Play all audios:
ABSTRACT In recent years, microRNAs (miRNAs) have emerged as a major class of regulatory genes, present in most metazoans and important for a diverse range of biological functions. Because
experimental identification of miRNA targets is difficult, there has been an explosion of computational target predictions. Although the initial round of predictions resulted in very diverse
results, subsequent computational and experimental analyses suggested that at least a certain class of conserved miRNA targets can be confidently predicted and that this class of targets is
large, covering, for example, at least 30% of all human genes when considering about 60 conserved vertebrate miRNA gene families. Most recent approaches have also shown that there are
correlations between domains of miRNA expression and mRNA levels of their targets. Our understanding of miRNA function is still extremely limited, but it may be that by integrating mRNA and
miRNA sequence and expression data with other comparative genomic data, we will be able to gain global and yet specific insights into the function and evolution of a broad layer of
post-transcriptional control. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read
our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS GGA-MIRNOME, A MICRORNA-SEQUENCING DATASET FROM CHICK EMBRYONIC TISSUES Article Open access 31 January 2022
MICRORNAS IN ACTION: BIOGENESIS, FUNCTION AND REGULATION Article 28 June 2023 THE BIOGENESIS AND REGULATION OF ANIMAL MICRORNAS Article 19 December 2024 REFERENCES * Brennecke, J., Hipfner,
D.R., Stark, A., Russell, R.B. & Cohen, S.M. Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in _Drosophila_.
_Cell_ 113, 25–36 (2003). Article CAS Google Scholar * Stark, A., Brennecke, J., Russell, R.B. & Cohen, S.M. Identification of _Drosophila_ microRNA targets. _PLoS Biol._ 1, E60
(2003). Article Google Scholar * Enright, A.J. et al. MicroRNA targets in _Drosophila_. _Genome Biol._ 5, R1 (2003). Article Google Scholar * Rajewsky, N. & Socci, N.D. Computational
identification of microRNA targets. _Dev. Biol._ 267, 529–535 (2004). Article CAS Google Scholar * John, B. et al. Human MicroRNA targets. _PLoS Biol._ 2, e363 (2004). Article Google
Scholar * Kiriakidou, M. et al. A combined computational-experimental approach predicts human microRNA targets. _Genes Dev._ 18, 1165–1178 (2004). Article CAS Google Scholar * Lewis,
B.P., Shih, I.H., Jones-Rhoades, M.W., Bartel, D.P. & Burge, C.B. Prediction of mammalian microRNA targets. _Cell_ 115, 787–798 (2003). Article CAS Google Scholar * Lai, E.C. Micro
RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. _Nat. Genet._ 30, 363–364 (2002). Article CAS Google Scholar * Bartel, D.P.
MicroRNAs: genomics, biogenesis, mechanism, and function. _Cell_ 116, 281–297 (2004). Article CAS Google Scholar * Bentwich, I. Prediction and validation of microRNAs and their targets.
_FEBS Lett._ 579, 5904–5910 (2005). Article CAS Google Scholar * Sood, P., Krek, A., Zavolan, M., Macino, G. & Rajewsky, N. Cell-type-specific signatures of microRNAs on target mRNA
expression. _Proc. Natl. Acad. Sci. USA_, published online 13 February 2006 (doi:10.1073/pnas.0511045103). * Wu, L., Fan, J. & Belasco, J.G. MicroRNAs direct rapid deadenylation of mRNA.
_Proc. Natl. Acad. Sci. USA_ 103, 4034–4039 (2006). Article CAS Google Scholar * Giraldez, A.J. et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. _Science_
312, 75–79 (2006). Article CAS Google Scholar * Robins, H. & Press, W.H. Human microRNAs target a functionally distinct population of genes with AT-rich 3′ UTRs. _Proc. Natl. Acad.
Sci. USA_ 102, 15557–15562 (2005). Article CAS Google Scholar * Brennecke, J., Stark, A., Russell, R.B. & Cohen, S.M. Principles of microRNA-target recognition. _PLoS Biol._ 3, e85
(2005). Article Google Scholar * Grün, D., Wang, Y.L., Langenberger, D., Gunsalus, K.C. & Rajewsky, N. microRNA target predictions across seven _Drosophila_ species and comparison to
mammalian targets. _PLoS Comput. Biol._ 1, e13 (2005). Article Google Scholar * Krek, A. et al. Combinatorial microRNA target predictions. _Nat. Genet._ 37, 495–500 (2005). Article CAS
Google Scholar * Lewis, B.P., Burge, C.B. & Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. _Cell_ 120,
15–20 (2005). Article CAS Google Scholar * Stark, A., Brennecke, J., Bushati, N., Russell, R.B. & Cohen, S.M. Animal MicroRNAs confer robustness to gene expression and have a
significant impact on 3′UTR evolution. _Cell_ 123, 1133–1146 (2005). Article CAS Google Scholar * Xie, X. et al. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs
by comparison of several mammals. _Nature_ 434, 338–345 (2005). Article CAS Google Scholar * Lall, S. et al. A genome-wide map of conserved microRNA targets in _C. elegans_. _Curr. Biol._
16, 460–471 (2006). Article CAS Google Scholar * Robins, H., Li, Y. & Padgett, R.W. Incorporating structure to predict microRNA targets. _Proc. Natl. Acad. Sci. USA_ 102, 4006–4009
(2005). Article CAS Google Scholar * Zhao, Y., Samal, E. & Srivastava, D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. _Nature_
436, 214–220 (2005). Article CAS Google Scholar * Farh, K.K. et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. _Science_ 310, 1817–1821 (2005). Article
CAS Google Scholar * Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. _Nucleic Acids Res._ 31, 3406–3415 (2003). Article CAS Google Scholar *
Rehmsmeier, M., Steffen, P., Hochsmann, M. & Giegerich, R. Fast and effective prediction of microRNA/target duplexes. _RNA_ 10, 1507–1517 (2004). Article CAS Google Scholar * Chan,
C.S., Elemento, O. & Tavazoie, S. Revealing posttranscriptional regulatory elements through network-level conservation. _PLoS Comput. Biol._ 1, e69 (2005). Article Google Scholar *
Watanabe, Y. et al. Computational analysis of microRNA targets in _Caenorhabditis elegans_. _Gene_ 365, 2–10 (2006). Article CAS Google Scholar * Sethupathy, P., Corda, B. &
Hatzigeorgiou, A.G. TarBase: A comprehensive database of experimentally supported animal microRNA targets. _RNA_ 12, 192–197 (2006). Article CAS Google Scholar * Shahi, P. et al.
Argonaute–a database for gene regulation by mammalian microRNAs. _Nucleic Acids Res._ 34, D115–D118 (2006). Article CAS Google Scholar * Hsu, P.W. et al. miRNAMAP: genomic maps of
microRNA genes and their target genes in mammalian genomes. _Nucleic Acids Res._ 34, D135–D139 (2006). Article CAS Google Scholar * Montgomery, S.B. et al. ORegAnno: an open access
database and curation system for literature-derived promoters, transcription factor binding sites and regulatory variation. _Bioinformatics_ 22, 637–640 (2006). Article CAS Google Scholar
* Doench, J.G. & Sharp, P.A. Specificity of microRNA target selection in translational repression. _Genes Dev._ 18, 504–511 (2004). Article CAS Google Scholar * Lindblad-Toh, K. et
al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. _Nature_ 438, 803–819 (2005). Article CAS Google Scholar * Lim, L.P. et al. Microarray analysis
shows that some microRNAs downregulate large numbers of target mRNAs. _Nature_ 433, 769–773 (2005). Article CAS Google Scholar * Bagga, S. et al. Regulation by let-7 and lin-4 microRNAs
results in target mRNA degradation. _Cell_ 122, 553–563 (2005). Article CAS Google Scholar * Krutzfeldt, J. et al. Silencing of microRNAs _in vivo_ with 'antagomirs'. _Nature_
438, 685–689 (2005). Article Google Scholar * Bartel, D.P. & Chen, C.Z. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. _Nat. Rev. Genet._
5, 396–400 (2004). Article CAS Google Scholar * Bussemaker, H.J., Li, H. & Siggia, E.D. Regulatory element detection using correlation with expression. _Nat. Genet._ 27, 167–171
(2001). Article CAS Google Scholar * Foat, B.C., Houshmandi, S.S., Olivas, W.M. & Bussemaker, H.J. Profiling condition-specific, genome-wide regulation of mRNA stability in yeast.
_Proc. Natl. Acad. Sci. USA_ 102, 17675–17680 (2005). Article CAS Google Scholar * Aravin, A.A. et al. The small RNA profile during _Drosophila melanogaster_ development. _Dev. Cell_ 5,
337–350 (2003). Article CAS Google Scholar * Chen, P.Y. et al. The developmental miRNA profiles of zebrafish as determined by small RNA cloning. _Genes Dev._ 19, 1288–1293 (2005). Article
CAS Google Scholar * Baskerville, S. & Bartel, D.P. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. _RNA_ 11, 241–247 (2005).
Article CAS Google Scholar * Barad, O. et al. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. _Genome Res._
14, 2486–2494 (2004). Article CAS Google Scholar * Bartel, D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. _Cell_ 116, 281–297 (2004). Article CAS Google Scholar *
Hammond, S.M. microRNA detection comes of age. _Nat. Methods_ 3, 12–3 (2006). Article CAS Google Scholar * Burgler, C. & Macdonald, P.M. Prediction and verification of microRNA
targets by MovingTargets, a highly adaptable prediction method. _BMC Genomics_ 6, 88 (2005). Article Google Scholar * Poy, M.N. et al. A pancreatic islet-specific microRNA regulates
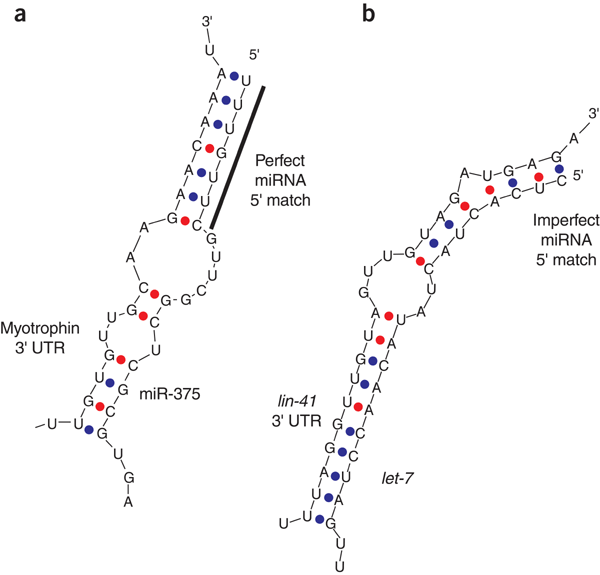
insulin secretion. _Nature_ 432, 226–230 (2004). Article CAS Google Scholar * Vella, M.C. et al. The _C. elegans_ microRNA let-7 binds to imperfect let-7 complementary sites from the
lin-41 3′ UTR. _Genes Dev._ 18, 132–137 (2004). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS Writing a perspective in a new and fast-moving field is necessarily
selective on which papers are being cited. However, I would like to apologize to all authors whose papers were not included. I also want to thank A. Stark, U. Ohler and S. Lall for a
critical reading of the manuscript and helpful suggestions, and my collaborators and members of the miRNA/RNAi community for many interesting and stimulating discussions. J. Thierry-Mieg and
D. Thierry-Mieg as well as W. Majoros and U. Ohler shared unpublished data with me. Finally, I thank A. Stark and S. Cohen for permission to use Figure 2. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Center for Comparative Functional Genomics Department of Biology, 100 Washington Square East, New York, 10003, New York, USA Nikolaus Rajewsky Authors * Nikolaus Rajewsky View
author publications You can also search for this author inPubMed Google Scholar ETHICS DECLARATIONS COMPETING INTERESTS The author declares no competing financial interests. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Rajewsky, N. microRNA target predictions in animals. _Nat Genet_ 38 (Suppl 6), S8–S13 (2006).
https://doi.org/10.1038/ng1798 Download citation * Published: 30 May 2006 * Issue Date: June 2006 * DOI: https://doi.org/10.1038/ng1798 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative