Play all audios:
ABSTRACT The small intestine epithelium renews every 2 to 5 days, making it one of the most regenerative mammalian tissues. Genetic inducible fate mapping studies have identified two
principal epithelial stem cell pools in this tissue. One pool consists of columnar _Lgr5_-expressing cells that cycle rapidly and are present predominantly at the crypt base1. The other pool
consists of _Bmi1-_expressing cells that largely reside above the crypt base2. However, the relative functions of these two pools and their interrelationship are not understood. Here we
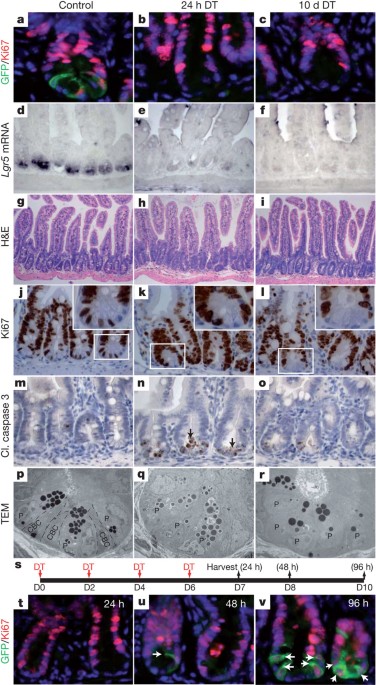
specifically ablated _Lgr5_-expressing cells in mice using a human diphtheria toxin receptor (_DTR_) gene knocked into the _Lgr5_ locus. We found that complete loss of the _Lgr5_-expressing
cells did not perturb homeostasis of the epithelium, indicating that other cell types can compensate for the elimination of this population. After ablation of _Lgr5_-expressing cells,
progeny production by _Bmi1_-expressing cells increased, indicating that _Bmi1_-expressing stem cells compensate for the loss of _Lgr5_-expressing cells. Indeed, lineage tracing showed that
_Bmi1_-expressing cells gave rise to _Lgr5_-expressing cells, pointing to a hierarchy of stem cells in the intestinal epithelium. Our results demonstrate that _Lgr5_-expressing cells are
dispensable for normal intestinal homeostasis, and that in the absence of these cells, _Bmi1_-expressing cells can serve as an alternative stem cell pool. These data provide the first
experimental evidence for the interrelationship between these populations. The _Bmi1_-expressing stem cells may represent both a reserve stem cell pool in case of injury to the small
intestine epithelium and a source for replenishment of the _Lgr5-_expressing cells under non-pathological conditions. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only
$3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MTGR1 IS REQUIRED TO MAINTAIN
SMALL INTESTINAL STEM CELL POPULATIONS Article Open access 25 July 2024 CELL FATE SPECIFICATION AND DIFFERENTIATION IN THE ADULT MAMMALIAN INTESTINE Article 21 September 2020 CREPT IS
REQUIRED FOR MURINE STEM CELL MAINTENANCE DURING INTESTINAL REGENERATION Article Open access 11 January 2021 REFERENCES * Barker, N. et al. Identification of stem cells in small intestine
and colon by marker gene _Lgr5_ . _Nature_ 449, 1003–1007 (2007) Article ADS CAS Google Scholar * Sangiorgi, E. & Capecchi, M. R. _Bmi1_ is expressed _in vivo_ in intestinal stem
cells. _Nature Genet._ 40, 915–920 (2008) Article CAS Google Scholar * Li, L. & Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. _Science_ 327, 542–545
(2010) Article ADS CAS Google Scholar * Fuchs, E. The tortoise and the hair: slow-cycling cells in the stem cell race. _Cell_ 137, 811–819 (2009) Article CAS Google Scholar * Zhu, L.
et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. _Nature_ 457, 603–607 (2009) Article ADS CAS Google Scholar * Furuyama, K. et al.
Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. _Nature Genet._ 43, 34–41 (2011) Article CAS Google Scholar * Sato, T. et
al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. _Nature_ 469, 415–418 (2011) Article ADS CAS Google Scholar * Cheng, H. & Leblond, C. P. Origin,
differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. _Am. J. Anat._ 141,
537–561 (1974) Article CAS Google Scholar * Montgomery, R. K. et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. _Proc. Natl
Acad. Sci. USA_ 108, 179–184 (2011) Article ADS CAS Google Scholar * Muncan, V. et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene _c-Myc_ .
_Mol. Cell. Biol._ 26, 8418–8426 (2006) Article CAS Google Scholar * van der Flier, L. G. et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. _Cell_ 136,
903–912 (2009) Article CAS Google Scholar * Garcia, M. I. et al. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine.
_Dev. Biol._ 331, 58–67 (2009) Article CAS Google Scholar * Crosnier, C., Stamataki, D. & Lewis, J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial
control. _Nature Rev. Genet._ 7, 349–359 (2006) Article CAS Google Scholar * Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures _in vitro_ without a mesenchymal niche.
_Nature_ 459, 262–265 (2009) Article ADS CAS Google Scholar * Park, I. K., Morrison, S. J. & Clarke, M. F. Bmi1, stem cells, and senescence regulation. _J. Clin. Invest._ 113,
175–179 (2004) Article CAS Google Scholar * Hosen, N. et al. Bmi-1-green fluorescent protein-knock-in mice reveal the dynamic regulation of bmi-1 expression in normal and leukemic
hematopoietic cells. _Stem Cells_ 25, 1635–1644 (2007) Article CAS Google Scholar * van der Flier, L. G., Haegebarth, A., Stange, D. E., van de Wetering, M. & Clevers, H. OLFM4 is a
robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. _Gastroenterology_ 137, 15–17 (2009) Article Google Scholar * Lobachevsky, P. N. &
Radford, I. R. Intestinal crypt properties fit a model that incorporates replicative ageing and deep and proximate stem cells. _Cell Prolif._ 39, 379–402 (2006) Article CAS Google Scholar
* Buske, P. et al. A comprehensive model of the spatio-temporal stem cell and tissue organisation in the intestinal crypt. _PLOS Comput. Biol._ 7, e1001045 (2011) Article CAS Google
Scholar * Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. _Cell_ 135, 1118–1129 (2008) Article CAS Google Scholar
* Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. _Nature Med._ 11, 1351–1354 (2005) Article CAS Google Scholar
* Hsu, Y. C., Pasolli, H. A. & Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. _Cell_ 144, 92–105 (2011) Article CAS Google Scholar * Bastide, P. et
al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. _J. Cell Biol._ 178, 635–648 (2007) Article CAS Google Scholar *
Garabedian, E. M., Roberts, L. J., McNevin, M. S. & Gordon, J. I. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. _J. Biol. Chem._ 272,
23729–23740 (1997) Article CAS Google Scholar * Warming, S., Rachel, R. A., Jenkins, N. A. & Copeland, N. G. _Zfp423_ is required for normal cerebellar development. _Mol. Cell. Biol._
26, 6913–6922 (2006) Article CAS Google Scholar * Liu, P., Jenkins, N. A. & Copeland, N. G. A highly efficient recombineering-based method for generating conditional knockout
mutations. _Genome Res._ 13, 476–484 (2003) Article CAS Google Scholar * Kissenpfennig, A. et al. Dynamics and function of Langerhans cells _in vivo_: dermal dendritic cells colonize
lymph node areas distinct from slower migrating Langerhans cells. _Immunity_ 22, 643–654 (2005) Article CAS Google Scholar * Warming, S., Costantino, N., Court, D. L., Jenkins, N. A.
& Copeland, N. G. Simple and highly efficient BAC recombineering using galK selection. _Nucleic Acids Res._ 33, e36 (2005) Article Google Scholar * Lee, E. C. et al. A highly efficient
_Escherichia coli_-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. _Genomics_ 73, 56–65 (2001) Article CAS Google Scholar * Van
Keuren, M. L., Gavrilina, G. B., Filipiak, W. E., Zeidler, M. G. & Saunders, T. L. Generating transgenic mice from bacterial artificial chromosomes: transgenesis efficiency, integration
and expression outcomes. _Transgenic Res._ 18, 769–785 (2009) Article Google Scholar * Gregorieff, A. & Clevers, H. _In situ_ hybridization to identify gut stem cells. _Curr. Protoc.
Stem Cell Biol._ Ch. 2, Unit 2F.1. (2010) * Potten, C. S., Gandara, R., Mahida, Y. R., Loeffler, M. & Wright, N. A. The stem cells of small intestinal crypts: where are they? _Cell
Prolif._ 42, 731–750 (2009) Article CAS Google Scholar * Bjerknes, M. & Cheng, H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult
mouse. _Am. J. Anat._ 160, 51–63 (1981) Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We gratefully acknowledge efforts by all the members of the Genentech mouse
facility, in particular R. Ybarra and G. Morrow. We are grateful to N. Strauli, D.-K. Tran and A. Rathnayake for assistance with mouse breeding. We thank M. Roose-Girma, X. Rairdan and the
members of the embryonic stem cell and microinjection groups for embryonic stem cell work and transgenic line generation and members of the F.J.d.S. laboratory for discussions and ideas.
This work was funded in part by the National Institutes of Health through the NIH Director’s New Innovator Award Program, 1-DP2-OD007191 and by R01-DE021420, both to O.D.K. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Molecular Biology, Genentech Inc., 1 DNA Way, South San Francisco, California 94080, USA, Hua Tian, Søren Warming & Frederic J. de
Sauvage * Departments of Orofacial Sciences and Pediatrics, Institute for Human Genetics and Program in Craniofacial and Mesenchymal Biology, UCSF, 513 Parnassus Avenue, San Francisco,
California 94143-0442, USA, Brian Biehs & Ophir D. Klein * Department of Research Oncology, Genentech Inc., 1 DNA Way, South San Francisco, California 94080, USA, Kevin G. Leong *
Department of Pathology, Genentech Inc., 1 DNA Way, South San Francisco, California 94080, USA, Linda Rangell Authors * Hua Tian View author publications You can also search for this author
inPubMed Google Scholar * Brian Biehs View author publications You can also search for this author inPubMed Google Scholar * Søren Warming View author publications You can also search for
this author inPubMed Google Scholar * Kevin G. Leong View author publications You can also search for this author inPubMed Google Scholar * Linda Rangell View author publications You can
also search for this author inPubMed Google Scholar * Ophir D. Klein View author publications You can also search for this author inPubMed Google Scholar * Frederic J. de Sauvage View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.T., B.B., S.W., K.G.L. and L.R. designed, performed experiments and collected data. H.T., B.B.,
O.D.K. and F.J.d.S. designed experiments, analysed the data and wrote the manuscript. O.D.K. and F.J.d.S. are joint senior authors. All authors discussed results and edited the manuscript.
CORRESPONDING AUTHORS Correspondence to Ophir D. Klein or Frederic J. de Sauvage. ETHICS DECLARATIONS COMPETING INTERESTS H.T., S.W., K.G.L., L.R. and F.J.d.S. are employees of Genentech
Inc., a member of the Roche Group, and may have an equity interest in Roche. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES The file contains Supplementary Figures 1-8 with legends. (PDF
388 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Tian, H., Biehs, B., Warming, S. _et al._ A reserve stem cell population in small intestine renders _Lgr5_-positive cells dispensable. _Nature_ 478,
255–259 (2011). https://doi.org/10.1038/nature10408 Download citation * Received: 19 April 2011 * Accepted: 01 August 2011 * Published: 18 September 2011 * Issue Date: 13 October 2011 * DOI:
https://doi.org/10.1038/nature10408 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative