Play all audios:
ABSTRACT Mutations in homeoprotein NKX2-5 are linked to human congenital heart disease, resulting in various cardiac anomalies, as well as in postnatal progressive conduction defects and
occasional left ventricular dysfunction; yet the function of Nkx2-5 in the postnatal period is largely unexplored. In the heart, the majority of cardiomyocytes are believed to complete
cell-cycle withdrawal shortly after birth, which is generally accompanied by a re-organization of chromatin structure shown in other tissues. We reasoned that the effects of the loss of
Nkx2-5 in mice may be different after cell-cycle withdrawal compared with those of the perinatal loss of Nkx2-5, which results in rapid conduction and contraction defects within 4 days after
the deletion of Nkx2-5 alleles (_Circ Res._ 2008;103:580). In this study, floxed-Nkx2-5 alleles were deleted using tamoxifen-inducible Cre transgene (Cre-ER) beginning at 2 weeks of age.
The loss of Nkx2-5 beginning at 2 weeks of age resulted in conduction and contraction defects similar to the perinatal loss of Nkx2-5, however, with a substantially slower disease
progression shown by 1° atrioventricular block at 6 weeks of age (4 weeks after tamoxifen injections) and heart enlargement after 12 weeks of age (10 weeks after tamoxifen injections). The
phenotypes were accompanied by a slower and smaller degree of reduction of several critical Nkx2-5 downstream targets that were observed in mice with a perinatal loss of Nkx2-5. These
results suggest that Nkx2-5 is necessary for proper conduction and contraction after 2 weeks of age, but with a substantially distinct level of necessity at 2 weeks of age compared with that
in the perinatal period. SIMILAR CONTENT BEING VIEWED BY OTHERS DECREASE OF _PDZRN3_ IS REQUIRED FOR HEART MATURATION AND PROTECTS AGAINST HEART FAILURE Article Open access 07 January 2022
LOSS OF CONNECTIN NOVEX-3 LEADS TO HEART DYSFUNCTION ASSOCIATED WITH IMPAIRED CARDIOMYOCYTE PROLIFERATION AND ABNORMAL NUCLEAR MECHANICS Article Open access 14 June 2024 TROPONIN T AMINO
ACID MUTATION (ΔK210) KNOCK-IN MICE AS A NEONATAL DILATED CARDIOMYOPATHY MODEL Article 20 June 2020 MAIN Nkx2-5 is a homeodomain-containing transcription factor, highly conserved among
species and is one of the earliest cardiogenic markers1, 2 with expression continuing throughout adulthood.3, 4, 5 The targeted disruption of Nkx2-5 in mice causes embryonic lethality around
embryonic (E) day 10.5, with retarded cardiac development.6, 7 Mice with ventricular-restricted Nkx2-5 deletion from E 8.0–8.5 survive but show progressive and advanced conduction defects,
as well as left ventricular hypertrophy postnatally.8 Heterozygous mutations in human NKX2-5 cause various cardiac anomalies and postnatal progressive conduction defects and occasional left
ventricular dysfunction.9, 10, 11, 12 The Nkx2-5 function has predominantly been studied in mouse embryos or stem cells when cardiomyocytes are proliferating.13, 14 To address the Nkx2-5
function at distinct stages of development, we recently generated tamoxifen-inducible Nkx2-5 knockout mice. Using mice with a perinatal loss of Nkx2-5, we have demonstrated that Nkx2-5 is
critically important for cardiac conduction and contraction in the perinatal period as well. In these mice, atrioventricular (AV) block and heart enlargement appeared within 4 days after
tamoxifen injection, leading to premature death due to cardiac dysfunction.15 Rapid disease progression was accompanied by the rapid decrease of several gene products important for both
conduction and contraction, including the cardiac Na+ channel, Nav1.5(_α_), ryanodine receptor 2 and cardiac myosin light chain kinase (MLCK).15, 16 Our ongoing hypothesis is that Nkx2-5
actively regulates a critical set of genes in postnatal cardiomyocytes to maintain proper cardiac function. Less well understood is the function of Nkx2-5 from the time between perinatal
development to adulthood. Recent studies demonstrate the formation of a population of new myocytes in the adult heart;17 although perhaps the majority of cardiomyocytes may be considered to
lose their ability for proliferation (G1 cell-cycle arrest) and DNA synthesis during the first week after birth, and transit into cells with a highly specialized structure and function.18
Because G1 cell-cycle arrest is generally linked to morphological, metabolic and gene expression changes with a re-organization of the nuclear architecture and chromatin structure,19, 20
cardiomyocytes at G1 cell-cycle arrest may share these properties. We reasoned that this transition might change the cardiomyocyte responses to cardiac transcription factors, including
Nkx2-5. In this study, we deleted the Nkx2-5 gene beginning at 2 weeks of age, which resulted in a slow progressive conduction defect and heart enlargement, accompanied by a slow
downregulation of critical genes that were important determinants for the phenotypes observed in the perinatal loss of Nkx2-5 mice. MATERIALS AND METHODS INDUCIBLE NKX2-5 KNOCKOUT MICE
Floxed-Nkx2-5 mice8 were bred with transgenic mice carrying the Cre-ER gene under CMV promoter.21 Details of mouse generation have been described previously.15 To delete the floxed-Nkx2-5
gene, tamoxifen (1 mg/g body weight, i.p.) was injected into mice beginning at 2 weeks of age for 4 consecutive days. All animal care protocols fully conformed to the Association for the
Assessment and Accreditation of Laboratory Animal Care, with approvals from the University of Florida Institutional Animal Care and Use Committee. TELEMETRY ECG RECORDINGS AND ECHOCARDIOGRAM
Recording of telemetry ECG (Data Sciences International) was performed 3 days after implantation of wireless radiofrequency telemetry devices to avoid the effects of anesthesia on ECG.
Telemetry data were analyzed using PowerLab software (ADInstruments) as described previously.22 An M-mode ultrasound imaging of the left ventricle of anesthetized mice (pentobarbital 60
mg/kg, i.p.) was obtained at the level of the papillary muscle from a parasternal window using an ultrasound biomicroscope with a single transducer with a frequency of 40 MHz (VisualSonics,
Toronto, Canada). NORTHERN BLOTTING, IMMUNOSTAINING AND HISTOLOGICAL ANALYSES Northern blot analyses were carried out using the following probes: an Nkx2-5-coding probe, a _Pfl_MI-_Eco_RI
fragment of mouse Nkx2-5 cDNA; atrial natriuretic factor (ANF) (330 bp PCR products for rat ANF, F, 5′-GGGGTAGGATTGACAGGATTGG-3′; R, 5′-CCGTGGTGCTGAAGTTTATTCG-3′); brain natriuretic peptide
(BNP) (429 bp PCR products, F, 5′-TGGGGAGGCGAGACAAGGG-3′; R, 5′-TCTTCCTACAACAACTTCAGTGCG-3′); _β_-myosin heavy chain (_β_MHC) (oligonucleotide probe,
5′-GCTTTATTCTGCTTCCACCTAAAGGGCTGTTGCAAAGGCTCCAGGTCTGAGGGCTTC-3′); GAPDH (450 bp PCR products, F, 5′-TTCATTGACCTCAACTACAT-3′; R, 5′-GTGGCAGTGATGGCATGGAC-3′). Immunostaining was carried out
with the following primary antibodies: anti-Nkx2-5 pAb,5 and sarcomeric actinin (Sigma A7811). Fluorescent microscopic images were obtained using ZEISS Axiovert200M with or without Apotome.
Histological analyses, including hematoxylin/eosin and Masson's trichrome staining, acetylcholine esterase staining in frozen tissue sections and whole-mount acetylcholine esterase
staining, were carried out as described previously.15 Digitalized images from the AV node, the LV free wall in the longitudinal section at the level of nuclei and isolated ventricular
myocytes were utilized for measurement using Image J software as described previously.23, 24, 25 SIMULTANEOUS RECORDING OF CARDIAC CONTRACTION AND CA2+ MEASUREMENTS Rod-shaped cardiomyocytes
with clear cross striations, staircase ends and surface membranes free from blebs isolated from approximately 24-week-old flox/flox or flox/flox/Cre mice were studied for simultaneous
measurements of cardiomyocyte cell shortening and intracellular free calcium concentration, as described previously.16 REAL-TIME RT-PCR Real-time RT-PCR was performed using inventoried
Taqman Gene Expression Assays (Applied Biosystems): cardiac MLCK, Mm00615292; Scn5a, Mm00451971; RyR2, Mm0046587; ANF, Mm01255748; BNP, Mm00435304; _β_MHC (MHC7), Mm00600555. Data were
normalized to _β_-actin expression (no. 4352933E). Duplicate experiments were averaged. STATISTICAL ANALYSIS Values among groups were compared using ANOVA and the Fisher PLSD _post hoc_ test
(StatView version 5.01). _P_<0.05 was considered significant. RESULTS SLOW PROGRESSIVE CONDUCTION DEFECTS AND HEART ENLARGEMENT IN NKX2-5 KNOCKOUT MICE FROM 2 WEEKS OF AGE Recently, we
generated a mouse model to delete the Nkx2-5 gene by tamoxifen injection15 by mating mice homozygous for floxed-Nkx2-5 alleles8 and heterozygous for the Cre-ER transgene under the control of
the CMV enhancer and the chicken _β_-globin promoter.21 Tamoxifen injection within 24 h before birth induced rapid progressive conduction and contraction defects within 4 days, which leads
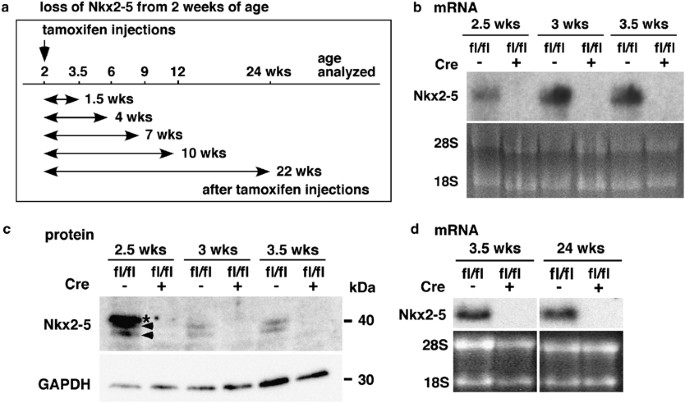
to premature death.15 In this study, tamoxifen was injected at 2 weeks of age for 4 consecutive days. Time course studies at multiple time points are shown in Figure 1a. Measurements of
heart weight/tibial length and telemetry ECG recordings of Nkx2-5 knockout mice were determined at multiple time points from 3.5 to 24 weeks of age (1.5 and 22 weeks from the initiation of
tamoxifen injections, respectively). Deletion of the Nkx2-5 gene, followed by a reduction of Nkx2-5 mRNA (Figure 1b) and proteins (Figure 1c), was demonstrated 1 day after four consecutive
tamoxifen injections (approximately 2.5 weeks of age), as well as 3 days (approximately 3 weeks of age) and 6 days (approximately 3.5 weeks of age) after injections. The reduction of Nkx2-5
mRNA continued up to 24 weeks of age by tamoxifen injections at 2 weeks of age, as demonstrated in northern blotting (Figure 1d). Telemetry ECG recordings of Nkx2-5 knockout mice and
tamoxifen-injected control mice (flox/flox or flox/wild/Cre) were performed at multiple time points from 3.5 to 24 weeks of age (1.5 and 22 weeks after tamoxifen injections, respectively)
(Table 1). Nkx2-5 knockout mice first showed PR prolongation at 9 weeks of age, accompanied by an intermittent 2° AV block. The PR interval was progressively prolonged, and wide QRS was
observed at 24 weeks of age (Table 1). With the exception of one mouse at 24 weeks of age with an intermittent 2° AV block, heterozygous Nkx2-5 knockout mice (flox/wild/Cre) did not show PR
prolongation or wide QRS (Table 1). Examples of telemetry ECG recordings normal at 3.5 weeks of age and with a demonstration of the 2° AV block and wide QRS at 24 weeks of age in Nkx2-5
knockout hearts are shown in Figure 2a. Heart weight/tibial length, an indicator of heart enlargement, was slightly but significantly increased in mice with Nkx2-5 knockout beginning at 12
weeks of age compared with that in tamoxifen-injected control mice (flox/flox or flox/wild/Cre) (Figure 2b). A reduction of cardiac contractile performance (% fraction shortening) and an
increase of left ventricular systolic dimension (LVDs) were observed in Nkx2-5 knockout mice at 24 weeks of age, using 40 MHz ultrasound imaging (Figures 2c and d). Taken together, mice with
loss of Nkx2-5 from 2 weeks of age show conduction and contraction defects. Although this phenotype is qualitatively similar to the phenotype observed with the perinatal loss of Nkx2-5,15
disease progression and severity are markedly distinct despite using a genetically identical mouse model. For instance, when tamoxifen was administered at 2 weeks of age, no phenotype was
apparent over a time span of 1.5 weeks after tamoxifen injection (3.5 weeks of age), in contrast to perinatal tamoxifen administration.15 In addition, mice with loss of Nkx2-5 from 2 weeks
of age, survive over 1.5 years of age, despite the presence of PR prolongation, wide QRS and a slight increase in heart weight/tibial length (data not shown), whereas mice with perinatal
loss of Nkx2-5 die prematurely.15 REDUCED EXPRESSION OF NKX2-5 PROTEIN IN CARDIOMYOCYTES ACCOMPANIED BY SMALL AV NODE Immunostaining confirmed a decreased Nkx2-5 expression in Nkx2-5
knockout ventricles at 3.5 weeks of age (Figure 3a, left panels). Despite a slight heart enlargement in Nkx2-5 knockout mice at 24 weeks of age, interstitial fibrosis was not apparent in
ventricles (Figure 3a, right panels). In adjacent tissue sections, including the acetylcholine esterase-positive AV node, positive Nkx2-5 staining was shown in the control heart, but not in
the Nkx2-5 knockout heart (Figure 3b). Although formation of the AV node is completed before birth, whole-mount acetylcholine esterase staining showed that the AV nodal surface area size was
smaller at 24 weeks of age in hearts with a loss of Nkx2-5 beginning at 2 weeks of age (Figures 3c and d). This observation is similar to previous studies using ventricular-specific
deletion of Nkx2-5 from the embryonic stage in MLC2v-Cre mice and in perinatal Nkx2-5 knockout mice;8 however, AV nodal fibrosis was not apparent in mice with Nkx2-5 knockout in perinatal15
and at 2 weeks of age (Figure 3e). In addition, nuclei in the AV node appeared more condensed and the nuclear number/mm2 was significantly increased in the AV node in Nkx2-5 knockout _vs_
control hearts at 24 weeks of age (Figure 3e and f). To examine whether this could be because of reduced cardiomyocyte cell size in the AV node of Nkx2-5 knockout mice, cell diameter was
measured in the longitudinal section at the position of nuclei. The AV nodal cell width in control flox/flox mice was increased from 3.5 to 24 weeks of age, but was not increased in
flox/flox/Cre mice, leading to a smaller AV nodal cell width in Nkx2-5 knockout _vs_ control mice at 24 weeks of age (Figure 3g, upper panels and h, left panel). In contrast, cell width in
non-AV nodal myocytes in the left ventricle was increased between 3.5 and 24 weeks of age in both Nkx2-5 knockout and control mice, and was slightly, but not significantly, wider in Nkx2-5
knockout at 24 weeks of age (Figure 3g, lower panels and h, right panel). This observation was further examined using isolated cardiomyocyte measurements that showed a significant increase
in the mean surface area, long axis and short axis length in Nkx2-5 knockout myocytes (Figures 4a–c). Taken together, Nkx2-5 knockout hearts at 24 weeks of age showed ventricular myocyte
hypertrophy, and a reduction in cardiomyocyte cell width in the AV node. NKX2-5 KNOCKOUT VENTRICULAR CARDIOMYOCYTES ARE MORPHOLOGICALLY AND FUNCTIONALLY COMPENSATED At 24 weeks of age,
cardiomyocytes isolated from mice with Nkx2-5 knockout from 2 weeks of age did not show significant differences in fractional shortening, +dL/dT (rate of contraction) and −dL/dT (rate of
relaxation) compared with cells from control mice at a Ca2+ superfusate concentration of 1.2 mM. (Figure 4d). Notably, the Ca2+ transient amplitude and systolic fluorescence ratio were
significantly lower in Nkx2-5 knockout cardiomyocytes (Figure 4d), which is similar to perinatal Nkx2-5 knockout cardiomyocytes with a reduction of ryanodine receptor 2.15 When the Ca2+
superfusate concentration was increased from 1.2 to 2.5 mM, the increase in fractional shortening was blunted in Nkx2-5 knockout cardiomyocytes compared with that in control cardiomyocytes
(Figure 4e). These results indicate that cardiomyocytes from Nkx2-5 knockout hearts were morphologically and functionally compensated despite reduced Ca2+handling, and a reduction in
contraction appeared only under conditions of increased demand. SLOW REDUCTION OF NKX2-5 DOWNSTREAM TARGETS IN HEARTS WITH LOSS OF NKX2-5 FROM 2 WEEKS OF AGE The expressions of cardiac
hypertrophic markers, ANF and BNP are regulated by Nkx2-5 shown in embryos7, as well as at PD4 in the perinatal loss of Nkx2-5 (Figure 5a). In hearts from mice with Nkx2-5 knockout from 2
weeks of age, the expression of ANF and BNP was also downregulated early after tamoxifen injections at 3.5 weeks of age (1.5 weeks after tamoxifen injection) and further decreased at 6 weeks
of age (4 weeks after tamoxifen injection) compared with that in control flox/flox hearts (Figure 5b). In contrast, the expression of another hypertrophic marker, _β-_MHC, was induced 4
days after tamoxifen injection in hearts with a perinatal loss of Nkx2-5 (Figure 5a), whereas it was barely detected at 3.5 weeks of age in mice with Nkx2-5 knockout from 2 weeks of age, but
was upregulated at 6 weeks of age (Figure 5b). Thus, the delayed downregulation of ANF and BNP, as well as the delayed upregulation of _β_MHC, in hearts from mice with Nkx2-5 knockout from
2 weeks of age contrasts with the rapid changes in expression within 4 days after tamoxifen injection in hearts with a perinatal loss of Nkx2-5. At 24 weeks of age, when marked cardiomyocyte
hypertrophy was demonstrated (Figures 4a–c), the expression of ANF was increased to the level of control (fold difference flox/flox/Cre _vs_ flox/flox, means±s.e., 1.11±0.02, _n_=2);
however, the expression of BNP remained reduced (0.27±0.00, _n_=2) and that of _β_MHC remained upregulated (5.12±0.39, _n_=2). In hearts with perinatal loss of Nkx2-5, a marked reduction
(80–90%) of three additional gene products important for cardiac conduction and contraction, including the Na+ channel, Nav1.5(_α_), ryanodine receptor 2 and cardiac MLCK, was also
demonstrated by PD12.15, 16 A reduction in expression was also observed in hearts from mice with loss of Nkx2-5 beginning at 2 weeks of age (Figure 5c); however, the magnitude of the
reduction was less and the time course was prolonged in hearts with Nkx2-5 knockout from 2 weeks of age compared with those with a perinatal loss of Nkx2-5. Taken together, a slower
progression and decreased magnitude of reduction of Nkx2-5 downstream targets characterize the phenotype of hearts with loss of Nkx2-5 from 2 weeks of age. This observation is one potential
mechanism explaining the slower disease progression. DISCUSSION Nkx2-5 is a critical factor in fetal cardiac development when cardiomyocytes are proliferating as demonstrated in various
studies using mouse embryos or stem cells.13, 14 Nkx2-5 is also important in perinatal hearts, as demonstrated by rapid conduction and contraction defects observed within 4 days after the
deletion of Nkx2-5 following tamoxifen injection, leading to premature death.15 The loss of Nkx2-5 from 2 weeks of age results in an overall similar phenotype, but with a distinct disease
progression and severity despite using a genetically identical mouse model with a 2-week difference in the administration of tamoxifen injection. For example, the perinatal loss of Nkx2-5
results in premature death, but the loss of Nkx2-5 from 2 weeks of age does not. An apparent phenotype emerges within 4 days with the perinatal loss of Nkx2-5 _vs_ over 7 weeks with the loss
beginning at 2 weeks of age. With respect to the pathogenesis of human congenital AV block and occasional LV dysfunction associated with NKX2-5 mutations, our previous15 and current studies
reveal that Nkx2-5 is necessary for proper conduction and contraction postnatally, but is more critical in the perinatal heart and is necessary for survival in mice. Haploinsufficiency
(loss of one allele) of Nkx2-5 is considered to be an underlying cause of human congenital heart disease as shown in several germline heterozygous-null Nkx2-5 mice.26, 27, 28, 29 Although
substantially different degrees of defects among studies were reported, PR prolongation accompanied with wide QRS was detected as early as 7 weeks of age.28 When the Nkx2-5 gene was deleted
after 2 weeks of age, heterozygous-null mice did not apparently develop heart enlargement, PR prolongation or wide QRS by 24 weeks of age, with the exception of one mouse with an
intermittent 2° AV block at 24 weeks of age. We initiated this study on the basis of the hypothesis that cardiomyocytes before and after G1 cell-cycle arrest may demonstrate morphological,
metabolic and gene expression changes with a re-organization of nuclear architecture and chromatin structure.19, 20 In fact, we found a global increase of DNA methylation in hearts within 2
weeks after birth using 5-methylcytosine staining (data not shown).30 The question remains as to how an increase in broad DNA methylation relates to slower and milder phenotypes in mice with
loss of Nkx2-5 from 2 weeks age. It might be argued that a compact chromatin DNA structure due to DNA methylation may delay transcriptional re-regulation after the loss of Nkx2-5 from 2
weeks of age. Another mechanism is that other transcription factors might compensate for the absence of Nkx2-5 after 2 weeks of age. The mRNA half-life of Nkx2-5 targets after 2 weeks of age
might be substantially longer compared with that at the neonatal stage. To our knowledge, a limited number of cardiac transcription factors have been studied using postnatal-specific gene
deletion. The loss of serum responsive factor (SRF) from 2 months of age leads to lethal heart failure 10 weeks after tamoxifen injection.31 Although a 70% reduction of the SRF downstream
target, _α_-actin, was demonstrated 5 days after tamoxifen injections, its expression did not change appreciably 30 and 60 days after tamoxifen injection. The expression of other SRF
targets, such as vinculin and zyxin, demonstrated in ES cells, as well as in Nkx2-5, MEF2C, GATA4 and TEF1, demonstrated after the loss of SRF in embryonic hearts (floxed-SRF mice crossed
with _β_MHC-Cre transgenic mice), was unchanged or fluctuated 5, 30 and 60 days after tamoxifen injection.31 Thus, this study also demonstrated a differential regulation of transcription in
adult _vs_ proliferating cardiomyocytes. Additional studies using postnatal-specific gene targeting of cardiac transcription factors will reveal whether the developmental stage-dependent
effects are a general property of cardiac transcription factors. Nkx2-5 has been shown to be involved in cardiac hypertrophy;32, 33, 34 however, this study shows that without Nkx2-5,
ventricular cardiomyocytes have compensatory hypertrophy at 24 weeks of age. Thus, Nkx2-5 may not be critically important for adult ventricular cardiomyocyte hypertrophy as was suggested in
a recent review.35 In contrast, Nkx2-5 might be important for cellular enlargement in AV nodal cardiomyocytes during postnatal development, as demonstrated in this study, which are not
involved in contraction nor are they affected by compensatory hypertrophic stimuli. It is noteworthy that defects in AV bundle development during embryonic stages have been shown in
heterozygous Nkx2-5 knockout mice.29 Morphological changes in ventricular conduction systems, including AV bundle, remain to be analyzed in postnatal Nkx2-5 knockout hearts. Cardiomyocyte
hypertrophy is usually accompanied by an increase in the expression of ANF and BNP. The expression of those markers in hypertrophic cardiomyocytes with a loss of their upstream regulatory
transcription factor, Nkx2-5, is somewhat difficult to interpret and is interesting. We found that soon after the loss of Nkx2-5 (3.5 and 6 weeks of age, Figure 5b), the expression of ANF
and BNP was decreased because of the loss of their transactivator, Nkx2-5. At 24 weeks of age, when cardiomyocyte hypertrophy was evident, ANF expression was increased to the normal level,
but BNP expression remained downregulated. One potential interpretation of the time-dependent changes in ANF expression (downregulation soon after the loss of Nkx2-5 and normalization at 24
weeks of age), which are highly induced and overcome the loss of Nkx2-5 function in the transcription of the ANF gene, would be because of an Nkx2-5-independent regulatory mechanism at 24
weeks of age, resulting in an increase in ANF expression to the normal control level. In summary, we demonstrated that Nkx2-5 actively regulates a critical set of genes in postnatal
cardiomyocytes to maintain proper cardiac function. However an age-related phenotypic difference after the loss of Nkx2-5 between 2 weeks of age _vs_ perinatal stage was identified, which is
accompanied by a slower and smaller reduction of several critical Nkx2-5 responsive genes. REFERENCES * Harvey RP . NK-2 homeobox genes and heart development. _Dev Biol_ 1996;178:203–216.
Article CAS PubMed Google Scholar * Harvey RP, Rosenthal N . _Heart Development_. Academic Press: San Diego, CA, USA, 1999. Google Scholar * Lints TJ, Parsons LM, Hartley L, _et al_.
Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. _Development_ 1993;119:419–431. CAS PubMed Google Scholar * Komuro I, Izumo
S . Csx: a murine homeobox-containing gene specifically expressed in the developing heart. _Proc Natl Acad Sci USA_ 1993;90:8145–8149. Article CAS PubMed PubMed Central Google Scholar
* Kasahara H, Bartunkova S, Schinke M, _et al_. Cardiac and extracardiac expression of Csx/Nkx2.5 homeodomain protein. _Circ Res_ 1998;82:936–946. Article CAS PubMed Google Scholar *
Lyons I, Parsons LM, Hartley L, _et al_. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. _Genes Dev_ 1995;9:1654–1666. Article CAS
PubMed Google Scholar * Tanaka M, Chen Z, Bartunkova S, _et al_. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development.
_Development_ 1999;126:1269–1280. CAS PubMed Google Scholar * Pashmforoush M, Lu JT, Chen H, _et al_. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage
specification leads to progressive cardiomyopathy and complete heart block. _Cell_ 2004;117:373–386. Article CAS PubMed Google Scholar * Schott JJ, Benson DW, Basson CT, _et al_.
Congenital heart disease caused by mutations in the transcription factor NKX2-5. _Science_ 1998;281:108–111. Article CAS PubMed Google Scholar * Benson DW, Silberbach GM,
Kavanaugh-McHugh A, _et al_. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. _J Clin Invest_ 1999;104:1567–1573. Article CAS PubMed
PubMed Central Google Scholar * Kasahara H, Benson DW . Biochemical analyses of eight NKX2.5 homeodomain missense mutations causing atrioventricular block and cardiac anomalies.
_Cardiovasc Res_ 2004;64:40–51. Article CAS PubMed Google Scholar * Konig K, Will JC, Berger F, _et al_. Familial congenital heart disease, progressive atrioventricular block and the
cardiac homeobox transcription factor gene NKX2.5: identification of a novel mutation. _Clin Res Cardiol_ 2006;95:499–503. Article CAS PubMed Google Scholar * Sachinidis A, Fleischmann
BK, Kolossov E, _et al_. Cardiac specific differentiation of mouse embryonic stem cells. _Cardiovasc Res_ 2003;58:278–291. Article CAS PubMed Google Scholar * Puceat M . Rb and LEK1: a
‘pas de deux’ in cardiogenesis. _Cell Cycle_ 2005;4:1030–1032. Article CAS PubMed Google Scholar * Briggs LE, Takeda M, Cuadra AE, _et al_. Perinatal loss of Nkx2-5 results in rapid
conduction and contraction defects. _Circ Res_ 2008;103:580–590. Article CAS PubMed PubMed Central Google Scholar * Chan JY, Takeda M, Briggs LE, _et al_. Identification of
cardiac-specific myosin light chain kinase. _Circ Res_ 2008;102:571–580. Article CAS PubMed PubMed Central Google Scholar * Anversa P, Leri A, Kajstura J . Cardiac regeneration. _J Am
Coll Cardiol_ 2006;47:1769–1776. Article PubMed Google Scholar * Ahuja P, Sdek P, MacLellan WR . Cardiac myocyte cell cycle control in development, disease, and regeneration. _Physiol
Rev_ 2007;87:521–544. Article CAS PubMed Google Scholar * Oberdoerffer P, Sinclair DA . The role of nuclear architecture in genomic instability and ageing. _Nat Rev Mol Cell Biol_
2007;8:692–702. Article CAS PubMed Google Scholar * Rowat AC, Lammerding J, Herrmann H, _et al_. Towards an integrated understanding of the structure and mechanics of the cell nucleus.
_Bioessays_ 2008;30:226–236. Article PubMed Google Scholar * Hayashi S, McMahon AP . Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally
regulated gene activation/inactivation in the mouse. _Dev Biol_ 2002;244:305–318. Article CAS PubMed Google Scholar * Wakimoto H, Kasahara H, Maguire CT, _et al_. Developmentally
modulated cardiac conduction failure in transgenic mice with fetal or postnatal overexpression of DNA nonbinding mutant Nkx2.5. _J Cardiovasc Electrophysiol_ 2002;13:682–688. Article PubMed
Google Scholar * Scherrer-Crosbie M, Ullrich R, Bloch KD, _et al_. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. _Circulation_
2001;104:1286–1291. Article CAS PubMed Google Scholar * Janssens S, Pokreisz P, Schoonjans L, _et al_. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left
ventricular performance and reduces compensatory hypertrophy after myocardial infarction. _Circ Res_ 2004;94:1256–1262. Article CAS PubMed Google Scholar * Minhas KM, Saraiva RM,
Schuleri KH, _et al_. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. _Circ Res_ 2006;98:271–279. Article CAS PubMed Google Scholar *
Biben C, Weber R, Kesteven S, _et al_. Cardiac septal and valvular dysmorphogenesis in mice heterozygous for mutations in the homeobox gene Nkx2-5. _Circ Res_ 2000;87:888–895. Article CAS
PubMed Google Scholar * Tanaka M, Berul CI, Ishii M, _et al_. A mouse model of congenital heart disease: cardiac arrhythmias and atrial septal defect caused by haploinsufficiency of the
cardiac transcription factor Csx/Nkx2.5. _Cold Spring Harb Symp Quant Biol_ 2002;67:317–325. Article CAS PubMed Google Scholar * Jay PY, Harris BS, Maguire CT, _et al_. Nkx2-5 mutation
causes anatomic hypoplasia of the cardiac conduction system. _J Clin Invest_ 2004;113:1130–1137. Article CAS PubMed PubMed Central Google Scholar * Moskowitz IP, Kim JB, Moore ML, _et
al_. A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. _Cell_ 2007;129:1365–1376. Article CAS PubMed Google Scholar * Sedmera D,
Pexieder T, Vuillemin M, _et al_. Developmental patterning of the myocardium. _Anat Rec_ 2000;258:319–337. Article CAS PubMed Google Scholar * Parlakian A, Charvet C, Escoubet B, _et
al_. Temporally controlled onset of dilated cardiomyopathy through disruption of the SRF gene in adult heart. _Circulation_ 2005;112:2930–2939. Article CAS PubMed Google Scholar *
Thompson JT, Rackley MS, O'Brien TX . Upregulation of the cardiac homeobox gene Nkx2-5 (CSX) in feline right ventricular pressure overload. _Am J Physiol_ 1998;274 (5 Part
2):H1569–H1573. CAS PubMed Google Scholar * Saadane N, Alpert L, Chalifour LE . Expression of immediate early genes, GATA-4, and Nkx-2.5 in adrenergic-induced cardiac hypertrophy and
during regression in adult mice. _Br J Pharmacol_ 1999;127:1165–1176. Article CAS PubMed PubMed Central Google Scholar * Bar H, Kreuzer J, Cojoc A, _et al_. Upregulation of embryonic
transcription factors in right ventricular hypertrophy. _Basic Res Cardiol_ 2003;98:285–294. Article PubMed Google Scholar * Oka T, Xu J, Molkentin JD . Re-employment of developmental
transcription factors in adult heart disease. _Semin Cell Dev Biol_ 2007;18:117–131. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We greatly appreciate E Chan
and P Sayeski for their valuable suggestions and technical support. This study was supported by the National Institutes of Health (HL081577 to HK) and the American Heart Association-National
SDG grant (0035258N to HK). AUTHOR INFORMATION Author notes * Morihiko Takeda, Laura E Briggs and Hiroko Wakimoto: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS *
Department of Physiology and Functional Genomics, University of Florida College of Medicine, Gainesville, FL, USA Morihiko Takeda, Laura E Briggs, Melissa H Marks, Sonisha A Warren &
Hideko Kasahara * Department of Pediatrics, Tokyo Medical and Dental School, Tokyo, Japan Hiroko Wakimoto * Cardiology Division, UCSF, San Francisco, CA, USA Jonathan T Lu * Cardiovascular
Research, Boston University Medical Center, Boston, MA, USA Ellen O Weinberg * Department of Biochemistry and Molecular Biology, University of Florida College of Medicine, Gainesville, FL,
USA Keith D Robertson * Cardiovascular Research Center, Massachusetts General Hospital, Boston, MA, USA Kenneth R Chien Authors * Morihiko Takeda View author publications You can also search
for this author inPubMed Google Scholar * Laura E Briggs View author publications You can also search for this author inPubMed Google Scholar * Hiroko Wakimoto View author publications You
can also search for this author inPubMed Google Scholar * Melissa H Marks View author publications You can also search for this author inPubMed Google Scholar * Sonisha A Warren View author
publications You can also search for this author inPubMed Google Scholar * Jonathan T Lu View author publications You can also search for this author inPubMed Google Scholar * Ellen O
Weinberg View author publications You can also search for this author inPubMed Google Scholar * Keith D Robertson View author publications You can also search for this author inPubMed Google
Scholar * Kenneth R Chien View author publications You can also search for this author inPubMed Google Scholar * Hideko Kasahara View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Hideko Kasahara. ADDITIONAL INFORMATION DUALITY OF INTEREST The authors declare no conflict of interest. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Takeda, M., Briggs, L., Wakimoto, H. _et al._ Slow progressive conduction and contraction defects in loss of Nkx2-5
mice after cardiomyocyte terminal differentiation. _Lab Invest_ 89, 983–993 (2009). https://doi.org/10.1038/labinvest.2009.59 Download citation * Received: 17 February 2009 * Revised: 29
April 2009 * Accepted: 21 May 2009 * Published: 22 June 2009 * Issue Date: September 2009 * DOI: https://doi.org/10.1038/labinvest.2009.59 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative KEYWORDS * Nkx2-5 * cardiomyocyte * conduction defects * gene targeting * hypertrophy