Play all audios:
ABSTRACT Rett syndrome (RTT) is unique among genetic, chromosomal and other developmental disorders because of its extreme female gender bias, early normal development, and subsequent
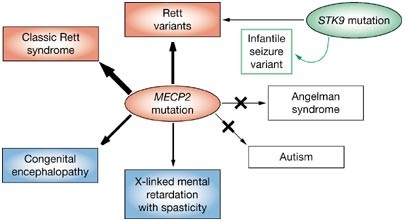
developmental regression with loss of motor and language skills. RTT is caused by heterozygosity for mutations in the X-linked gene _MECP2_, which encodes methyl-CpG binding protein 2. MeCP2
is a multifunctional protein that can act as an architectural chromatin-binding protein, a function that is unrelated to its ability to bind methyl-CpG and to attract chromatin modification
complexes. Inactivating mutations that cause RTT in females are not prenatally lethal in males, but lead to profound congenital encephalopathy. Molecular diagnoses of RTT, through
demonstration of a _MECP2_ mutation, made at an early stage of the disorder, usually confirm the sporadic nature and very low recurrence risk of the condition. A positive DNA test result,
however, also predicts the inevitable clinical course, given the lack of effective intervention. Initial hypotheses indicating that the MeCP2 protein acts as a genome-wide transcriptional
repressor were not confirmed by global gene expression studies in various tissues of individuals with RTT and mouse models of MeCP2 deficiency. Rather, recent evidence points to
low-magnitude effects of a small number of genes—including the brain-derived neurotrophic factor pathway and glucocorticoid response genes—that might affect formation and maturation of
synapses or synaptic function in postmitotic neurons. KEY POINTS * Rett syndrome (RTT) in females is caused by heterozygous _de novo_ mutations in the _MECP2_ (methyl-CpG binding protein 2)
gene * Null mutations in _MECP2_ that cause classic RTT in females are not prenatally lethal in boys, but cause a distinct form of congenital encephalopathy * Functional genetic studies in
mouse models of MeCP2 deficiency identified postmitotic neurons as the site for pathogenetic mechanisms, and highlighted the need for tight control of MeCP2 levels in the brain * MeCP2 can
act as an architectural chromatin binding protein, a function that is unrelated to its ability to bind methyl-CpG and attract chromatin modification complexes * Although differential CpG
methylation patterns are essential for epigenetic regulation of gene expression, there is no confirmed evidence that loss of _MeCP2_ function leads to epigenetic dysregulation _in vivo_ in
any of the systems studied * Brain-derived neurotrophic factor (BDNF), a direct MeCP2 target, is able to modulate onset and disease progression in _Mecp2_ mutant mice, indicating potential
therapeutic opportunities Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read
our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EXPLORING THE COMPLEXITY OF MECP2 FUNCTION IN RETT SYNDROME Article 13 May 2025 RETT SYNDROME Article 07 November
2024 MYT1L IN THE MAKING: EMERGING INSIGHTS ON FUNCTIONS OF A NEURODEVELOPMENTAL DISORDER GENE Article Open access 22 July 2022 REFERENCES * Rett A (1966) Uber ein eigenartiges
hirnatrophisches Syndrom bei Hyperammonamie im Kindesalter. _Wien Med Wochenschr_ 116: 723–738 CAS PubMed Google Scholar * Einspieler C _ et al_. (2005) Abnormal general movements in
girls with Rett disorder: the first four months of life. _Brain Dev_ 27 (Suppl 1): S8–S13 Article Google Scholar * Schanen NC _ et al_. (1997) A new Rett syndrome family consistent with
X-linked inheritance expands the X chromosome exclusion map. _Am J Hum Genet_ 61: 634–641 Article CAS Google Scholar * Schanen NC and Francke U (1998) A severely affected male born into a
Rett syndrome kindred supports X-linked inheritance and allows extension of the exclusion map. _Am J Hum Genet_ 63: 267–269 Article CAS Google Scholar * Sirianni N _ et al_. (1998) Rett
syndrome: confirmation of X-linked dominant inheritance, and localization of the gene to Xq28. _Am J Hum Genet_ 63: 1552–1558 Article CAS Google Scholar * Amir RE _ et al_. (1999) Rett
syndrome is caused by mutations in X-linked _MECP2_, encoding methyl-CpG-binding protein 2. _Nat Genet_ 23: 185–188 Article CAS Google Scholar * Wan M _ et al_. (1999) Rett syndrome and
beyond: recurrent spontaneous and familial _MECP2_ mutations at CpG hotpots. _Am J Hum Genet_ 65: 1520–1529 Article CAS Google Scholar * Ravn K _ et al_. (2005) Large genomic
rearrangements in _MECP2_. _Hum Mutat_ 25: 324 Article Google Scholar * Mnatzakanian GN _ et al_. (2004) A previously unidentified _MECP2_ open reading frame defines a new protein isoform
relevant to Rett syndrome. _Nat Genet_ 36: 339–341 Article CAS Google Scholar * Kriaucionis S and Bird A (2004) The major form of MeCP2 has a novel N-terminus generated by alternative
splicing. _Nucleic Acids Res_ 32: 1818–1823 Article CAS Google Scholar * Amir RE _ et al_. (2005) Mutations in exon 1 of _MECP2_ are a rare cause of Rett syndrome. _J Med Genet_ 42: e15
Article CAS Google Scholar * Hoffbuhr K _ et al_. (2001) _MeCP2_ mutations in children with and without the phenotype of Rett syndrome. _Neurology_ 56: 1486–1495 Article CAS Google
Scholar * Weaving LS _ et al_. (2003) Effects of _MECP2_ mutation type, location and X-inactivation in modulating Rett syndrome phenotype. _Am J Med Genet A_ 118: 103–114 Article Google
Scholar * Huppke P _ et al_. (2002) Influence of mutation type and location on phenotype in 123 patients with Rett Syndrome. _Neuropediatrics_ 33: 63–68 Article CAS Google Scholar *
Colvin L _ et al_. (2004) Refining the phenotype of common mutations in Rett syndrome. _J Med Genet_ 41: 25–30 Article CAS Google Scholar * Smeets E _ et al_. (2005) Rett syndrome in
females with CTS hot spot deletions: a disorder profile. _Am J Med Genet A_ 132: 117–120 Article Google Scholar * Shibayama A _ et al_. (2004) MECP2 structural and 3'-UTR variants in
schizophrenia, autism and other psychiatric diseases: a possible association with autism. _Am J Med Genet B Neuropsychiatr Genet_ 128: 50–53 Article Google Scholar * Imessaoudene B _ et
al_. (2001) _MECP2_ mutation in non-fatal, non-progressive encephalopathy in a male. _J Med Genet_ 38: 171–174 Article CAS Google Scholar * Hitchins MP _ et al_. (2004) Investigation of
_UBE3A_ and _MECP2_ in Angelman syndrome (AS) and patients with features of AS. _Am J Med Genet A_ 125: 167–172 Article Google Scholar * Kleefstra T _ et al_. (2004) MECP2 analysis in
mentally retarded patients: implications for routine DNA diagnostics. _Eur J Hum Genet_ 12: 24–28 Article CAS Google Scholar * Maiwald R _ et al_. (2002) _De novo MECP2_ mutation in a
46,XX male patient with Rett syndrome. _Neurogenetics_ 4: 107–108 Article Google Scholar * Clayton-Smith J _ et al_. (2000) Somatic mutation in _MECP2_ as a non-fatal neurodevelopmental
disorder in males. _Lancet_ 356: 830–832 Article CAS Google Scholar * Topcu M _ et al_. (2002) Somatic mosaicism for a _MECP2_ mutation associated with classic Rett syndrome in a boy.
_Eur J Hum Genet_ 10: 77–81 Article CAS Google Scholar * Villard L _ et al_. (2000) Two affected boys in a Rett syndrome family: clinical and molecular findings. _Neurology_ 55: 1188–1193
Article CAS Google Scholar * Zeev BB _ et al_. (2002) Rett syndrome: clinical manifestations in males with _MECP2_ mutations. _J Child Neurol_ 17: 20–24 Article Google Scholar * Lynch
SA _ et al_. (2003) Sporadic case of fatal encephalopathy with neonatal onset associated with a T158M missense mutation in MECP2. _Arch Dis Child Fetal Neonatal Ed_ 88: F250–252 Article CAS
Google Scholar * Leuzzi V _ et al_. (2004) Early-onset encephalopathy and cortical myoclonus in a boy with _MECP2_ gene mutation. _Neurology_ 63: 1968–1970 Article CAS Google Scholar *
Meloni I _ et al_. (2000) A mutation in the Rett syndrome gene, _MECP2_, causes X-linked mental retardation and progressive spasticity in males. _Am J Hum Genet_ 67: 982–985 Article CAS
Google Scholar * Yntema HG _ et al_. (2002) Low frequency of _MECP2_ mutations in mentally retarded males. _Eur J Hum Genet_ 10: 487–490 Article CAS Google Scholar * Ylisaukko-Oja T _ et
al_. (2005) _MECP2_ mutation analysis in patients with mental retardation. _Am J Med Genet A_ 132: 121–124 Article Google Scholar * Van Esch H _ et al_. (2005) Duplication of the _MECP2_
region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. _Am J Hum Genet_ 77: 442–453 Article CAS Google Scholar * Evans JC _ et al_. (2005)
Early onset seizures and Rett-like features associated with mutations in _CDKL5_. _Eur J Hum Genet_ 13: 1113–1120 Article CAS Google Scholar * Tate P _ et al_. (1996) The methyl-CpG
binding protein MeCP2 is essential for embryonic development in the mouse. _Nature Genet_ 12: 205–208 Article CAS Google Scholar * Chen RZ _ et al_. (2001) Deficiency of methyl-CpG
binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. _Nat Genet_ 27: 327–331 Article CAS Google Scholar * Guy J _ et al_. (2001) A mouse _Mecp2_-null mutation causes
neurological symptoms that mimic Rett syndrome. _Nat Genet_ 27: 322–326 Article CAS Google Scholar * Shahbazian M _ et al_. (2002) Mice with truncated MeCP2 recapitulate many Rett
syndrome features and display hyperacetylation of histone H3. _Neuron_ 35: 243–254 Article CAS Google Scholar * Luikenhuis S _ et al_. (2004) Expression of MeCP2 in postmitotic neurons
rescues Rett syndrome in mice. _Proc Natl Acad Sci USA_ 101: 6033–6038 Article CAS Google Scholar * Collins AL _ et al_. (2004) Mild overexpression of MeCP2 causes a progressive
neurological disorder in mice. _Hum Mol Genet_ 13: 2679–2689 Article CAS Google Scholar * Traynor J _ et al_. (2002) Gene expression patterns vary in clonal cell cultures from Rett
syndrome females with eight different _MECP2_ mutations. _BMC Med Genet_ 3: 12 Article Google Scholar * Ronnett GV _ et al_. (2003) Olfactory biopsies demonstrate a defect in neuronal
development in Rett's syndrome. _Ann Neurol_ 54: 206–218 Article CAS Google Scholar * Shahbazian MD _ et al_. (2002) Insight into Rett syndrome: MeCP2 levels display tissue- and
cell-specific differences and correlate with neuronal maturation. _Hum Mol Genet_ 11: 115–124 Article CAS Google Scholar * Jung BP _ et al_. (2003) The expression of methyl CpG binding
factor MeCP2 correlates with cellular differentiation in the developing rat brain and in cultured cells. _J Neurobiol_ 55: 86–96 Article CAS Google Scholar * Nagai K _ et al_. (2005) A
transcriptional repressor MeCP2 causing Rett syndrome is expressed in embryonic non-neuronal cells and controls their growth. _Brain Res Dev Brain Res_ 157: 103–106 Article CAS Google
Scholar * Kishi N and Macklis JD (2004) MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. _Mol Cell
Neurosci_ 27: 306–321 Article CAS Google Scholar * Matarazzo V _ et al_. (2004) The transcriptional repressor Mecp2 regulates terminal neuronal differentiation. _Mol Cell Neurosci_ 27:
44–58 Article CAS Google Scholar * Lewis JD _ et al_. (1992) Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. _Cell_ 69:
905–914 Article CAS Google Scholar * Nan X _ et al_. (1997) MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. _Cell_ 88: 471–481 Article CAS Google
Scholar * Kokura K _ et al_. (2001) The Ski protein family is required for MeCP2-mediated transcriptional repression. _J Biol Chem_ 276: 34115–34121 Article CAS Google Scholar *
Harikrishnan KN _ et al_. (2005) Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing. _Nat Genet_ 37: 254–264 Article CAS Google Scholar *
Klose RJ and Bird AP (2004) MeCP2 behaves as an elongated monomer that does not stably associate with the Sin3a chromatin remodeling complex. _J Biol Chem_ 279: 46490–46496 Article CAS
Google Scholar * Fuks F _ et al_. (2003) The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. _Nucleic Acids Res_ 31: 2305–2312 Article CAS Google
Scholar * Kimura H and Shiota K (2003) Methyl-CpG-binding protein, MeCP2, is a target molecule for maintenance DNA methyltransferase, Dnmt1. _J Biol Chem_ 278: 4806–4812 Article CAS
Google Scholar * Jeffery L and Nakielny S (2004) Components of the DNA methylation system of chromatin control are RNA-binding proteins. _J Biol Chem_ 279: 49479–49487 Article CAS Google
Scholar * Buschdorf JP and Stratling WH (2004) A WW domain binding region in methyl-CpG-binding protein MeCP2: impact on Rett syndrome. _J Mol Med_ 82: 135–143 Article CAS Google Scholar
* Young JI _ et al_. (2005) Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. _Proc Natl Acad Sci USA_ 102: 17551–17558
Article CAS Google Scholar * von Kries JP _ et al_. (1991) A matrix/scaffold attachment region binding protein: identification, purification, and mode of binding. _Cell_ 64: 123–135
Article CAS Google Scholar * Chandler SP _ et al_. (1999) The methyl-CpG binding transcriptional repressor MeCP2 stably associates with nucleosomal DNA. _Biochemistry_ 38: 7008–7018
Article CAS Google Scholar * Georgel PT _ et al_. (2003) Chromatin compaction by human MeCP2: assembly of novel secondary chromatin structures in the absence of DNA methylation. _J Biol
Chem_ 278: 32181–32188 Article CAS Google Scholar * Horike S _ et al_. (2005) Loss of silent-chromatin looping and impaired imprinting of _DLX5_ in Rett syndrome. _Nat Genet_ 37: 31–40
Article CAS Google Scholar * Razin A and Shemer R (1999) Epigenetic control of gene expression. _Results Probl Cell Differ_ 25: 189–204 Article CAS Google Scholar * Balmer D _ et al_.
(2002) _MECP2_ mutations in Rett syndrome adversely affect lymphocyte growth, but do not affect imprinted gene expression in blood or brain. _Hum Genet_ 110: 545–552 Article CAS Google
Scholar * Kimura MI _ et al_. (2004) _Dlx5_, the mouse homologue of the human-imprinted _DLX5_ gene, is biallelically expressed in the mouse brain. _J Hum Genet_ 49: 273–277 Article CAS
Google Scholar * Samaco RC _ et al_. (2005) Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. _Hum Mol
Genet_ 14: 483–492 Article CAS Google Scholar * Makedonski K _ et al_. (2005) MeCP2 deficiency in Rett syndrome causes epigenetic aberrations at the PWS/AS imprinting center that affects
UBE3A expression. _Hum Mol Genet_ 14: 1049–1058 Article CAS Google Scholar * Gartler SM _ et al_. (2004) Normal histone modifications on the inactive X chromosome in ICF and Rett syndrome
cells: implications for methyl-CpG binding proteins. _BMC Biol_ 2: 21 Article Google Scholar * Colantuoni C _ et al_. (2001) Gene expression profiling in postmortem Rett syndrome brain:
differential gene expression and patient classification. _Neurobiol Dis_ 8: 847–865 Article CAS Google Scholar * Tudor M _ et al_. (2002) Transcriptional profiling of a mouse model for
Rett syndrome reveals subtle transcriptional changes in the brain. _Proc Natl Acad Sci USA_ 99: 15536–15541 Article CAS Google Scholar * Ballestar E _ et al_. (2005) The impact of _MECP2_
mutations in the expression patterns of Rett syndrome patients. _Hum Genet_ 116: 91–104 Article CAS Google Scholar * Stancheva I _ et al_. (2003) A mutant form of MeCP2 protein
associated with human Rett syndrome cannot be displaced from methylated DNA by notch in _Xenopus_ embryos. _Mol Cell_ 12: 425–435 Article CAS Google Scholar * Chen WG _ et al_. (2003)
Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. _Science_ 302: 885–889 Article CAS Google Scholar * Martinowich K _ et al_. (2003) DNA
methylation-related chromatin remodeling in activity-dependent _BDNF_ gene regulation. _Science_ 302: 890–893 Article CAS Google Scholar * Chang Q _ et al_. (2006) The disease progression
of Mecp2 mutant mice is affected by the level of BDNF expression. _Neuron_ 49: 341–348 Article CAS Google Scholar * Gorski JA _ et al_. (2003) Brain-derived neurotrophic factor is
required for the maintenance of cortical dendrites. _J Neurosci_ 23: 6856–6865 Article CAS Google Scholar * Salama-Cohen P _ et al_. (2005) NGF controls dendrite development in
hippocampal neurons by binding to p75NTR and modulating the cellular targets of _Notch_. _Mol Biol Cell_ 16: 339–347 Article CAS Google Scholar * Armstrong DD (1995) The neuropathology of
Rett syndrome—overview 1994. _Neuropediatrics_ 26: 100–104 Article CAS Google Scholar * Belichenko PV _ et al_. (2005) Dendritic alterations in the brains of mouse models of Rett
syndrome. In _Proceedings of the RSRF 6th Annual Rett Syndrome Symposium_, 2005 June 27–29; Itasca, IL Google Scholar * Nuber UA _ et al_. (2005) Up-regulation of glucocorticoid-regulated
genes in a mouse model of Rett syndrome. _Hum Mol Genet_ 14: 2247–2256 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The author thanks Hong-Hua Li, ChaRandle Jordan,
Birgitt Schüle and Pavel Belichenko for helpful discussions, Rick Cuevas for assistance with the manuscript, and the March of Dimes Birth Defects Foundation and the International Rett
Syndrome Association for financial support. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * a professor of genetics and pediatrics at Stanford University School of Medicine, Stanford, CA, USA
Uta Francke Authors * Uta Francke View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Uta Francke. ETHICS DECLARATIONS
COMPETING INTERESTS The author declares no competing financial interests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Francke, U. Mechanisms of
Disease: neurogenetics of MeCP2 deficiency. _Nat Rev Neurol_ 2, 212–221 (2006). https://doi.org/10.1038/ncpneuro0148 Download citation * Received: 05 August 2005 * Accepted: 24 January 2006
* Issue Date: April 2006 * DOI: https://doi.org/10.1038/ncpneuro0148 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative