2009) and the largest reservoir of oceanic methane (Sansone et al., 2001, 2004; Reeburgh, 2007; Naqvi et al., 2010). Here, the methane is thought to be
formed by a combination of decomposing sinking organic matter and coastal or benthic sources but neither have been directly measured (Sansone et al., 2001, 2004). Porewater and bottom-water
methane concentrations along with stable isotope ratio data suggested the sediments were the source of the pelagic methane and the flux was greatest where the anoxic core of the OMZ touched
the sediment (western Mexican margin, Sansone et al. (2004)). Although these studies offer useful insights, there are no direct measurements of sediment methanogenesis or methane efflux in a
marine OMZ. Pelagic methane oxidation in marine environments is a rarely quantified process but on the margins of an OMZ, where methane intersects traces of oxygen, it could be a
significant process. Published rates span ~0.001–10 nmol l−1day−1 and all studies used either 3H-CH4 or LL-14C as a tracer (Mau et al., 2013). The only study to have successfully measured
methane oxidation in the ETNP OMZ (Pack et al., 2015) found exceptionally slow rates (0.000034–4 nmol l−1day−1), which could explain how the methane, if of benthic origin, can be sustained
hundreds of kilometres offshore. We used high-resolution water column profiles to show that methane concentrations peak in the anoxic core of the ETNP OMZ. We then used a combination of
water and sediment incubations, along with stable isotope tracers and molecular analyses, to quantify sediment methane flux, methanogenic potential and pelagic methane oxidation potentials.
We hypothesised that all sediments would contain active methanogens, but that their activity would be controlled by the oxygen concentration in the bottom-water. Further, the methane
released from the sediments is then oxidised by aerobic and/or anaerobic methanotrophs in the water column as it moves towards the OMZ margins. To the best of our knowledge, this is the
first study to combine biogeochemical with molecular microbial data, in order to better understand the origins and fate of methane in the ocean’s largest OMZ. MATERIALS AND METHODS SAMPLE
SITES This study comprised two cruises in the ETNP: the first (D373, 11 December 2011–13 January 2012), which focused on the water column (0–4000 m), was structured around 6 ‘offshore’ sites
located along 92.5oW, between 8 and 13°N (Supplementary Figure S1). The second (JC097, 28 December 2013–10 February 2014), concentrated on the continental shelf and slope, 70–150 km off the
Guatemalan coast (Supplementary Figure S1) and here, both sediments and water column samples were collected. A standard conductivity–temperature–depth rosette, comprising 24 Niskin (20
litre) bottles and a Sea-Bird 24 electronics system (fluorimeter, altimeter, photosynthetically active radiation and oxygen sensors, and so on) was used to collect water and a multi-corer
(Mega Corer, OSIL, Havant, UK) was used to recover intact cores of sediment and overlying water. WATER COLUMN GAS AND NUTRIENT PROFILES High-resolution (5–15 m) water column profiles
(_n_=21) were constructed to define the OMZ and locate the methane. To minimise atmospheric contamination, water for methane analysis was discharged from the Niskin bottles into 12.5 ml
gas-tight vials (Labco, Lampeter, UK) via Tygon tubing and allowed to overflow three times before capping, temperature equilibration and head-spacing (2 ml helium (BOC, Guildford, UK)).
Methane was measured on-board using a gas chromatograph fitted with a flame ionisation detector (gas chromatography/flame ionization detector Agilent Technologies (Santa Clara, CA, USA), see
Sanders et al. (2007) for details). Oxygen concentrations were measured by the Sea-Bird sensor (Bellevue, WA, USA) (with a limit of detection (LOD) ~1.4 μmol l−1) and nitrite was measured
using a segmented flow auto-analyser (Skalar, Breda, Netherlands; LOD=0.05 μmol l−1, Nicholls et al., 2007). SEDIMENTS AS A METHANE SOURCE Sediment-water flux was determined using intact
cores and the methanogenic potential of discrete layers was quantified using slurries. As the conductivity–temperature–depth could not sample closer than ~10 m from the seabed, the water
overlying the sediment (_n_=3 from the least disturbed core) was sampled, as above, to measure the methane concentration as close to the seabed as possible (<15 cm). Next, six sediment
mini-cores were subsampled from three of the large cores (using Perspex tubes, 3.4 × 25 cm), sealed with rubber bungs and transferred to a temperature controlled (10 °C) tank. This was
repeated at 16 locations ranging in seabed depth from 100 to 900 m. Methane flux was quantified by measuring methane in the overlying water before and after a sealed 24-h incubation. First,
the overlying water was degassed by bubbling (2 min) with oxygen-free nitrogen (BOC), to ensure all cores were incubated under the same hypoxic conditions (precise concentration verified
using an oxygen micro-sensor, Unisense, Aarhus, Denmark) and that the majority of ambient methane was removed (previous experiment had demonstrated that 2 min was sufficient to remove
>90% methane). Water samples were taken from each mini-core after degassing (T0), they were then sealed with bungs with inbuilt magnetic stirrers, and left for 24 h in the dark until a
second water sample (Tfinal) was taken for methane analysis. The daily flux of methane was calculated as the increase between T0 and Tfinal. To identify the sediment layer with the greatest
methanogenic potential, additional large sediment cores (six locations, Table 1) were carefully extruded and ~4 ml of sediment and 3 ml of bottom water (overlying the cores) was transferred
to gas-tight vials using a truncated 1 ml syringe (to minimise air contamination) to create a slurry. The headspace and water was purged with helium for 2 min to deoxygenate the vials and
optimise conditions for methanogenesis. The methane concentration in the headspace was measured by gas chromatography/ flame ionization detector 4–8 times over the following 4–12 days and
between measurements vials were kept at 12 °C in the dark. Following the first two experiments (550 m and 650 m), only the top 5 cm was used for further sites. The concentration of sulphate,
nitrite and nitrate in the sediment porewater was measured in eight large cores from four different locations (150, 350, 550 and 750 m seabed depth) by ion chromatography (Dionex,
Sunnyvale, CA, USA; for sulphate) and segmented flow auto-analyser (Skalar for nitrite and nitrate), after separating the porewater from the sediment by centrifugation. Finally, hydrogen
sulphide was measured in the cores by inserting a calibrated, miniaturised amperometric sensor (Unisense) into an extruded portion of the core, from the side, at 2 cm intervals. AEROBIC AND
ANAEROBIC WATER COLUMN METHANE OXIDATION We set up four experiments using 13C-labelled methane to quantify the potential for aerobic and anaerobic methane oxidation in the water column
(Supplementary Figure S2). First, we set up short time experiments with water from the upper margin of the OMZ, where oxygen is at the LOD (200 and 226 m). Seawater saturated with 99.9%
13C-CH4 was used to spike the samples with 3.3 nmol 13C-CH4 (264 nmol L−1) to avoid the need for a headspace and, thus, maintain ambient oxygen conditions. Samples were fixed (100 μl of 12.2
m HCl) at 3–5 time points over 10–15 days to track the accumulation of dissolved inorganic carbon (13C-DIC). Second, we set up dose-response experiments (65 and 200 m), whereby we varied
the injection volume to give a range of methane concentrations (44–790 nmol l−1) to assess the extent to which the methanotrophic community was substrate limited. These were left for the
duration that the samples took to get back to the UK (5 months), without a headspace, before fixing. Third, we started more widespread long-term incubations (eight locations) at one methane
concentration (264 nmol l−1) and after fixing a sample within 2 min (control), we incubated the remaining samples for 5 months at 12 °C in the dark. Finally, to test for the potential for
nitrite-dependent anaerobic methane oxidation (N-DAMO) we incubated water from five depths spanning the upper boundary and into the core of the OMZ, where nitrite and methane were both
present (235–412 m, Supplementary Figure S2 and Table 2) with 13C-CH4 (3.4 μmol l−1) and 15N-NO2 (11.4 μmol l−1), just 13C-CH4 or no spike. These samples were taken from depths with no
detectable oxygen but, to ensure complete anoxia in the water, we introduced a 2 ml helium headspace before adding the gas spikes. For all four types of incubations, we included a reference
sample (no spike, fixed at same time as samples), a spiked-control (spiked with 13C-CH4 and/or 15NO2 and killed at the beginning of the experiment) and three technical replicates of each
treatment. Microbial activity was stopped, and any resulting 13C-DIC converted to 13C-CO2 for analysis, by injecting HCl through the septa (as above). See Supplementary Figure S2 for sample
location details. Upon return to the UK, all unfixed samples were fixed and a 2 ml helium headspace was introduced into those incubated without one. To confirm the initial CH4 concentration,
spiked-control samples were analysed on a gas chromatography/flame ionization detector and then the 13C-DIC (and 15N-N2 where necessary) was quantified using an elemental analyser
interfaced with a continuous flow isotope ratio mass spectrometer (Sercon 20–22, Sercon Group, Crewe, UK), calibrated against sodium bicarbonate (0–4 mM for DIC) or air (for N2). MOLECULAR
ANALYSIS Water (see Supplementary Table S1 for details) was filtered either through stand alone pumps or Nalgene filtration units (Supor, Pall, Port Washington, NY, USA; ø 293 mm for stand
alone pumps or ø 47 mm for Nalgene units, 0.2 μm pore size filters). Filters were immediately frozen in liquid nitrogen, placed in –80 °C freezer and transferred to the UK for DNA
extraction. Sediments (seabed depth 222 , 342, 550, 650 and 657 m) were collected from the top 2 cm of the sediment cores into 2 ml cryovials and frozen (as above) until DNA extraction.
Details of the extraction process and downstream analysis are given in Supplementary Information. ACCESSION NUMBERS The DNA sequences reported in this study were deposited in the EMBL
database under the accession numbers LT575999–LT576028. RESULTS WATER COLUMN PROFILES Over the two cruises, we constructed 21 water column profiles covering seabed depths ranging from 55 to
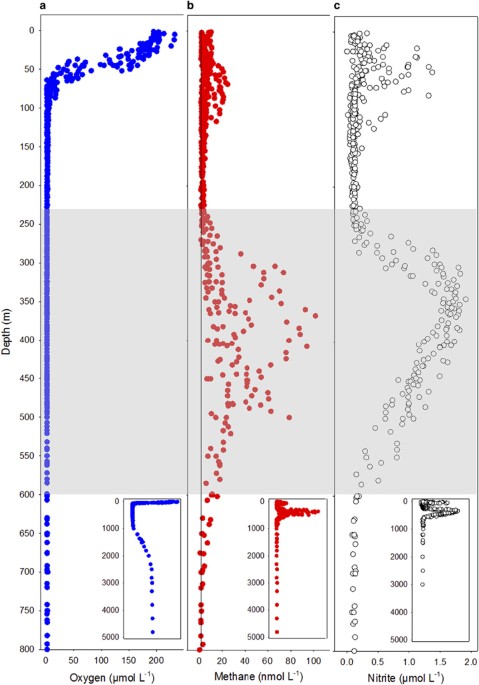
5320 m. Oxygen declined steeply from an average of 193 μmol l−1 in the top 20 m, to 6.4 μmol l−1 at 80 m and then slowly until it went below our LOD (1.4 μmol l−1) at 230 m (Figure 1). Below
800 m, oxygen returned to detectable concentrations and reached 100 μmol l−1 (30% saturation) at 2500 m where it remained stable until the seabed (>5000 m). The baseline nitrite
concentrations were 0.05 (LOD) to 0.2 μmol l−1 but within the OMZ, at 275–600 m, there was a large, secondary nitrite maximum of up to 1.8 μmol l−1 at 345 m and in the epipelagic waters, a
smaller, primary maximum at around 50 m (max 1.37 μmol l−1) in most profiles (Figure 1). The true anoxic core of the OMZ was where oxygen was below the LOD and a clear secondary nitrite
maximum was present (230–600 m). Methane was supersaturated relative to the atmosphere throughout the water column and there was a clear peak around 250–600 m (Figure 1). The maximum
concentration (102 nmol l−1) was measured at 368 m on the continental shelf where the seabed depth was 506 m, although very close to the sediments (<15 cm) 254 nmol l−1 was measured.
Outside the core of the OMZ, the methane was consistently above atmospheric equilibration at 3–5 nmol l−1, except for a small epipelagic methane peak (Figure 1), which was only found in some
of the profiles (maximum concentration 25 nmol l−1 at 65 m). METHANE FLUX, METHANOGENESIS AND THE METHANOGEN COMMUNITY In the intact, anoxic core incubations, methane flux averaged 262±65.5
nmol m−2day−1, peaking at 1007 nmol m−2day−1 at 550 m and with the slowest of 162 nmol m−2day−1 measured at 300 m (Figure 2a). Although all sediment cores were degassed to remove oxygen
(that is, optimal conditions for methanogenesis), methane efflux was greater in sediments from locations where the OMZ intersected the shelf (indicated by shaded area on Figure 2a) compared
with those with oxygenated (2–4.6 μmol l−1) bottom-water (_X_2(1)=13.261, _P_<0.0001). The efflux of methane from the sediments was positively correlated with the concentration of methane
in the bottom-water (_X_2(1)=23.233, _P_<0.0001), which ranged from 6 to 254 nmol l−1 (Figure 2b). Further, there was a strong, non-linear inverse relationship between the concentration
of methane and oxygen in the bottom-water (Figure 2b, inset, LOD~1.4 μM O2). Incubating anoxic sediment slurries from discrete depth intervals from two locations revealed that the bulk of
the methanogenic potential was in the surface sediments (Figure 2c) and so all further experiments were focused on this layer (Table 1). Methanogenesis was also detected in the overlying
water (0–5 cm above sediment) and in sediments down to 25 cm, but in the uppermost layer the rate was at least an order of magnitude higher than any other depth (Figure 2c). The greatest
potential was measured in sediment from 750 m (88 nmol g−1day−1, Table 1). Hydrogen sulphide was detected in two out of the six cores in which it was measured; at 550 m the concentration
reached 59 μmol l−1 at 25 cm (Figure 2c) and in a core from 350 m it reached 219 μmol l−1 at 23 cm. The porewater profiles (Supplementary Figure S3) revealed that in the top 2 cm of
sediment, where methane production was most active, sulphate (23 mmol l−1), nitrite (2.8 μmol l−1) and nitrate (86 μmol l−1) were similar to, or above, bottom-water concentrations. A total
of 126 303 _mcrA_ gene sequences from the top 2 cm were retrieved and clustered into 16 operational taxonomic units (OTUs), hereafter named as ETNP_MG. The majority of sequences (50% of
sequences represented by ETNP_MG2 and 35% represented by ETNP_MG1) were 96–97% similar to _Methanococcoides_ sp. (Supplementary Table S2). Most of the OTUs (13 out of 16) clustered within
the order Methanosarcinales, whereas two OTUs (ETNP_MG3 and ETNP_MG5) clustered within the order Methanomicrobiales and one (ETNP_MG14) within the order Methanocellales (Figure 3a). The
_Methanococcoides_-like species dominated all five seabed samples, which displayed a similar level of intra-sample diversity (assessed by Shannon and Simpson indices, Supplementary Table
S2). Principal coordinate analysis indicates that most of the variation (91.2%) in the methanogen community is explained by the first two principal coordinates (58.5% of the variation
explained by axis 1 and 32.7% by axis 2, Figure 3b). The most separated communities, by the first coordinate, are those from 650 to 222 m, because of unique Methanomicrobiales (ETNP_MG5) and
Methanocellales (ETNP_MG14) sequences at 650 m and Methanosarcinales at 222 m. The community at 342 m is also somewhat separated from the others, as it is the only one with ETNP_MG3
Methanomicrobiales-like sequences. METHANE OXIDATION AND METHANOTROPHS Methane oxidation was measured, through the accumulation of 13C-DIC, in short-term (10–15 days) time series incubations
with water from the uppermost margins of the OMZ (Figure 4a and Supplementary Figure S2); 13C-DIC was produced at a rate of 3.0–5.9 nmol l−1 day−1, and, after 15 days, 26% of the 13C-CH4
had been oxidised to 13C-DIC. In the epipelagic zone, we could not measure any methane oxidation at 47 m but we did at 65 m (Table 2). Our dose-response experiments indicate that the
methanotrophs can oxidise methane at concentrations much higher than the ambient concentration (Figure 4b). Water from both the epipelagic (65 m) and mesopelagic waters (200 m, where oxygen
was below detection) oxidised increasing amounts of 13C-CH4 to 13DIC with increasing initial methane spike (Figure 4b) and there was a good correlation between methane oxidised and 13C-DIC
produced (R2=0.96). This relationship between 13C-DIC produced and starting methane concentration was linear (_R_2(200m)=0.94 and _R_2(65m)=0.54) for the range of concentrations tested
(85–760 nmol l−1) and the slope (b1(200m)=0.0055 and b1(65m)=0.0057) of the relationship was similar for the two different water samples (Figure 4b). Long-term (5 months) incubations from
eight different locations (47–228 m), yielded mixed results, with methane oxidation being undetectable in some vials (Table 2). The greatest amount of 13C-DIC produced was at 200 m where,
following a 3.3 nmol spike of 13C-CH4, 0.6 nmol 13C-DIC was measured in the water after 5 months. In the shorter incubations, water from the same location, produced a similar amount in only
10 days (Figure 4a), which indicates methane oxidation did not continue linearly during the 5 months. For comparison, if a total of 0.6 nmol 13C-DIC in the vial accumulated linearly, after
150 days, it would equate to 0.42 nmol l−1 day−1, which is 14 times slower than that measured in the short-term incubations. Water incubated with 13CH4 and 15NO2− did produce both 13DIC
(0.9–15.4 nmol) and 29+30N2 (8.9–80.5 nmol), and the relative proportions produced varied across depths with high rates of 29+30N2 production at 235 and 264 m, indicative of nitrite
reduction alongside methanotrophy (Table 2 and Supplementary Figure S2). Methanotrophic bacteria were targeted in waters offshore (30–1250 m depth) and closer to the coast (200 and 228 m).
Analysed aerobic _pmoA_ sequences in the offshore samples (6202 in total) were clustered into six OTUs, hereafter called ETNP_Offshore_MO, and in the inshore samples (363 816 in total) were
clustered into six OTUs, hereafter called ETNP_Inshore_MO. The sequences from both the offshore and inshore samples were highly similar (97–100% BLAST similarity) to uncultured bacteria from
marine environments (Supplementary Table S3, Figure 5a). The vast majority of the offshore sequences are represented by two OTUs, that is, ETNP_Offshore_MO1 (44.16% of sequences) and
ETNP_Offshore_MO2 (47.19% of sequences). Similarly, ETNP_Inshore_MO1 represents the majority (89.43%) of the analysed inshore sequences. Phylogenetic analysis shows that all the OTUs cluster
within known type I methanotrophs (Figure 5a). Among them, three OTUs of the offshore samples (ETNP_Offshore_MO1/MO3/MO5) sit within a sub-cluster of the family Methylococcaceae including
_Methylococcus_ and _Methylomonas_ species. The diversity (based on Shannon and Simpson indices) within all the analysed samples and particularly of the inshore ones is small, with the most
diverse sample being that of 290 m offshore (Shannon=1.19, Simpson=0.64; Supplementary Table S3). The principal coordinate analysis plot also shows a very close proximity of all the inshore
samples (that is, green circles on Figure 5b practically overlap), whereas there is some variance among the offshore samples, as indicated by their good separation along the second principal
coordinate, that is, axis 2, explaining 26.9% of the observed variance (triangles in Figure 5b). However, most of the principal coordinate analysis variance is explained by the first
principal coordinate (axis 1, explaining 70.8% of the variance), which is mainly driven by the divergence of the anaerobic methanotroph community (overlapping purple circles on Figure 5b)
and, to a lesser extent, by the divergence of two offshore aerobic methanotroph samples (30 and 645 m; blue triangles on Figure 5b). The diversity within the two OTUs of the anaerobic
methanotrophs (ETNP_NDAMO1 and ETNP_NDAMO2) is minimal (see Shannon and Simpson indices, Supplementary Table S3). Indeed, phylogenetic analysis placed both of these OTUs into a separate and
well-defined cluster, related to the _Candidatus_ Methylomirabilis oxyfera anaerobic methanotroph (Figure 5a). DISCUSSION Here we have shown that biological methanogenesis, in the surface
layer of the seabed sediments, is a major source of methane to the ETNP OMZ. These are the first direct measurements of methane production in sediments from this region. The reactivity of
these sulphate and nitrate-rich surface sediments highlights the potential importance of non-competitive methanogenesis to the marine methane pool. Our oxygen profiles agree with previously
published data for the ETNP OMZ (Burke et al., 1983; Sansone et al., 2001, 2004; Pack et al., 2015) and show oxygen to be ⩽1.4 μmol l−1 between 200 and 800 m. However, as a secondary nitrite
maximum occurs when oxygen is below 0.05 μmol l−1 (Thamdrup et al., 2012), we used this profile to define the true core of the OMZ (230–600 m). In addition, we have presented evidence for
microbial methane oxidation, which can be sustained under a wide range of oxygen (<1.4–65 μmol l−1) and methane (44–790 nmol l−1) concentrations, potentially controlling the release of
methane emissions from the OMZ. The highest potential for methanogenesis is in the top 2 cm of seabed, in the presence of ample sulphate, nitrate and nitrite (as alternative electron
acceptors) and >20 cm above the hydrogen sulphide peak (Figure 2c, Table 1). The co-occurrence of the greatest potential for methanogenesis and highest concentration of sulphate indicates
that this is likely to be non-competitive methanogenesis and this is supported by the methanogen community findings. The majority of methanogens in all the analysed samples (97.31% of total
sequences) clustered within the family Methanosarcinaceae and the dominant OTUs were similar to _Methanococcoides_ sp. deriving from sub-seafloor sediments (Imachi et al., 2011) or
estuarine mudflats (Watkins et al., 2012). _Methanococcoides_ sp. have often been isolated from marine sediments (for example, Singh et al., 2005; Lazar et al., 2011; Webster et al., 2015)
and they are obligatory methylotrophic methanogens, that is, utilising only non-competitive substrates, such as methanol or methylamines (Garcia et al., 2000; Ferry, 2010). Although we could
not find other direct measurements of methanogenesis in the ETNP OMZ, there are data reported from other locations. Krüger et al. (2005) reported rates of methanogenesis in sediment surface
slurries from eight different marine sites in the Atlantic, Pacific and Arctic Oceans and the North and Baltic Seas, and their results (0.01–0.1 μmol g−1 day−1) agree well with our
measurements using the same technique (0.001–0.09 μmol g−1 day−1, Table 1). They noted that the highest methanogenic potentials were measured in regions with high input of organic matter
from the water column (Krüger et al., 2005). The only intact core experiment (Crill and Martens, 1983) to report marine methane flux was performed on coastal sediments from Cape Lookout
Bight, and showed a similar range (0.18–1.56 μmol m−2 day−1) to those found in our ETNP sediments (0.16–1.01 μmol m−2 day−1). The potential for methanogenesis was markedly reduced below the
top 2 cm and we propose that this is linked to organic carbon supply raining down from above, which the surface methanogens can preferentially access. Continental shelves are known for high
productivity and therefore, the delivery of carbon to the seafloor is high relative to less productive areas of the ocean (Ramaswamy et al., 2008; Fennel, 2010). We propose that this benthic
methanogenesis supplies the water column with methane, which persists far offshore. The location of the methane peak (250–700 m), agrees well with other ETNP studies but the magnitude in
our study (102 nmol l−1 using the conductivity–temperature–depth and 254 nmol l−1 very close to the sediment surface using Mega-Cores) was considerably higher than previously reported
(maximum 5–80 nmol l−1, Burke et al., 1983; Sansone et al., 2001, 2004 and Pack et al., 2015). Variation in maximum concentrations found across the ETNP is likely due to proximity to the
source of methane, dilution and slow microbial oxidation. The flux was greatest when oxygen concentration in the bottom-water was below the LOD (Figure 2a) and a clear plume, originating in
the continental shelf slope and extending offshore, can be seen in our profiles (Supplementary Figure S1), both of which support our theory. Even when there was a wedge of oxygenated water
between the OMZ and the seafloor, methane was supersaturated in the OMZ and the maximum concentration of methane decreased with distance offshore. Indeed, methane was only found to be over
35 nmol l−1 when the maximum water depth was between 350 and 650 m, and in the deeper water (seabed >750 m) the methane did not exceed 25 nmol l−1 even when oxygen and nitrite indicated
true OMZ conditions. The close agreement between our potential methanogenesis rates and the flux data show that benthic methanogenesis could be responsible for all the methane measured in
the bottom-water without the need to invoke additional methane sources, for example, seeps or dissociation of hydrates. Further, to the best of our knowledge, there are no reports of methane
seeps in this OMZ. The spatial alignment of the methane and nitrite peaks suggests that methane could be oxidised, in the presence of nitrite and the absence of measureable oxygen, that is,
anaerobically. Our attempts to measure the potential for N-DAMO were inconclusive, and others (Padilla et al., 2016) using a similar dual-isotope incubation technique, recently tried and
failed to fully quantify this process in the ETNP OMZ. However, in our experiments, water incubated with 13C-CH4 and 15N-NO2 did produce 13C-DIC and 29+30N-N2 but the stoichiometry (Table 2)
was not indicative of pure N-DAMO (3CH4 and 8NO2 produce 3CO2 and 4N2, Ettwig et al., 2010), nor were the rates of 13C-DIC production consistently stimulated by addition of nitrite.
Nonetheless, sequences from N-DAMO-like bacteria were detected in all the targeted water depths. The sequences belonged to just two closely related phylotypes (ETNP_NDAMO_1 and ETNP_NDAMO_2;
Figure 5,Supplementary Table S3) affiliated with uncultured anaerobic methanotrophs from South China Sea sediments (Chen et al., 2014, 2015). They clustered within the _Candidatus_
Methylomirabilis oxyfera lineage, which is known to couple anaerobic methane oxidation to the reduction of nitrite (Ettwig et al., 2010; Haroon et al., 2013) and, although they are well
described in lakes (Deutzmann and Schink, 2011; Kojima et al., 2012), paddy soils (Wang et al., 2012) and peatlands (Zhu et al., 2012), the ecological role of these phylotypes in marine
environments has only recently been addressed (Chen et al., 2014; Li-Dong et al., 2014). More recently, Padilla et al. (2016) reported transcriptionally active Methylomirabilis-like NC10
phylotypes in all their ETNP sites, off the North Mexican coast, with the abundance of 16S rRNA transcripts peaking in the core of the OMZ, thereby confirming marine OMZs as a niche for such
phylotypes. In agreement with recent findings in the South China Sea (Chen et al., 2015), we show that these marine phylotypes form a separate cluster from their equivalent freshwater
phylotypes. We were able to confirm the potential for aerobic methane oxidation in the OMZ of ETNP by measuring the conversion of 13C-CH4 to 13C-DIC over relatively short timescales (<2
weeks). We artificially raised the methane concentration, to ensure that 13C-CH4 (rather than 12C-CH4) constituted the overwhelming majority of the methane available for oxidation. However,
we can use our dose-response experiment to approximate ambient rates of methane oxidation. For example, at 200 m the average methane concentration was 3.3 nmol l−1 so although we measured
4.5 nmol l−1 day−1 (incubated with 300 nmol l−1 CH4) _in situ_ we would expect 0.0495 nmol l−1 day−1 with a turnover time of 67 days. Even in anoxic incubations (LOD for oxygen), 13C-DIC was
produced following a spike of 13C-CH4 and so it is reasonable to predict that methanotrophs are oxidising methane right at the margin of the OMZ core and our measurements fall within the
range recently reported for methane oxidation in the ETNP (0.000034–4 nmol l−1 day−1, Pack et al., 2015). Molecular analysis confirmed the presence of aerobic methane oxidisers at a wide
range of depths (ranging from 30 to 1250 m) in both offshore (ETNP_Offsshore_MO) and coastal (ETNP_Inshore_MO) waters. The majority of methanotrophs from inshore waters (99.96% of sequences)
were phylogenetically related (>97% similarity, Figure 5,Supplementary Table S3) to uncultured bacteria detected in the ETNP (Hayashi et al., 2007). The methanotrophs in the offshore
samples were somewhat more diverse, with some similar to those in the inshore samples and others forming a separate sub-cluster with known Methylococcaceae species (for example,
_Methylomonas methanica_ and _Methylococcus capsulatus_ str. Bath, Figure 5). This could be partly attributed to the difference in the range of depths from which the samples were obtained,
that is, offshore samples were collected from depths between 30 and 1250 m, whereas inshore samples were collected from a much narrower range of depths (200–228 m). Depth-related differences
in the aerobic methanotroph community along vertical water horizons have been reported elsewhere (for example, Tavormina et al., 2010, 2013). Such differences may be related to the physical
transport of waters, harbouring distinct microbial communities, which, along with environmental selection and spatial separation, has been shown to shape the distribution of marine microbes
(Wilkins et al., 2013; Steinle et al., 2015). The diversity of methanotroph phylotypes in the water column is likely controlled by environmental factors rather than geographical proximity,
and the same phylotypes may be adapted to a range of methane concentrations (Tavormina et al., 2008). Indeed, a later study of Cu-MMO phylotypes from the Costa-Rican OMZ showed that methane
concentration did not predict the occurrence, abundance or distribution of any phylotypes; instead environmental factors such as depth, salinity, temperature and dissolved oxygen
concentrations accounted for most of the observed phylotype variance (Tavormina et al., 2013). Aerobic methanotrophs in both offshore and inshore samples clustered within type I, whereas no
sequences were affiliated to type II methanotrophs, which is in accordance with the findings in other marine environments (Schubert et al., 2006; Tavormina et al., 2008; Wasmund et al.,
2009; Schmale et al., 2012). The lack of close affiliation of marine phylotypes with established methanotroph lineages has been reported previously and it has been linked to specialisation
of these phylotypes in marine environments and to the rather small representation of marine methanotroph sequences in public databases (Tavormina et al., 2008; Wasmund et al., 2009). Here we
present clear evidence for microbial methanogenesis in the continental shelf sediments fuelling the ETNP OMZ methane plume, which is sustained several 100 km offshore, despite biological
oxidation. Molecular analyses support the methanogenesis and methanotrophy potentials presented, however, more studies are needed to fully unravel the diversity of pelagic methanotrophs and
to determine the precise electron acceptors for anaerobic methane oxidation. REFERENCES * Blumenberg M, Seifert R, Michaelis W . (2007). Aerobic methanotrophy in the oxic–anoxic transition
zone of the Black Sea water column. _Org Geochem_ 38: 84–91. Article CAS Google Scholar * Boetius A, Wenzhofer F . (2013). Seafloor oxygen consumption fuelled by methane from cold seeps.
_Nat Geosci_ 6: 725–734. Article CAS Google Scholar * Burke RA, Reid DF, Brooks JM, Lavoie DM . (1983). Upper water column methane geochemistry in the Eastern Tropical North Pacific.
_Limnol Oceanogr_ 28: 19–32. Article CAS Google Scholar * Chen J, Jiang X-W, Gu J-D . (2015). Existence of novel phylotypes of nitrite-dependent anaerobic methane-oxidizing bacteria in
surface and subsurface sediments of the South China Sea. _Geomicrobiol J_ 32: 1–10. Article Google Scholar * Chen J, Zhou Z-C, Gu J-D . (2014). Occurrence and diversity of
nitrite-dependent anaerobic methane oxidation bacteria in the sediments of the South China Sea revealed by amplification of both 16S rRNA and pmoA genes. _Appl Microbiol Biotechnol_ 98:
5685–5696. Article CAS Google Scholar * Crill PM, Martens CS . (1983). Spatial and temporal fluctuation of methane production in anoxic coastal marine sediments. _Limnol Oceanogr_ 28:
1117–1130. Article CAS Google Scholar * D’Hondt S, Rutherford S, Spivack AJ . (2002). Metabolic activity of subsurface life in deep-sea sediments. _Science_ 295: 2067–2070. Article
Google Scholar * Deutzmann JS, Schink B . (2011). Anaerobic oxidation of methane in sediments of Lake Constance, an oligotrophic freshwater lake. _Appl Environ Microbiol_ 77: 4429–4436.
Article CAS Google Scholar * Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MMM _et al_. (2010). Nitrite-driven anaerobic methane oxidation by oxygenic bacteria.
_Nature_ 464: 543–548. Article CAS Google Scholar * Fennel K . (2010). The role of continental shelves in nitrogen and carbon cycling: Northwestern North Atlantic case study. _Ocean Sci_
6: 539–548. Article CAS Google Scholar * Ferry JG . (2010). How to make a living by exhaling methane. _Annu Rev Microbiol_ 64: 453–473. Article CAS Google Scholar * Ferry JG, Lessner
DJ . (2008). Methanogenesis in marine sediments. _Ann N Y Acad Sci_ 1125: 147–157. Article CAS Google Scholar * Garcia JL, Patel BK, Ollivier B . (2000). Taxonomic, phylogenetic, and
ecological diversity of methanogenic Archaea. _Anaerobe_ 6: 205–226. Article CAS Google Scholar * Haroon MF, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P _et al_. (2013). Anaerobic
oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. _Nature_ 500: 567–570. Article CAS Google Scholar * Hayashi T, Obata H, Gamo T, Sano Y, Naganuma T . (2007).
Distribution and phylogenetic characteristics of the genes encoding enzymes relevant to methane oxidation in oxygen minimum zones of the Eastern Pacific Ocean. _Res J Environ Sci_ 1:
275–284. Article CAS Google Scholar * Heintz MB, Mau S, Valentine DL . (2012). Physical control on methanotrophic potential in waters of the Santa Monica Basin, Southern California.
_Limnol Oceanogr_ 57: 420–432. Article CAS Google Scholar * Helm KP, Bindoff NL, Church JA . (2011). Observed decreases in oxygen content of the global ocean. _Geophys Res Lett_ 38:
L23602. Article Google Scholar * Imachi H, Aoi K, Tasumi E, Saito Y, Yamanaka Y, Saito Y _et al_. (2011). Cultivation of methanogenic community from subseafloor sediments using a
continuous-flow bioreactor. _ISME J_ 5: 1913–1925. Article CAS Google Scholar * Keeling RE, Körtzinger A, Gruber N . (2010). Ocean deoxygenation in a warming world. _Ann Rev Mar Sci_ 2:
199–229. Article Google Scholar * Kessler JD, Valentine DL, Redmond MC, Du M, Chan EW, Mendes SD _et al_. (2011). A persistent oxygen anomaly reveals the fate of spilled methane in the
deep Gulf of Mexico. _Science_ 331: 312–315. Article CAS Google Scholar * Kirschke S, Bousquet P, Ciais P, Saunois M, Canadell JG, Dlugokencky EJ _et al_. (2013). Three decades of global
methane sources and sinks. _Nat Geosci_ 6: 813–823. Article CAS Google Scholar * Knittel K, Boetius A . (2009). Anaerobic oxidation of methane: progress with an unknown process. _Annu Rev
Microbiol_ 63: 311–334. Article CAS Google Scholar * Kojima H, Tsutsumi M, Ishikawa K, Iwata T, Mußmann M, Fukui M . (2012). Distribution of putative denitrifying methane oxidizing
bacteria in sediment of a freshwater lake, Lake Biwa. _Syst Appl Microbiol_ 35: 233–238. Article CAS Google Scholar * Krüger M, Treude T, Wolters H, Nauhaus K, Boetius A . (2005).
Microbial methane turnover in different marine habitats. _Palaeogeogr Palaeoclimatol Palaeoecol_ 227: 6–17. Article Google Scholar * Lashof DA, Ahuja DR . (1990). Relative contributions of
greenhouse gas emissions to global warming. _Nature_ 344: 529–531. Article CAS Google Scholar * Lazar CS, Parkes RJ, Cragg BA, L’Haridon S, Toffin L . (2011). Methanogenic diversity and
activity in hypersaline sediments of the centre of the Napoli mud volcano, Eastern Mediterranean Sea. _Environ Microbiol_ 13: 2078–2091. Article CAS Google Scholar * Li-Dong S, Qun Z,
Shuai L, Ping D, Jiang-Ning Z, Dong-Qing C _et al_. (2014). Molecular evidence for nitrite-dependent anaerobic methane-oxidising bacteria in the Jiaojiang Estuary of the East Sea (China).
_Appl Microbiol Biotechnol_ 98: 5029–5038. Article Google Scholar * Martens CS, Berner RA . (1977). Interstitial water chemistry of anoxic Long Island Sound sediments. 1. Dissolved gases.
_Limnol Oceanogr_ 22: 10–25. Article CAS Google Scholar * Mau S, Blees J, Helmke E, Niemann H, Damm E . (2013). Vertical distribution of methane oxidation and methanotrophic response to
elevated methane concentrations in stratified waters of the Arctic fjord Storfjorden (Svalbard, Norway). _Biogeosciences_ 10: 6267–6278. Article CAS Google Scholar * Mitterer RM . (2010).
Methanogenesis and sulfate reduction in marine sediments: a new model. _Earth Planet Sci Lett_ 295: 358–366. Article CAS Google Scholar * Naqvi SWA, Bange HW, Farías L, Monteiro PMS,
Scranton MI, Zhang J . (2010). Marine hypoxia/anoxia as a source of CH4 and N2O. _Biogeosciences_ 7: 2159–2190. Article CAS Google Scholar * Nicholls JC, Davies CA, Trimmer M . (2007).
High-resolution profiles and nitrogen isotope tracing reveal a dominant source of nitrous oxide and multiple pathways of nitrogen gas formation in the central Arabian Sea. _Limnol Oceanogr_
52: 156–168. Article CAS Google Scholar * Oremland RS, Marsh LM, Polcin S . (1982). Methane production and simultaneous sulphate reduction in anoxic, salt marsh sediments. _Nature_ 296:
143–145. Article CAS Google Scholar * Pack MA, Heintz MB, Reeburgh WS, Trumbore SE, Valentine DL, Xu X _et al_. (2015). Methane oxidation in the Eastern Tropical North Pacific Ocean water
column. _J Geophys Res Biogeosciences_ 120: 1078–1092. Article CAS Google Scholar * Padilla C, Bristow LA, Sarode N, Garcia-Robledo E, Gómez Ramirez E, Benson CR _et al_. (2016). NC10
bacteria in marine oxygen minimum zones. _ISME J_ 10: 2067–2071. Article CAS Google Scholar * Paulmier A, Ruiz-Pino D . (2009). Oxygen minimum zones (OMZs) in the modern ocean. _Prog
Oceanogr_ 80: 113–128. Article Google Scholar * Ramaswamy V, Gaye B, Shirodkar PV, Rao PS, Chivas AR, Wheeler D _et al_. (2008). Distribution and sources of organic carbon, nitrogen and
their isotopic signatures in sediments from the Ayeyarwady (Irrawaddy) continental shelf, northern Andaman Sea. _Mar Chem_ 111: 137–150. Article CAS Google Scholar * Reeburgh WS . (2007).
Oceanic methane biogeochemistry. _Chem Rev_ 107: 486–513. Article CAS Google Scholar * Reeburgh WS, Ward BB, Whalen SC, Sandbeck KA, Kilpatrickt KA, Kerkhof LJ . (1991). Black Sea
methane geochemistry. _Deep Sea Res Part A Oceanogr Res Pap_ 38: S1189–S1210. Article Google Scholar * Sanders IA, Heppell CM, Cotton JA, Wharton G, Hildrew AG, Flowers EJ _et al_. (2007).
Emission of methane from chalk streams has potential implications for agricultural practices. _Freshw Biol_ 52: 1176–1186. Article CAS Google Scholar * Sansone F, Graham A, Berelson W .
(2004). Methane along the western Mexican margin. _Limnol Oceanogr_ 49: 2242–2255. Article CAS Google Scholar * Sansone FJ, Popp BN, Gasc A, Graham AW, Rust TM . (2001). Highly elevated
methane in the Eastern Tropical North Pacific and associated isotopically enriched fluxes to the atmosphere. _Geophys Res Lett_ 28: 4567–4570. Article CAS Google Scholar * Schmale O,
Blumenberg M, Kießlich K, Jakobs G, Berndmeyer C, Labrenz M _et al_. (2012). Aerobic methanotrophy within the pelagic redox-zone of the Gotland Deep (central Baltic Sea). _Biogeosciences_ 9:
4969–4977. Article CAS Google Scholar * Schubert CJ, Coolen MJL, Neretin LN, Schippers A, Abbas B, Durisch-Kaiser E _et al_. (2006). Aerobic and anaerobic methanotrophs in the Black Sea
water column. _Environ Microbiol_ 8: 1844–1856. Article CAS Google Scholar * Shakhova N, Semiletov I, Panteleev G . (2005). The distribution of methane on the Siberian Arctic shelves:
Implications for the marine methane cycle. _Geophys Res Lett_ 32: 1–4. Article Google Scholar * Singh N, Kendall M, Liu Y, Boone D . (2005). Isolation and characterization of
methylotrophic methanogens from anoxic marine sediments in Skan Bay, Alaska: description of Methanococcoides alaskense sp nov., and emended description of Methanosarcina baltica. _Int J Syst
Evol Microbiol_ 55: 2531–2538. Article CAS Google Scholar * Steinle L, Graves CA, Treude T, Ferré B, Biastoch A, Bussmann I _et al_. (2015). Water column methanotrophy controlled by a
rapid oceanographic switch. _Nat Geosci_ 8: 378–382. Article CAS Google Scholar * Stramma L, Johnson GC, Sprintall J, Mohrholz V . (2008). Expanding oxygen-minimum zones in the tropical
oceans. _Science_ 320: 655–658. Article CAS Google Scholar * Tavormina PL, Ussler W, Steele JA, Connon SA, Klotz MG, Orphan VJ . (2013). Abundance and distribution of diverse
membrane-bound monooxygenase (Cu-MMO) genes within the Costa Rica oxygen minimum zone. _Environ Microbiol Rep_ 5: 414–423. Article CAS Google Scholar * Tavormina PL, Ussler W, Joye SB,
Harrison BK, Orphan VJ . (2010). Distributions of putative aerobic methanotrophs in diverse pelagic marine environments. _ISME J_ 4: 700–710. Article Google Scholar * Tavormina PL, Ussler
W, Orphan VJ . (2008). Planktonic and sediment-associated aerobic methanotrophs in two seep systems along the North American margin. _Appl Environ Microbiol_ 74: 3985–3995. Article CAS
Google Scholar * Thamdrup B, Dalsgaard T, Revsbech NP . (2012). Widespread functional anoxia in the oxygen minimum zone of the Eastern South Pacific. _Deep Sea Res Part I Oceanogr Res Pap_
65: 36–45. Article CAS Google Scholar * Valentine DL . (2011). Emerging topics in marine methane biogeochemistry. _Ann Rev Mar Sci_ 3: 147–171. Article Google Scholar *
Vázquez-Domínguez E, Vaqué D, Gasol JM . (2007). Ocean warming enhances respiration and carbon demand of coastal microbial plankton. _Glob Chang Biol_ 13: 1327–1334. Article Google Scholar
* Wang Y, Zhu G, Harhangi HR, Zhu B, Jetten MSM, Yin C _et al_. (2012). Co-occurrence and distribution of nitrite-dependent anaerobic ammonium and methane-oxidizing bacteria in a paddy
soil. _FEMS Microbiol Lett_ 336: 79–88. Article CAS Google Scholar * Wasmund K, Kurtböke DI, Burns KA, Bourne DG . (2009). Microbial diversity in sediments associated with a shallow
methane seep in the tropical Timor Sea of Australia reveals a novel aerobic methanotroph diversity. _FEMS Microbiol Ecol_ 68: 142–151. Article CAS Google Scholar * Watkins AJ, Roussel EG,
Webster G, Parkes RJ, Sass H . (2012). Choline and N,N-dimethylethanolamine as direct substrates for methanogens. _Appl Environ Microbiol_ 78: 8298–8303. Article CAS Google Scholar *
Webster G, O’Sullivan LA, Meng Y, Williams AS, Sass AM, Watkins AJ _et al_. (2015). Archaeal community diversity and abundance changes along a natural salinity gradient in estuarine
sediments. _FEMS Microbiol Ecol_ 91: 1–18. Article Google Scholar * Whiticar MJ . (2002). Diagenetic relationships of methanogenesis, nutrients, acoustic turbidity, pockmarks and
freshwater seepages in Eckernförde Bay. _Mar Geol_ 182: 29–53. Article CAS Google Scholar * Wilkins D, van Sebille E, Rintoul SR, Lauro FM, Cavicchioli R . (2013). Advection shapes
Southern Ocean microbial assemblages independent of distance and environment effects. _Nat Commun_ 4: 2457. Article Google Scholar * Wright JJ, Konwar KM, Hallam SJ . (2012). Microbial
ecology of expanding oxygen minimum zones. _Nat Rev Microbiol_ 10: 381–394. Article CAS Google Scholar * Zhang X, Hester KC, Ussler W, Walz PM, Peltzer ET, Brewer PG . (2011). _In situ_
Raman-based measurements of high dissolved methane concentrations in hydrate-rich ocean sediments. _Geophys Res Lett_ 38: L08605. Google Scholar * Zhu B, van Dijk G, Fritz C, Smolders AJP,
Pol A, Jetten MSM _et al_. (2012). Anaerobic oxidization of methane in a minerotrophic peatland: enrichment of nitrite-dependent methane-oxidizing bacteria. _Appl Environ Microbiol_ 78:
8657–8665. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was funded by the Natural Environment Research Council (grant NE/E01559X/1). We thank Dr Ian Sanders
for general technical assistance, John Green for support with the Dionex and Nele Thijs for DNA extraction of the ETNP_Offshore_MO samples. We appreciate the support of the Captain and crew
of RRS Discovery (cruise D373) and RRS James Cook (cruise JC097). AUTHOR INFORMATION Author notes * Panagiota-Myrsini Chronopoulou Present address: 3Current address: Department of
Environmental Sciences, University of Helsinki, PO Box (Viikinkaari 1), 00014, Helsinki, Finland., * William J Pritchard Present address: 4Current address: Faculty of Life Sciences,
University of Manchester, A4051 Michael Smith Building, Dover Street, Manchester, M13 9PT, UK., * Susanna T Maanoja Present address: 5Current address: Department of Chemistry and
Bioengineering, Tampere University of Technology, PO Box 541, FI-33101, Tampere, Finland., * Panagiota-Myrsini Chronopoulou and Felicity Shelley: These authors contributed equally to this
work. AUTHORS AND AFFILIATIONS * School of Biological and Chemical Sciences, Queen Mary University of London, London, UK Panagiota-Myrsini Chronopoulou, Felicity Shelley, William J
Pritchard, Susanna T Maanoja & Mark Trimmer Authors * Panagiota-Myrsini Chronopoulou View author publications You can also search for this author inPubMed Google Scholar * Felicity
Shelley View author publications You can also search for this author inPubMed Google Scholar * William J Pritchard View author publications You can also search for this author inPubMed
Google Scholar * Susanna T Maanoja View author publications You can also search for this author inPubMed Google Scholar * Mark Trimmer View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Mark Trimmer. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL
INFORMATION Supplementary Information accompanies this paper on The ISME Journal website SUPPLEMENTARY INFORMATION SUPPLEMENTARY METHODS AND TABLES (DOCX 40 KB) SUPPLEMENTARY FIGURE S1 (PDF
1438 KB) SUPPLEMENTARY FIGURE S2 (PDF 63 KB) SUPPLEMENTARY FIGURE S3 (JPG 1292 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International
License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is
not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chronopoulou, PM., Shelley, F., Pritchard, W. _et al._ Origin and fate of methane in
the Eastern Tropical North Pacific oxygen minimum zone. _ISME J_ 11, 1386–1399 (2017). https://doi.org/10.1038/ismej.2017.6 Download citation * Received: 22 June 2016 * Revised: 06 December
2016 * Accepted: 09 January 2017 * Published: 28 February 2017 * Issue Date: June 2017 * DOI: https://doi.org/10.1038/ismej.2017.6 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative