Play all audios:
ABSTRACT Innate immunity serves as the first line of defence against invading pathogens such as bacteria and viruses1. Toll-like receptors (TLRs) are examples of innate immune receptors,
which sense specific molecular patterns from pathogens and activate immune responses2. TLR9 recognizes bacterial and viral DNA containing the cytosine–phosphate–guanine (CpG)
dideoxynucleotide motif3,4. The molecular basis by which CpG-containing DNA (CpG-DNA) elicits immunostimulatory activity via TLR9 remains to be elucidated. Here we show the crystal
structures of three forms of TLR9: unliganded, bound to agonistic CpG-DNA, and bound to inhibitory DNA (iDNA). Agonistic-CpG-DNA-bound TLR9 formed a symmetric TLR9–CpG-DNA complex with 2:2
stoichiometry, whereas iDNA-bound TLR9 was a monomer. CpG-DNA was recognized by both protomers in the dimer, in particular by the amino-terminal fragment (LRRNT–LRR10) from one protomer and
the carboxy-terminal fragment (LRR20–LRR22) from the other. The iDNA, which formed a stem-loop structure suitable for binding by intramolecular base pairing, bound to the concave surface
from LRR2–LRR10. This structure serves as an important basis for improving our understanding of the functional mechanisms of TLR9. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per
year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during
checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS TLR3 FORMS A HIGHLY
ORGANIZED CLUSTER WHEN BOUND TO A POLY(I:C) RNA LIGAND Article Open access 12 November 2022 REGULATION OF THE NUCLEIC ACID-SENSING TOLL-LIKE RECEPTORS Article 16 July 2021 STRUCTURAL INSIGHT
INTO TLR4/MD-2 ACTIVATION BY SYNTHETIC LPS MIMETICS WITH DISTINCT BINDING MODES Article Open access 05 May 2025 ACCESSION CODES PRIMARY ACCESSIONS PROTEIN DATA BANK * 3WPB * 3WPC * 3WPD *
3WPE * 3WPF * 3WPG * 3WPH * 3WPI DATA DEPOSITS The coordinates and structure-factor data of horse TLR9 (unliganded form), TLR9–DNA1668_12mer, TLR9–iDNA4084, bovine TLR9–DNA1668_12mer, mouse
TLR9 (unliganded form), TLR9–iDNA4084 (form1), TLR9–iDNA4084 (form2) and TLR9–iDNA_super have been deposited in the Protein Data Bank under the accession numbers 3WPB, 3WPC, 3WPD, 3WPE,
3WPF, 3WPG, 3WPH and 3WPI, respectively. REFERENCES * Janeway, C. A., Jr & Medzhitov, R. Innate immune recognition. _Annu. Rev. Immunol._ 20, 197–216 (2002) Article CAS Google Scholar
* Akira, S. & Takeda, K. Toll-like receptor signalling. _Nature Rev. Immunol._ 4, 499–511 (2004) Article CAS Google Scholar * Bauer, S. et al. Human TLR9 confers responsiveness to
bacterial DNA via species-specific CpG motif recognition. _Proc. Natl Acad. Sci. USA_ 98, 9237–9242 (2001) Article ADS CAS Google Scholar * Hemmi, H. et al. A Toll-like receptor
recognizes bacterial DNA. _Nature_ 408, 740–745 (2000) Article ADS CAS Google Scholar * Krieg, A. M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. _Nature_ 374,
546–549 (1995) Article ADS CAS Google Scholar * Ewald, S. E. et al. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine
endopeptidase. _J. Exp. Med._ 208, 643–651 (2011) Article CAS Google Scholar * Sepulveda, F. E. et al. Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling
in dendritic cells. _Immunity_ 31, 737–748 (2009) Article CAS Google Scholar * Park, B. et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of
Toll-like receptor 9. _Nature Immunol._ 9, 1407–1414 (2008) Article CAS Google Scholar * Ewald, S. E. et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional
receptor. _Nature_ 456, 658–662 (2008) Article ADS CAS Google Scholar * Onji, M. et al. An essential role for the N-terminal fragment of Toll-like receptor 9 in DNA sensing. _Nature
Commun._ 4, 1949 (2013) Article ADS Google Scholar * Lenert, P. S. Classification, mechanisms of action, and therapeutic applications of inhibitory oligonucleotides for Toll-like
receptors (TLR) 7 and 9. _Mediators Inflamm._ 2010, 986596 (2010) Article Google Scholar * Jin, M. S. et al. Crystal structure of the TLR1–TLR2 heterodimer induced by binding of a
tri-acylated lipopeptide. _Cell_ 130, 1071–1082 (2007) Article CAS Google Scholar * Kang, J. Y. et al. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6
heterodimer. _Immunity_ 31, 873–884 (2009) Article CAS Google Scholar * Liu, L. et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. _Science_ 320, 379–381
(2008) Article ADS CAS Google Scholar * Park, B. S. et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. _Nature_ 458, 1191–1195 (2009) Article ADS
CAS Google Scholar * Tanji, H., Ohto, U., Shibata, T., Miyake, K. & Shimizu, T. Structural reorganization of the Toll-like receptor 8 dimer induced by agonistic ligands. _Science_ 339,
1426–1429 (2013) Article ADS CAS Google Scholar * Yoon, S. I. et al. Structural basis of TLR5-flagellin recognition and signaling. _Science_ 335, 859–864 (2012) Article ADS CAS
Google Scholar * Kubarenko, A. V. et al. A naturally occurring variant in human TLR9, P99L, is associated with loss of CpG oligonucleotide responsiveness. _J. Biol. Chem._ 285, 36486–36494
(2010) Article CAS Google Scholar * Peter, M. E., Kubarenko, A. V., Weber, A. N. & Dalpke, A. H. Identification of an N-terminal recognition site in TLR9 that contributes to
CpG-DNA-mediated receptor activation. _J. Immunol._ 182, 7690–7697 (2009) Article CAS Google Scholar * Ho, K. L. et al. MeCP2 binding to DNA depends upon hydration at methyl-CpG. _Mol.
Cell_ 29, 525–531 (2008) Article CAS Google Scholar * Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. _Methods Enzymol._ 276, 307–326
(1997) Article CAS Google Scholar * Battye, T. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. iMOSFLM: a new graphical interface for diffraction-image processing
with MOSFLM. _Acta Crystallogr. D_ 67, 271–281 (2011) Article CAS Google Scholar * Vagin, A. & Teplyakov, A. Molecular replacement with MOLREP. _Acta Crystallogr. D_ 66, 22–25 (2010)
Article CAS Google Scholar * Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. _Acta Crystallogr. D_ 60, 2126–2132 (2004) Article Google Scholar *
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. _Acta Crystallogr. D_ 53, 240–255 (1997) Article CAS Google
Scholar * Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. _Acta Crystallogr. D_ 58, 1948–1954 (2002) Article Google Scholar *
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. _Acta Crystallogr. D_ 66, 12–21 (2010) Article CAS Google Scholar * DeLano, W. L. The
PyMOL Molecular Graphics System. DeLano Scientific LLC. http://www.pymol.org (2008) * Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation
and lamm equation modeling. _Biophys. J._ 78, 1606–1619 (2000) Article ADS CAS Google Scholar * Muta, T. & Takeshige, K. Essential roles of CD14 and lipopolysaccharide-binding
protein for activation of toll-like receptor (TLR)2 as well as TLR4 reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. _FEBS J._ 268, 4580–4589
(2001) CAS Google Scholar * Xu, C., Bian, C., Lam, R., Dong, A. & Min, J. The structural basis for selective binding of non-methylated CpG islands by the CFP1 CXXC domain. _Nat.
Commun._ 2, 227 (2011) Article ADS Google Scholar * Arita, K., Ariyoshi, M., Tochio, H., Nakamura, Y. & Shirakawa, M. Recognition of hemi-methylated DNA by the SRA protein UHRF1 by a
base-flipping mechanism. _Nature_ 455, 818–821 (2008) Article ADS CAS Google Scholar * Avvakumov, G. V., Walker, J. R. & Xue, S. Li, Y. Duan, S., Bronner, C., Arrowsmith, C. H. &
Dhe-Paganon, S. Structural basis for recognition of hemi-methylated DNA by the SRA domain of human UHRF1. _Nature_ 455, 822–825 (2008) Article ADS CAS Google Scholar * Hashimoto, H.,
Horton, J. R., Zhang, X. & Cheng, X. The SRA domain of UHRF1 flips 5-methylcytosine out of the DNA helix. _Nature_ 455, 826–829 (2008) Article ADS CAS Google Scholar Download
references ACKNOWLEDGEMENTS We thank the beamline staff members at the Photon Factory and SPring-8 for their assistance with data collection. This work was supported by a Grant-in-Aid from
the Japanese Ministry of Education, Culture, Sports, Science, and Technology (U.O., S.U., K.M. and T.S.); the JSPS Japanese–German Graduate Externship (S.U.); the Senri-Life Science
Foundation (S.U.); the Takeda Science Foundation (U.O. and T.S.); and the Mochida Memorial Foundation for Medical and Pharmaceutical Research (U.O.). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Umeharu Ohto, Hiromi Tanji, Hanako Ishida & Toshiyuki Shimizu
* Division of Innate Immunity, Department of Microbiology and Immunology, Laboratory of Innate Immunity, Center for Experimental Medicine and Systems Biology, The Institute of Medical
Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan, Takuma Shibata & Kensuke Miyake * Core Research for Evolutional Science and Technology (CREST),
Japan Science and Technology Agency (JST), Saitama 332-0012, Japan, Takuma Shibata & Toshiyuki Shimizu * Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka
565-0871, Japan, Elena Krayukhina & Susumu Uchiyama * U-Medico Corporation, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan, Elena Krayukhina Authors * Umeharu Ohto View author publications
You can also search for this author inPubMed Google Scholar * Takuma Shibata View author publications You can also search for this author inPubMed Google Scholar * Hiromi Tanji View author
publications You can also search for this author inPubMed Google Scholar * Hanako Ishida View author publications You can also search for this author inPubMed Google Scholar * Elena
Krayukhina View author publications You can also search for this author inPubMed Google Scholar * Susumu Uchiyama View author publications You can also search for this author inPubMed Google
Scholar * Kensuke Miyake View author publications You can also search for this author inPubMed Google Scholar * Toshiyuki Shimizu View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS U.O. and H.T. expressed and purified recombinant proteins. U.O. performed crystallization and structure determination. T. Shibata and K.M.
performed cellular assays. E.K. and S.U. performed AUC analyses. U.O. and H.I. performed ITC experiments. U.O. and T. Shimizu directed the research and wrote the paper with assistance from
all other authors. CORRESPONDING AUTHOR Correspondence to Toshiyuki Shimizu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. EXTENDED DATA
FIGURES AND TABLES EXTENDED DATA FIGURE 1 SEQUENCE ALIGNMENT OF HUMAN, HORSE, BOVINE AND MOUSE TLR9. Sequence alignments are displayed for each LRR module. The agonist DNA interfaces 1 and 2
deduced from the EcTLR9–DNA1668_12mer complex are indicated by blue and yellow highlighting, respectively. The antagonist DNA interface deduced from the EcTLR9–iDNA4084 complex is indicated
with boxes. The protein–protein interface in the EcTLR9–DNA1668_12mer complex is indicated by bold orange bars below each LRR module. Alignments were performed using Clustal Omega software
(EMBL-European Bioinformatics Institute). Residues are coloured to indicate the degree of similarity: red residues are those with the highest similarity, followed by green, blue and black
(lowest similarity). EXTENDED DATA FIGURE 2 THE NF-ΚB ACTIVATION EXPERIMENTS. A, DNA1668_12mer retains agonistic activity to TLR9. The NF-κB activation of wild-type mouse TLR9 induced by
DNA1668 (TCCATGACGTTCCTGATGCT), DNA1668_12mer (CATGACGTTCCT), DNA1668 or DNA1668_12mer with a CpG to GpC inversion (DNA1668_GC, DNA1668_12mer_GC). DNAs were all complexed with
_N_-[1-(2,3-Dioleoyloxy)propyl]-_N_, _N_, _N_-trimethylammonium methyl-sulphate (DOTAP) and added at concentration of 1 μM. The activities were analysed with an NF-κB-dependent luciferase
reporter assay using HEK293T cells co-expressing mouse TLR9 and mouse Unc93B1. A two-tailed _t_-test was used to determine the statistical significance of differences between control (Ctrl)
and stimulated cells, or between each group. **_P_ < 0.01. Data from three independent experiments are shown. B, Horse and bovine TLR9 responses against agonistic and inhibitory DNAs. The
NF-κB activation of wild-type horse (left) or bovine (right) TLR9 induced by indicated DNAs. The activities were analysed with an NF-κB-dependent luciferase reporter assay using HEK293T
cells co-expressing horse or bovine TLR9 and human Unc93B1. The concentration of agonistic DNAs (DNA1668, DNA1668_GC and DNA1668_met) and inhibitory DNAs (iDNA4084 and iDNA_super) were 10 μM
and 1 μM, respectively. Data represent the mean fold induction of NF-κB activity +s.d. (_n_ = 3). A two-tailed _t_-test was used to determine the statistical significance of differences
between control (Ctrl) and stimulated cells, or between each group. **_P_ < 0.01. Data from three independent experiments are shown. EXTENDED DATA FIGURE 3 DIMERIZATION INTERACTION OF
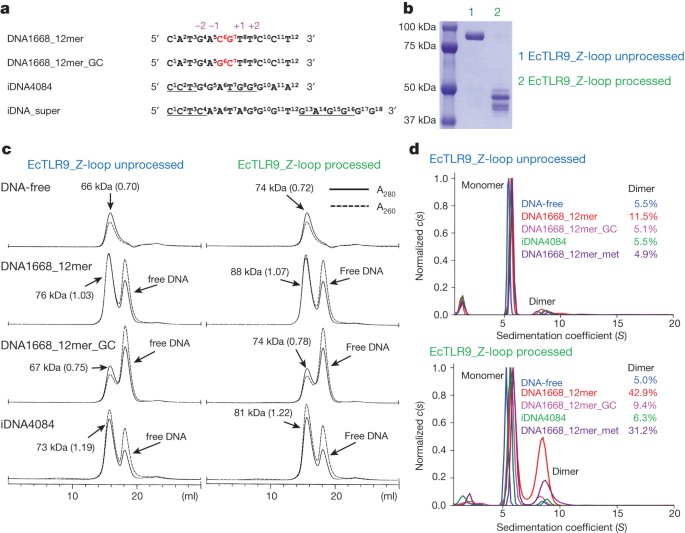
TLR9. A, B, The oligomerization states of EcTLR9 with an unprocessed Z-loop (A) or a processed Z-loop (B) were analysed by SV–AUC at various concentrations of TLR9–DNA1668_12mer (equimolar).
The weight-average sedimentation coefficients (Sw) were plotted against TLR9–DNA1668_12mer concentration to determine the _K_d value for the dimerization. The dissociation constant for the
dimerization of the processed TLR9 is estimated to be 20 μM. EXTENDED DATA FIGURE 4 ELECTRON DENSITIES OF DNA BOUND TO TLR9. A–E, The _F_o–_F_c omit difference electron densities of
DNA1668_12mer bound to EcTLR9 (A) and BtTLR9 (B), iDNA4084 bound to EcTLR9 (C) and MmTLR9 (D), and iDNA_super bound to MmTLR9 (E) contoured at the 3.0_σ_ level. The residues coloured blue in
the sequence are not visible in the electron density map. The core hexamer could be unambiguously modelled into the continuous electron density map in the EcTLR9–DNA1668_12mer and
BtTLR9–DNA1668_12mer complexes, whereas flanking bases were obscure or not visible. The A5–T12 loop connecting the base-paired region of iDNA_super was not visible in the electron density
map, whereas the G4–A6 sequence of iDNA4084 was well defined. EXTENDED DATA FIGURE 5 STRUCTURES OF TLR9. A, Monomer structure of EcTLR9, derived from the EcTLR9–DNA1668_12mer complex. The
structure and binding mode of TLR9 are markedly different from those of other CpG-binding proteins20,31,32,33,34. B, Monomer structure of human TLR8, derived from the human TLR8–CL097
(2-(ethoxymethyl)-1H-imidazo[4,5-c]quinolin-4-amine) complex (PDB ID: 3W3J)16. The Z-loops in TLR9 and TLR8 are oriented differently with respect to the concave face of TLR and engage in
different interactions with it. The latter half of the Z-loop of TLR8 extends towards the N-terminus, whereas the Z-loop of TLR9 extends towards the C-terminus to interact with LRR15–21.
TLR8 has three ordered _N_-glycans attached to Asn293, Asn511 and Asn590 that project into the inner space of the ring structure, whereas EcTLR9 has only one _N_-glycan attached to Asn731
that projects inward. As a result, the ring structure of TLR9 has more unoccupied inner space than that of TLR8, an arrangement that is suitable for ligand binding on the concave interior
surface. C, Superposition of the overall ligand-induced dimer structures of EcTLR9 (DNA1668_12mer complex, green) and human TLR8 (CL097 complex, purple) by PyMol28, yielding an
root-mean-square deviation (r.m.s.d.) value of 2.3 Å. D, Superposition of the overall dimer structures of EcTLR9 (DNA1668_12mer complex, green) and BtTLR9 (DNA1668_12mer complex, purple) by
PyMol28, yielding an r.m.s.d. value of 0.7 Å. E, Magnified view of the CpG-binding groove of EcTLR9 and BtTLR9. The amino acid at position 109 was proline for human and serine for mouse
(rodents). From the structure, Pro109 made a van der Waals contact with A at the –1 position, but its contact was somewhat close. If A is changed to T, this contact is weakened. Serine at
position 109 would accommodate a larger base. F, Magnified view of the G10 of iDNA4084 recognition by TLR9 (EcTLR9–iDNA4084 complex). G10 of iDNA4084 makes three hydrogen bonds with TLR9:
N2, N1 and O6 atoms of G10 with the side chains of Ser205, Asp175 and Ser151, respectively. To examine the functional importance of this base, we substituted it with other bases and examined
the binding affinity by ITC. The affinity of TLR9 for G10A (_K_d = 6 nM) was reduced from that of the original sequence (_K_d = 3 nM), but TLR9 exhibited much lower affinity for DNAs with a
pyrimidine at this position (_K_d = 41 nM for G10C and _K_d = 76 nM for G10T), suggesting that this position favours purine over pyrimidine. G, Superposition of iDNA4084 bound to EcTLR9
(green) and MmTLR9 (purple). The binding mode of iDNA4048 is perfectly conserved between EcTLR9 and MmTLR9. EXTENDED DATA FIGURE 6 BINDING INTERFACES FOR AGONISTIC AND INHIBITORY DNA. A,
Superposition of the structures of unliganded (grey), DNA1668_12mer-bound (green), and iDNA4084-bound (blue), EcTLR9. TLR9 and DNA are shown in Cα-trace and stick representation,
respectively. No significant conformational change was observed upon agonistic DNA binding, as indicated by the small r.m.s.d. value of 0.8 Å between EcTLR9 (unliganded) and the
EcTLR9–DNA1668_12mer complex. Instead, the conversion of EcTLR9 into the activated form appears to involve local conformational changes in the loop regions of LRR8, LRR11 and LRR18, all of
which are involved in formation of the dimer. No significant structural change was induced by binding to iDNA4084, as indicated by the small r.m.s.d. values of 0.49 Å and 0.45 Å between the
unliganded and iDNA4084-bound forms of EcTLR9 and MmTLR9, respectively. B, Surface representations of EcTLR9 structures in the DNA1668_12mer (upper) and iDNA4084 complexes (lower). The
protein–protein interface, TLR9_DNA1668_12mer interfaces 1 and 2, and TLR9_iDNA4084 interface are shown in orange, blue, cyan and yellow, respectively. The bound DNAs are shown in stick
representation with their 5′ and 3′ ends indicated. DNA1668_12mer buries approximately 1,136 Å2 and 294 Å2 of the accessible surface area of TLR9 and TLR9*, respectively, suggesting that the
N-terminal binding site of TLR9 for DNA1668_12mer makes a relatively larger contribution to binding. The binding site for iDNA partially overlaps with the binding site for agonistic DNA.
Specifically, LRR4 and LRR5 are both involved in the binding sites for agonistic DNA and iDNAs, although the binding modes of DNA1668_12mer and iDNA4084 are completely different: Arg152
(LRR4), Tyr179 and Lys181 (LRR5) interact with the phosphate of C11 of DNA1668_12mer but also with G8 of iDNA4084. C, Electrostatic potential map of DNA-binding region. The map was
calculated at basic and acidic conditions by PyMol28. Surface colours represent the potential from –20 _k_B_T_/e (red) to 20 _k_B_T_/e (blue), where _k_B is the Boltzman constant and _T_ is
the absolute temperature. The DNA molecule is shown as a stick model. EXTENDED DATA FIGURE 7 ITC THERMOGRAMS FOR ITC DATA (RELATED TO EXTENDED DATA TABLE 2). Representative ITC thermograms
for the ITC data are shown with their pH condition and _K_d values for EcTLR9. EXTENDED DATA FIGURE 8 SIGNIFICANCE OF THE CONSENSUS SEQUENCE IN THE CPG-DNA. Gel-filtration chromatography of
EcTLR9 with FITC-labelled DNA. DNA binding to TLR9 was monitored by FITC-fluorescence (excitation 495 nm, emission 520 nm). The parenthesized values indicate the ratios of the fluorescence
peak height of the derivative DNA to the original DNA containing the consensus sequence of GACGTT (top left). In each experiment, 0.5 μM EcTLR9 (total volume of 45 μl) with DNA (equimolar)
was injected into a Superdex 200 Increase 5/150 GL (GE healthcare) gel-filtration column. The running buffer was 10 mM 2-morpholinoethanesulfonic acid and 250 mM NaCl at pH 5.5. DNAs used in
the analyses are shown in each panel. Bases that are changed from the original sequence are highlighted in red. Conversion of the purine–purine sequence (GA) at the –1 and –2 positions of
the CpG motif (underlined) to AA and GG resulted in DNAs with affinities similar to the wild-type DNA, but conversion to a pyrimidine–pyrimidine sequence (TT and CC) weakened the affinity,
demonstrating that a purine–purine sequence is preferable at these positions. Conversion of the base at the +1 position into C, A or G led to weaker binding, suggesting that T is preferable
at the +1 position, although T is not specifically recognized. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG.
4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ohto, U., Shibata, T., Tanji, H. _et al._ Structural basis of CpG and inhibitory DNA recognition by
Toll-like receptor 9. _Nature_ 520, 702–705 (2015). https://doi.org/10.1038/nature14138 Download citation * Received: 18 February 2014 * Accepted: 03 December 2014 * Published: 09 February
2015 * Issue Date: 30 April 2015 * DOI: https://doi.org/10.1038/nature14138 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative