Play all audios:
ABSTRACT Metal nanoclusters have recently attracted extensive interest not only for fundamental scientific research, but also for practical applications. For fundamental scientific research,
it is of major importance to explore the internal structure and crystallographic arrangement. Herein, we synthesize a gold nanocluster whose composition is determined to be Au60S6(SCH2Ph)36
by using electrospray ionization mass spectrometry and single crystal X-ray crystallography (SCXC). SCXC also reveals that Au60S6(SCH2Ph)36 consists of a fcc-like Au20 kernel protected by a
pair of giant Au20S3(SCH2Ph)18 staple motifs, which contain 6 tetrahedral-coordinate _μ_4-S atoms not previously reported in the Au–S interface. Importantly, the fourth crystallographic
closest-packed pattern, termed 6H left-handed helical (6HLH) arrangement, which results in the distinct loss of solid photoluminescence of amorphous Au60S6(SCH2Ph)36, is found in the
crystals of Au60S6(SCH2Ph)36. The solvent-polarity-dependent solution photoluminescence is also demonstrated. Overall, this work provides important insights about the structure, Au–S bonding
and solid photoluminescence of gold nanoclusters. SIMILAR CONTENT BEING VIEWED BY OTHERS HIGH-RESOLUTION CRYSTAL STRUCTURE OF A 20 KDA SUPERFLUORINATED GOLD NANOCLUSTER Article Open access
11 May 2022 INTERDEPENDENCE BETWEEN NANOCLUSTERS AUAG24 AND AU2AG41 Article Open access 03 February 2021 ATOMICALLY PRECISE COPPER DOPANTS IN METAL CLUSTERS BOOST UP STABILITY, FLUORESCENCE,
AND PHOTOCATALYTIC ACTIVITY Article Open access 08 February 2023 INTRODUCTION Bridging gold atom (or gold complexes)1,2,3 and nanocrystals (typically >3 nm)4,5,6,7,8, gold
nanoclusters9,10,11,12,13,14,15 have recently attracted increasing research interest due to their well-defined compositions and structures, unique properties and potential applications. In
particular, the structures of gold nanoclusters are of primary significance and have received the most extensive attention in the research community16,17,18,19,20,21. However, structure
achievements remain limited due to the difficulty of precise size control and structural resolution, especially for relatively large gold nanoclusters22,23,24,25,26. It is generally believed
that the gold nanoclusters adopt kernel-staple structures, and the staple motifs play a vital role in stabilizing the nanoclusters. The large staple motif Au8(SR)8 was reported in
Au20(SR)16 nanocluster27, but the question remains whether there is a larger staple motif. Although thiolate or sulfido (-S-) can be found in the surface of gold nanoclusters as protecting
ligands, both thiolate sulfur and sulfido (-S-) are always three-coordinate (mainly _μ_2-S and rarely _μ_3-S)28,29, naturally raising the question of whether there are sulfurs with any other
coordination numbers in gold nanoclusters. Notably, four-coordinate _μ_4-S and five-coordinate _μ_4-S have been reported in Cr(III) and Cu(I) complexes30,31, respectively, but such
high-coordinate sulfur has not been found in Au–S interfaces (not limited to gold nanoclusters). In addition to these internal structure questions, there are some other crystallographic
arrangement issues that need to be addressed. It is known that there are two classic crystallographic closest packings for atomic crystals: the fcc arrangement with a packing sequence of
‘ABC’ found in bulk Au, Ag, Pd, Pt and Ir metals32, FePt nanoparticles33, and others; and the hcp or 2H arrangement with a packing sequence of ‘AB’ revealed in Rh nanosheets34, Au square
sheets35, PbPt nanorods36, BiPt nanoplates37, among others. The third closest packing, named the 4H arrangement, has a packing sequence of ‘ABCD’ and was first observed in bulk Ag by
Novgorodova _et al_. in 1979 (ref. 38), then subsequently revealed in Ag nanocrystals and nanowires39,40, Au nanoribbons41 and more. The advances in gold nanocluster research provide
opportunities to discover novel closest-packed patterns. It is worth noting that in contrast to gold nanocrystals, molecule-like gold nanoclusters can grow high-quality single crystals for
X-ray crystallography analyses. Thus, not only the internal structure (atom packing in every gold nanocluster) but also the crystallographic arrangement (the arrangement of gold nanoclusters
in single crystals) of gold nanoclusters can be resolved by single crystal X-ray crystallography (SCXC). Jin _et al_. reported the fcc crystallographic arrangement of Au30(SR)18
nanoclusters in early 2016 (ref. 42), and very recently, Wu _et al_. reported the 4H crystallographic arrangement in Au92 crystals43. It is currently unknown whether there are any other
intriguing arrangement patterns in gold nanoclusters. To address this question, together with the above mentioned issues, more gold nanoclusters with complex surfaces must be synthesized,
and their structures must be resolved. Herein, we report the synthesis, structure (including internal and crystallographic) and photoluminescence of a gold nanocluster whose composition is
determined to be Au60S6(SCH2Ph)36 using electrospray ionization mass spectrometry (ESI-MS) and SCXC. In particular, we find a fourth closest packing in the crystal of Au60S6(SCH2Ph)36.
RESULTS SYNTHESIS AND CHARACTERIZATION The Au60S6(SCH2Ph)36 nanocluster was obtained via a thermal-induced ligand exchange reaction of molecularly pure Au38(SC2H4Ph)24 with excess
phenylmethanethiol (HSCH2Ph). Of note, the interesting thing in this synthesis is that the protecting ligand of the starting nanoclusters and the incoming ligand only have subtle difference
in composition (-CH2-, see Supplementary Fig. 1), which may have some implications to other nanoparticles (including quantum dots) synthesis. Briefly, the reaction was initialized by
dissolving 10 mg of Au38(SC2H4Ph)24 nanoclusters in 1 ml of toluene containing 0.5 ml of HSCH2Ph, with stirring. After proceeding overnight at 100 °C under nitrogen atmosphere, the reaction
was terminated by the addition of plenty of methanol. The crude product was thoroughly washed with petroleum ether and methanol for four times, then subjected to subsequent separation and
purification by preparative thin-layer chromatography (PTLC)19. Single crystals of the purified nanoclusters were grown by the vapour diffusion of acetonitrile into the toluene solution of
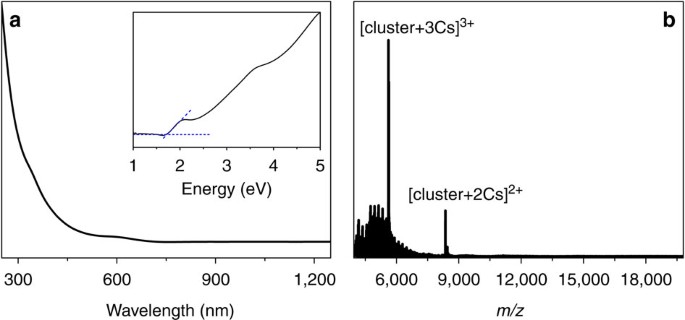
the purified nanoclusters at 5 °C. Black-coloured crystals were formed after one week. As shown in Fig. 1a, the optical absorption spectrum of the as-obtained nanocluster has no dominant
visible absorption peak and only shows a very weak absorption at∼345 nm (3.59 eV) and a step at∼600 nm (2.07 eV). The optical energy gap was determined to be ∼1.73 eV by extrapolating the
lowest-energy absorption peak to zero absorbance (see the inset in Fig. 1a). The composition of the as-obtained nanocluster was identified by ESI-MS. Of note, without the addition of cesium
acetate (CsOAc), no signal was observed in either positive or negative mode, which implies the charge neutrality of the nanocluster. To impart charges, CsOAc was added to the nanocluster
solution to form positively charged [cluster+_x_Cs]_x_+ adducts in the electrospray process. As shown in Fig. 1b, two intense peaks at mass/charge ratio (_m/z_) 8,356.22 and 5,615.49 are
observed, which can be readily assigned to [Au60S6(SCH2Ph)36Cs2]2+ (calculated: 8,356.31, deviation: 0.09) and [Au60S6(SCH2Ph)36Cs3]3+(calculated: 5,615.51, deviation: 0.02), respectively.
Thus, the as-obtained nanocluster should be Au60S6(SCH2Ph)36, which was further confirmed by the subsequent SCXC analysis. INTERNAL STRUCTURE The structure of the as-obtained nanocluster was
determined by SCXC. Figure 2 presents the total structure of the as-obtained nanocluster, which crystallizes in a hexagonal _P_6522 space group, has no centre or plane of symmetry and only
possesses a _C_2 rotation axis of symmetry. The as-obtained nanocluster consists of an Au20 kernel and a pair of giant Au20S3(SCH2Ph)18 staple motifs. The Au20 kernel can be viewed as a
fragment of the fcc structure in bulk gold or nanoparticles, typically >3 nm (diameter). The 16 gold atoms in the kernel constitute an Au16 tetrahedron without vertex (Fig. 3a,e), and
each facet of the tetrahedron is a distorted hexagon. The other 4 Au atoms (highlighted in blue and red, Fig. 3b,f) in the kernel are capped on the four facets of the tetrahedron in a
one-to-one fashion. Moreover, the Au20 kernel is protected by a pair of giant unanimous Au20S3(SCH2Ph)18 staple motifs: one Au20S3(SCH2Ph)18 staple connects to the Au20 kernel (Fig. 3c,g;
the back view can be found in Supplementary Fig. 2) by five terminal _μ_2-S atoms (binding to one kernel Au atom, one staple Au atom and one -CH2Ph group, indicated by white arrow, see Fig.
3i,j) and three bridging _μ_4-S atoms (binding to one kernel Au atom and three staple Au atoms, highlighted in the white circle, see Fig. 3i,j), and the other Au20S3(SCH2Ph)18 staple binds
to the Au20 kernel in the same fashion after rotating (180°) along the C2 axis of symmetry (Fig. 3d,h). It is worth noting that in addition to the common three-coordinate _μ_2-S atoms
(binding to one R group and two Au atoms (Au–SR–Au), see Fig. 3k), there are six tetrahedral-coordinate S atoms (_μ_4-S) (every _μ_4-S binds to four Au atoms, see Fig. 3l), indicating the
uniqueness of Au–S bonding in the Au60S6(SCH2Ph)36 nanocluster. Herein, the six surprising _μ_4-S atoms should come from the thiol, which may undergo S–C bond cleavage under heating
conditions during the ligand-exchange-induced structure transformation process42. A single Au20S3(SR)18 staple motif (branched at the site of the _μ_4-S atom) contains two -Au-SR-Au- and
three -Au-SR-Au-SR-Au- units between the five terminal _μ_2-S atoms and three bridging _μ_4-S atoms, and one -Au-SR-Au-SR-Au- and one -Au-SR-Au-SR-Au-SR-Au- units among the three bridging
_μ_4-S atoms (Fig. 3j). Of note, such a giant staple motif has not been found in thiolated gold nanoclusters, which is larger than the Au8(SR)8 staple motif in Au20(SR)16 nanoclusters27.
CRYSTALLOGRAPHIC ARRANGEMENT Interestingly, Au60S6(SCH2Ph)36 nanoclusters adopt the closest packing, and a very special stacking sequence of ‘ABCDEF’ along the close-packed [001] direction
is found in its single crystals (Fig. 4a). The Au60S6(SCH2Ph)36 nanoclusters in every stacking layer ((001) plane) are arranged uniformly, and each nanocluster is surrounded by six identical
nanoclusters with the same tropism (K-vector), as shown in Fig. 4b and Supplementary Fig. 3. Moreover, the stacking layer perpendicular to the [001] direction can overlap completely with
its neighboring layer after every nanocluster in the layer rotates (60°) clockwise or anti-clockwise along the _z_ axis (Supplementary Fig. 3), and thus the arrangement of Au60S6(SCH2Ph)36
nanoclusters in single crystals along the [001] direction is reminiscent of the left-handed helix (Supplementary Fig. 4). For clarity, a left-handed helical sequence, here termed the 6H
left-handed helical (6HLH) arrangement, is isolated from the crystal, as shown in Fig. 4c,d. Such a crystallographic arrangement is not only interesting but also exciting, as the third
closest packing in crystals, named 4H, was found in 1979 (ref. 38). PHOTOLUMINESCENCE The 6HLH arrangement indicates the unique interactions among Au60S6(SCH2Ph)36 nanoclusters in the single
crystals, which is supported by the photoluminescence intensity comparison between the amorphous and crystallized Au60S6(SCH2Ph)36 as shown in Fig. 5. Although the emission spectrum
profiles of the disordered (amorphous) Au60S6(SCH2Ph)36 is almost superimposable to that of the ordered (crystallized) Au60S6(SCH2Ph)36, the emission intensity of the former is ∼1.7 folds of
that of the latter. The lower photoluminescence intensity in crystallized Au60S6(SCH2Ph)36 might be caused by the energy transfer among the 6HLH arranged Au60S6(SCH2Ph)36 nanoclusters,
indicating the notable interactions among the 6HLH arranged Au60S6(SCH2Ph)36 nanoclusters. Although the 6HLH arrangement barely changes the emission spectrum, solvent does result in the
obvious blue-shift of the maximum emission peak, and interestingly, the blue-shift increases with the increase of solvent polarity, while the photoluminescence intensity of Au60S6(SCH2Ph)36
nanoclusters decreases with the increase of solvent polarity (see Fig. 6). Anyway, these facts indicate that the solution photoluminescence of Au60S6(SCH2Ph)36 is solvent-polarity dependent.
DISCUSSION In summary, a novel near-infrared-emissive gold nanocluster was synthesized via a thermal-induced ligand exchange process, and its composition was determined to be
Au60S6(SCH2Ph)36 using ESI-MS and SCXC. SCXC also revealed that the nanocluster consists of one fcc-like Au20 kernel protected by a pair of Au20S3(SCH2Ph)18 giant staple motifs, which are
larger than the existing staple motifs in structurally resolved gold nanoclusters. In particular, three tetrahedral-coordinate _μ_4-S atoms were found enclosed in every giant staple motif,
which challenges the conventional opinion that sulfur in the Au–S interface is always three-coordinate _μ_2-S. Most important of all, a 6HLH crystallographic arrangement was found in the
crystal of Au60S6(SCH2Ph)36 nanoclusters, and this finding also represents an advance in crystallographic packing research since the 4H phase finding in 1979. Interestingly, the 6HLH
arrangement gives rise to the obvious loss of solid photoluminescence of amorphous Au60S6(SCH2Ph)36, indicating the strong interaction among the uniquely arranged nanoclusters. Another
interesting finding is the solvent-polarity-dependent solution photoluminescence of Au60S6(SCH2Ph)36 nanoclusters. Briefly, this work provides new and exciting views to the structure, Au–S
bonding and photoluminescence (including solid state and solution) of gold nanoclusters, and our work is expected to stimulate further research on the structure and properties of
crystallized materials at the nanoscale. METHODS REAGENTS All chemicals are commercially available and used as received. Tetraoctylammonium bromide (TOAB, 98.0%), 2-Phenylethanethiol
(PhC2H4SH, 99.0%) and phenylmethanethiol (PhCH2SH, 99.0%) were purchased from Sigma-Aldrich. Tetrachloroauric(III) acid (HAuCl4·4H2O, 99.7%), sodium borohydride (NaBH4, 98.0%), acetonitrile
(99.0%, AR), dichloromethane (CH2Cl2, 99.0%, AR), tetrahydrofuran (THF, 99.0%, AR), toluene (99.5%, AR), methanol (CH3OH, 99.5%, AR), and petroleum ether (AR) were purchased from Sinopharm
Chemical Reagent Co., Ltd. SYNTHESIS OF AU60S6(SCH2PH)36 NANOCLUSTERS All chemicals and reagents were used as received. Ten milligrams of Au38(SC2H4Ph)24 nanoclusters was dissolved in 1 ml
of toluene containing 0.5 ml of PhCH2SH. Next, the reaction proceeded overnight at 100 °C under nitrogen atmosphere, and then was terminated by the addition of excess methanol. The crude
product was washed with petroleum ether and methanol four times, dissolved in dichloromethane (DCM), and then subjected to separation and purification by PTLC. Single crystals of the
purified nanoclusters were grown by the vapour diffusion of acetonitrile into a toluene solution of the purified nanoclusters at 5 °C, and black-coloured crystals formed after one week.
Au38(SC2H4Ph)24 were prepared according to previous reports44. CHARACTERIZATION Ultraviolet-visible-near-infrared absorption measurements were performed on a Shimadzu UV-3600
spectrophotometer (DCM as solvent). The single crystal diffraction data of Au60S6(SCH2Ph)36 was recorded on a Bruker APEXDUO X-ray Diffractometer (Bruker, Germany). ESI-MS was conducted on a
Waters Q-TOF mass spectrometer equipped with a Z-spray source, and the source temperature was kept at 70 °C. To prepare the samples for ESI-MS analysis, Au60S6(SCH2Ph)36 was dissolved in
toluene (∼0.5 mg ml−1) and then diluted (1/1, v/v) with an ethanol solution containing 0.5 mM CsOAc. The sample was directly infused into the chamber at 5 μl min−1. The spray voltage was
2.20 kV, and the cone voltage was kept at 60 V. The solution photoluminescence spectra of Au60S6(SCH2Ph)36 nanoclusters were recorded on a Fluorolog-3-21, Tempro-01 spectrofluorometer
(HORIBA Jobin Yvon), and the excitation wavelength was kept at 514 nm with a slit of 10 nm (OD514∼0.047, measured by Ultraviolet-visible-near-infrared spectrophotometer). The solid
photoluminescence spectra of Au60S6(SCH2Ph)36 nanoclusters were recorded on a laser confocal scanning Raman/fluorescence scope (HORIBA Jobin Yvon) and laser (514 nm) power is 0.5 mW. The
PTLC plates were eluted with DCM/petroleum ether mixture (1/1, v/v) at room temperature under air atmosphere. DATA AVAILABILITY The X-ray crystallographic coordinates for structures reported
in this article (see Supplementary Table 1 and Supplementary Data 1) have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1526120. The data
can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data are available from the authors on reasonable request.
ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Gan, Z. _et al_. The fourth crystallographic closest packing unveiled in the gold nanocluster crystal. _Nat. Commun._ 8, 14739 doi:
10.1038/ncomms14739 (2017). PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Ehlich, H.,
Schier, A. & Schmidbaur, H. Aurophilicity-based one-dimensional arrays of gold(I) phenylene-1,3-and-1,4-dithiolates. _Inorg. Chem._ 41, 3721–3727 (2002). Article CAS Google Scholar *
Vericat, C., Vela, M. E., Benitez, G., Carro, P. & Salvarezza, R. C. Self-assembled monolayers of thiols and dithiols on gold: new challenges for a well-known system. _Chem. Soc. Rev._
39, 1805–1834 (2010). Article CAS Google Scholar * Häkkinen, H. The gold-sulfur interface at the nanoscale. _Nat. Chem._ 4, 443–445 (2012). Article Google Scholar * Kim, F., Connor, S.,
Song, H., Kuykendall, T. & Yang, P. Platonic gold nanocrystals. _Angew. Chem. Int. Ed. Engl._ 43, 3673–3677 (2004). Article CAS Google Scholar * Xie, J., Lee, J. Y. & Wang, D. I.
C. Seedless, surfactantless, high-yield synthesis of branched gold nanocrystals in HEPES buffer solution. _Chem. Mater._ 19, 2823–2830 (2007). Article CAS Google Scholar * Zeng, J.,
Zhang, Q., Chen, J. & Xia, Y. A comparison study of the catalytic properties of Au-based nanocages, nanoboxes, and nanoparticles. _Nano Lett._ 10, 30–35 (2010). Article CAS ADS Google
Scholar * Chen, H. et al. Understanding the photothermal conversion efficiency of gold nanocrystals. _Small_ 6, 2272–2280 (2010). Article CAS Google Scholar * Angelome, P. C. et al.
Seedless synthesis of single crystalline Au nanoparticles with unusual shapes and tunable LSPR in the near-IR. _Chem. Mater._ 24, 1393–1399 (2012). Article CAS Google Scholar * Chen, S.
et al. Gold nanoelectrodes of varied size: transition to molecule-like charging. _Science_ 280, 2098–2101 (1998). Article CAS ADS Google Scholar * Shibu, E. S., Habeeb Muhammed, M. A.,
Tsukuda, T. & Pradeep, T. Ligand exchange of Au25SG18 leading to functionalized gold clusters: spectroscopy, kinetics, and luminescence. _J. Phys. Chem. C_ 112, 12168–12176 (2008).
Article CAS Google Scholar * Gruene, P. et al. Structures of neutral Au7, Au19, and Au20 clusters in the gas phase. _Science_ 321, 674–676 (2008). Article CAS ADS Google Scholar *
Dolamic, I., Knoppe, S., Dass, A. & Bürgi, T. First enantioseparation and circular dichroism spectra of Au38 clusters protected by achiral ligands. _Nat. Commun._ 3, 798 (2012). Article
ADS Google Scholar * Kurashige, W., Yamazoe, S., Kanehira, K., Tsukuda, T. & Negishi, Y. Selenolate-protected Au38 nanoclusters: isolation and structural characterization. _J. Phys.
Chem. Lett._ 4, 3181–3185 (2013). Article CAS Google Scholar * Zhang, X.-D. et al. Ultrasmall Au10-12(SG)10-12 nanomolecules for high tumor specificity and cancer radiotherapy. _Adv.
Mater._ 26, 4565–4568 (2014). Article CAS Google Scholar * Gan, Z. et al. Fluorescent gold nanoclusters with interlocked staples and a fully thiolate-bound kernel. _Angew. Chem. Int. Ed.
Engl._ 55, 11567–11571 (2016). Article CAS Google Scholar * Heaven, M. W., Dass, A., White, P. S., Holt, K. M. & Murray, R. W. Crystal structure of the gold nanoparticle
[N(C8H17)4][Au25(SCH2CH2Ph)18]. _J. Am. Chem. Soc._ 130, 3754–3755 (2008). Article CAS Google Scholar * Qian, H., Eckenhoff, W. T., Zhu, Y., Pintauer, T. & Jin, R. Total structure
determination of thiolate-protected Au38 nanoparticles. _J. Am. Chem. Soc._ 132, 8280–8281 (2010). Article CAS Google Scholar * Song, Y. et al. Crystal structure of selenolate-protected
Au24(SeR)20 nanocluster. _J. Am. Chem. Soc._ 136, 2963–2965 (2014). Article CAS Google Scholar * Tian, S. et al. Structural isomerism in gold nanoparticles revealed by X-ray
crystallography. _Nat. Commun._ 6, 8667 (2015). Article CAS Google Scholar * Liao, L. et al. Structure of chiral Au44(2,4-DMBT)26 nanocluster with an 18-electron shell closure. _J. Am.
Chem. Soc._ 138, 10425–10428 (2016). Article CAS Google Scholar * Zeng, C., Chen, Y., Kirschbaum, K. J., Lambright, K. J. & Jin, R. Emergence of hierarchical structural complexities
in nanoparticles and their assembly. _Science_ 354, 1580–1584 (2016). Article CAS ADS Google Scholar * Tsunoyama, R., Tsunoyama, H., Pannopard, P., Limtrakul, J. & Tsukuda, T. MALDI
mass analysis of 11 kDa gold clusters protected by octadecanethiolate Ligands. _J. Phys. Chem. C_ 114, 16004–16009 (2010). Article CAS Google Scholar * Zeng, C., Chen, Y., Li, G. &
Jin, R. Magic size Au64(S-_c_-C6H11)32 nanocluster protected by cyclohexanethiolate. _Chem. Mater._ 26, 2635–2641 (2014). Article CAS Google Scholar * Nimmala, P. R., Yoon, B., Whetten,
R. L., Landman, U. & Dass, A. Au67(SR)35 nanomolecules: characteristic size-specific optical, electrochemical, structural properties and first-principles theoretical analysis. _J. Phys.
Chem. A_ 117, 504–517 (2013). Article CAS Google Scholar * Negishi, Y., Sakamoto, C., Ohyama, T. & Tsukuda, T. Synthesis and the origin of the stability of thiolate-protected Au130
and Au187 clusters. _J. Phys. Chem. Lett._ 3, 1624–1628 (2012). Article CAS Google Scholar * Qian, H. & Jin, R. Ambient synthesis of Au144(SR)60 nanoclusters in methanol. _Chem.
Mater._ 23, 2209–2217 (2011). Article CAS Google Scholar * Zeng, C., Liu, C., Chen, Y., Rosi, N. L. & Jin, R. Gold-thiolate ring as a protecting motif in the Au20(SR)16 nanocluster
and implications. _J. Am. Chem. Soc._ 136, 11922–11925 (2014). Article CAS Google Scholar * Crasto, D., Malola, S., Brosofsky, G., Dass, A. & Häkkinen, H. Single crystal XRD structure
and theoretical analysis of the chiral Au30S(S-_t_-Bu)18 cluster. _J. Am. Chem. Soc._ 136, 5000–5005 (2014). Article CAS Google Scholar * Liu, C. et al. Observation of body-centered
cubic gold nanocluster. _Angew. Chem. Int. Ed. Engl._ 54, 9826–9829 (2015). Article CAS Google Scholar * Bino, A., Johnston, D. C. & Stiefel, E. I. Tetranuclear sulfido-bridged
complex of Cr(III) having a strongly magnetic ground state. US Patent 4,832,877 (1989). * Parish, R. V., Salehi, Z. & Pritchard, R. G. Five-coordinate sulfur in a polymeric copper(I)
thiolate complex. _Angew. Chem. Int. Ed. Engl._ 36, 251–253 (1997). Article CAS Google Scholar * Fan, Z. & Zhang, H. Crystal phase-controlled synthesis, properties and applications of
noble metal nanomaterials. _Chem. Soc. Rev._ 45, 63–82 (2016). Article CAS MathSciNet Google Scholar * Chen, M. et al. Synthesis and self-assembly of fcc phase FePt nanorods. _J. Am.
Chem. Soc._ 129, 6348–6349 (2007). Article CAS Google Scholar * Duan, H. et al. Ultrathin rhodium nanosheets. _Nat. Commun._ 5, 3093 (2014). Article Google Scholar * Huang, X. et al.
Synthesis of hexagonal close-packed gold nanostructures. _Nat. Commun._ 2, 292 (2011). Article Google Scholar * Yang, S., Peng, Z. & Yang, H. Platinum lead nanostructures: formation,
phase behavior, and electrocatalytic properties. _Adv. Funct. Mater._ 18, 2745–2753 (2008). Article CAS Google Scholar * Liao, H., Zhu, J. & Hou, Y. Synthesis and electrocatalytic
properties of PtBi nanoplatelets and PdBi nanowires. _Nanoscale_ 6, 1049–1055 (2014). Article CAS ADS Google Scholar * Novgorodova, M. I., Gorshkov, A. I. & Mokhov, A. V. Native
silver and its new structural modifications. _Zap. Vses. Mineral. Obschch._ 108, 552–563 (1979). CAS Google Scholar * Taneja, P., Banerjee, R. & Ayyub, P. Observation of a hexagonal 4H
phase in nanocrystalline silver. _Phys. Rev. B_ 64, 033405 (2001). Article ADS Google Scholar * Liu, X., Luo, J. & Zhu, J. Size effect on the crystal structure of silver nanowires.
_Nano Lett._ 6, 408–412 (2006). Article CAS ADS Google Scholar * Fan, Z. et al. Stabilization of 4H hexagonal phase in gold Nanoribbons. _Nat. Commun._ 6, 7684 (2015). Article CAS
Google Scholar * Higaki, T. et al. Controlling the atomic structure of Au30 nanoclusters by a ligand-based strategy. _Angew. Chem. Int. Ed. Engl._ 55, 6694–6697 (2016). Article CAS Google
Scholar * Liao, L. et al. Transition-sized Au92 nanoparticle bridging non-fcc-structured gold nanoclusters and fcc-structured gold nanocrystals. _Chem. Commun._ 52, 12036–12039 (2016).
Article CAS Google Scholar * Qian, H., Zhu, Y. & Jin, R. Size-focusing synthesis, optical and electrochemical properties of monodisperse Au38(SC2H4Ph)24 nanoclusters. _ACS Nano_ 3,
3795–3803 (2009). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank National Natural Science Foundation of China (Nos 21222301, 51502299, 21528303,
21171170, 21501182), Postdoctoral Science Foundation of China (No 2015M571951), National Basic Research Program of China (No 2013CB934302), the Ministry of Human Resources and Social
Security of China, Anhui Provincial Natural Science Foundation (1608085QB31), the Innovative Program of Development Foundation of Hefei Centre for Physical Science and Technology
(2014FXCX002), Hefei Science Center, CAS (user of potential: 2015HSC-UP003), the CAS/SAFEA International Partnership Program for Creative Research Teams, and the Hundred Talents Program of
the Chinese Academy of Sciences for financial support. AUTHOR INFORMATION Author notes * Zibao Gan and Jishi Chen: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS *
Key Laboratory of Materials Physics, Anhui Key Laboratory of Nanomaterials and Nanotechnology, CAS Center for Excellence in Nanoscience, Institute of Solid State Physics, Chinese Academy of
Sciences, Hefei, 230031, China Zibao Gan, Jishi Chen, Man-Bo Li, Chuanhao Yao, Shengli Zhuang & Zhikun Wu * Key Laboratory of Ion Beam Bioengineering, Institute of Technical Biology and
Agriculture Engineering, Chinese Academy of Sciences, Hefei, 230031, China Juan Wang & An Xu * Hefei National Laboratory for Physical Sciences at the Microscale, University of Science
and Technology of China, Hefei, 230026, China Chengming Wang * Instrumental Analysis Center, Shanghai Jiaotong University, Shanghai, 200240, China Lingling Li Authors * Zibao Gan View author
publications You can also search for this author inPubMed Google Scholar * Jishi Chen View author publications You can also search for this author inPubMed Google Scholar * Juan Wang View
author publications You can also search for this author inPubMed Google Scholar * Chengming Wang View author publications You can also search for this author inPubMed Google Scholar * Man-Bo
Li View author publications You can also search for this author inPubMed Google Scholar * Chuanhao Yao View author publications You can also search for this author inPubMed Google Scholar *
Shengli Zhuang View author publications You can also search for this author inPubMed Google Scholar * An Xu View author publications You can also search for this author inPubMed Google
Scholar * Lingling Li View author publications You can also search for this author inPubMed Google Scholar * Zhikun Wu View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS Z.G. conceived and carried out the experiment and UV/Vis/NIR characterization of Au60S6(SCH2Ph)36 with the assistance of C.Y. C.W. assisted the photoluminescence
data collection. J.C. analysed the single crystal structure of Au60S6(SCH2Ph)36. J.W. and A.X. assisted the crystallization of Au60S6(SCH2Ph)36. M.-B.L. and S.Z. assisted the ESI-MS
analysis. L.L. collected the single crystal data. Z.W. designed the study, supervised the project and analysed the data. All authors contributed to the preparation of the manuscript.
CORRESPONDING AUTHOR Correspondence to Zhikun Wu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION Supplementary Figures and Supplementary Table. (PDF 197 kb) SUPPLEMENTARY DATA 1 Crystal data of Au60S6(SCH2Ph)36 nanoclusters (CIF 19 kb) RIGHTS AND PERMISSIONS This work is
licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license,
unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce
the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gan, Z., Chen, J., Wang, J. _et
al._ The fourth crystallographic closest packing unveiled in the gold nanocluster crystal. _Nat Commun_ 8, 14739 (2017). https://doi.org/10.1038/ncomms14739 Download citation * Received: 09
December 2016 * Accepted: 27 January 2017 * Published: 24 March 2017 * DOI: https://doi.org/10.1038/ncomms14739 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative