Play all audios:
ABSTRACT Studies have suggested involvement of interleukin 17 (IL-17) in autoimmune diseases, although its effect on B cell biology has not been clearly established. Here we demonstrate that
IL-17 alone or in combination with B cell–activating factor controlled the survival and proliferation of human B cells and their differentiation into immunoglobulin-secreting cells. This
effect was mediated mainly through the nuclear factor-κB-regulated transcription factor Twist-1. In support of the relevance of our observations and the potential involvement of IL-17 in B
cell biology, we found that the serum of patients with systemic lupus erythematosus had higher concentrations of IL-17 than did the serum of healthy people and that IL-17 abundance
correlated with the disease severity of systemic lupus erythematosus. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article *
Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn
about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS LOW-DOSE IL-2 ENHANCES THE GENERATION OF IL-10-PRODUCING IMMUNOREGULATORY
B CELLS Article Open access 12 April 2023 THE SURVIVAL AND FUNCTION OF IL-10-PRODUCING REGULATORY B CELLS ARE NEGATIVELY CONTROLLED BY SLAMF5 Article Open access 25 March 2021 TOLL-LIKE
RECEPTOR SIGNALLING IN B CELLS DURING SYSTEMIC LUPUS ERYTHEMATOSUS Article 18 December 2020 CHANGE HISTORY * _ 12 JULY 2013 Despite many attempts to replicate these results, the authors have
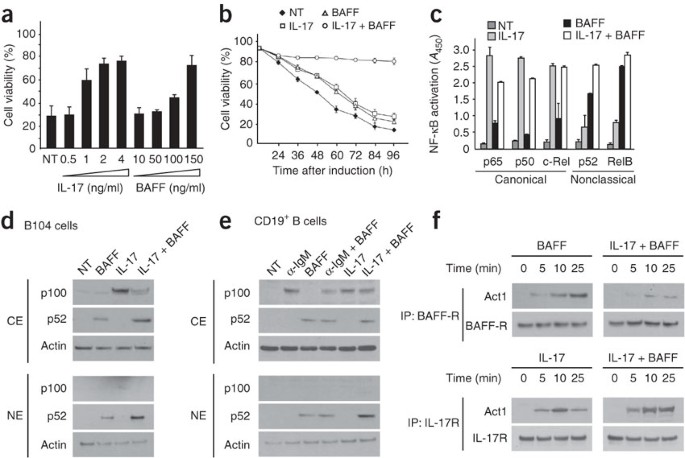
been unable to confirm the original data showing that IL-17 alone or in combination with B cell–activating factor controls the survival of human B cells (FIG. 1A,B). Because this weakens
the conclusions of the paper, all the authors (except A.D. and P.T., who could not be contacted) now retract this paper. _ REFERENCES * Yurasov, S., Hammersen, J., Tiller, T., Tsuiji, M.
& Wardemann, H. B-cell tolerance checkpoints in healthy humans and patients with systemic lupus erythematosus. _Ann. NY Acad. Sci._ 1062, 165–174 (2005). Article CAS Google Scholar *
Meffre, E. & Wardemann, H. B-cell tolerance checkpoints in health and autoimmunity. _Curr. Opin. Immunol._ 20, 632–638 (2008). Article CAS Google Scholar * Groom, J. & Mackay, F.
B cells flying solo. _Immunol. Cell Biol._ 86, 40–46 (2008). Article CAS Google Scholar * Nagata, S. & Golstein, P. The Fas death factor. _Science_ 267, 1449–1456 (1995). Article CAS
Google Scholar * Nagata, S. & Suda, T. Fas and Fas ligand: lpr and gld mutations. _Immunol. Today_ 16, 39–43 (1995). Article CAS Google Scholar * Hutcheson, J. et al. Combined
deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. _Immunity_ 28, 206–217 (2008). Article CAS Google Scholar * Rieux-Laucat, F., Le
Deist, F. & Fischer, A. Autoimmune lymphoproliferative syndromes: genetic defects of apoptosis pathways. _Cell Death Differ._ 10, 124–133 (2003). Article CAS Google Scholar * Andre,
J. et al. Overexpression of the antiapoptotic gene Bfl-1 in B cells from patients with familial systemic lupus erythematosus. _Lupus_ 16, 95–100 (2007). Article CAS Google Scholar *
Batten, M. et al. BAFF mediates survival of peripheral immature B lymphocytes. _J. Exp. Med._ 192, 1453–1466 (2000). Article CAS Google Scholar * Hsu, B.L., Harless, S.M., Lindsley, R.C.,
Hilbert, D.M. & Cancro, M.P. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. _J. Immunol._ 168, 5993–5996 (2002). Article CAS Google
Scholar * Cheema, G.S., Roschke, V., Hilbert, D.M. & Stohl, W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. _Arthritis
Rheum._ 44, 1313–1319 (2001). Article CAS Google Scholar * Zhang, J. et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. _J. Immunol._ 166, 6–10
(2001). Article CAS Google Scholar * Stohl, W. et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. _Arthritis Rheum._
48, 3475–3486 (2003). Article Google Scholar * Pene, J. et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. _J. Immunol._ 180, 7423–7430
(2008). Article CAS Google Scholar * Pan, H.F., Ye, D.Q. & Li, X.P. Type 17 T-helper cells might be a promising therapeutic target for systemic lupus erythematosus. _Nat. Clin. Pract.
Rheumatol._ 4, 352–353 (2008). Article CAS Google Scholar * Wong, C.K. et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for
Th17-mediated inflammation in auto-immunity. _Clin. Immunol._ 127, 385–393 (2008). Article CAS Google Scholar * Garrett-Sinha, L.A., John, S. & Gaffen, S.L. IL-17 and the Th17 lineage
in systemic lupus erythematosus. _Curr. Opin. Rheumatol._ 20, 519–525 (2008). Article CAS Google Scholar * Wong, C.K., Ho, C.Y., Li, E.K. & Lam, C.W. Elevation of proinflammatory
cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. _Lupus_ 9, 589–593 (2000). Article CAS Google Scholar * Crispin, J.C.
et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. _J. Immunol._ 181, 8761–8766 (2008). Article CAS Google
Scholar * Kang, H.K., Liu, M. & Datta, S.K. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T
cells and contraction of inflammatory Th17 cells. _J. Immunol._ 178, 7849–7858 (2007). Article CAS Google Scholar * Hsu, H.C. et al. Interleukin 17-producing T helper cells and
interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. _Nat. Immunol._ 9, 166–175 (2008). Article CAS Google Scholar * Jacob, N. et al. Accelerated
pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. _J.
Immunol._ 182, 2532–2541 (2009). Article CAS Google Scholar * Lai Kwan Lam, Q., King Hung Ko, O., Zheng, B.J. & Lu, L. Local BAFF gene silencing suppresses Th17-cell generation and
ameliorates autoimmune arthritis. _Proc. Natl. Acad. Sci. USA_ 105, 14993–14998 (2008). Article Google Scholar * Mackay, F. & Browning, J.L. BAFF: a fundamental survival factor for B
cells. _Nat. Rev. Immunol._ 2, 465–475 (2002). Article CAS Google Scholar * Li, X. Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling. _Cytokine_ 41,
105–113 (2008). Article CAS Google Scholar * Stadanlick, J.E. et al. Tonic B cell antigen receptor signals supply an NF-κB substrate for prosurvival BLyS signaling. _Nat. Immunol._ 9,
1379–1387 (2008). Article CAS Google Scholar * Chen, C., Edelstein, L.C. & Gelinas, C. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL . _Mol.
Cell. Biol._ 20, 2687–2695 (2000). Article Google Scholar * Zong, W.X., Edelstein, L.C., Chen, C., Bash, J. & Gelinas, C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct
transcriptional target of NF-κB that blocks TNFα-induced apoptosis. _Genes Dev._ 13, 382–387 (1999). Article CAS Google Scholar * Pham, C.G. et al. Upregulation of Twist-1 by NF-κB blocks
cytotoxicity induced by chemotherapeutic drugs. _Mol. Cell. Biol._ 27, 3920–3935 (2007). Article CAS Google Scholar * Maestro, R. et al. Twist is a potential oncogene that inhibits
apoptosis. _Genes Dev._ 13, 2207–2217 (1999). Article CAS Google Scholar * Ishigami, T. et al. Anti-IgM antibody-induced cell death in a human B lymphoma cell line, B104, represents a
novel programmed cell death. _J. Immunol._ 148, 360–368 (1992). CAS PubMed Google Scholar * Ruprecht, C.R. & Lanzavecchia, A. Toll-like receptor stimulation as a third signal required
for activation of human naive B cells. _Eur. J. Immunol._ 36, 810–816 (2006). Article CAS Google Scholar * John, S.A., Clements, J.L., Russell, L.M. & Garrett-Sinha, L.A. Ets-1
regulates plasma cell differentiation by interfering with the activity of the transcription factor Blimp-1. _J. Biol. Chem._ 283, 951–962 (2008). Article CAS Google Scholar * Ansieau, S.
et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. _Cancer Cell_ 14, 79–89 (2008). Article CAS Google Scholar *
Grumont, R.J., Rourke, I.J. & Gerondakis, S. Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. _Genes Dev._ 13,
400–411 (1999). Article CAS Google Scholar * Lee, H.H., Dadgostar, H., Cheng, Q., Shu, J. & Cheng, G. NF-κB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival
signaling in B lymphocytes. _Proc. Natl. Acad. Sci. USA_ 96, 9136–9141 (1999). Article CAS Google Scholar * Sims, G.P. et al. Identification and characterization of circulating human
transitional B cells. _Blood_ 105, 4390–4398 (2005). Article CAS Google Scholar * Ettinger, R., Kuchen, S. & Lipsky, P.E. Interleukin 21 as a target of intervention in autoimmune
disease. _Ann. Rheum. Dis._ 67 Suppl 3, iii83–iii86 (2008). Article CAS Google Scholar * Litinskiy, M.B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and
APRIL. _Nat. Immunol._ 3, 822–829 (2002). Article CAS Google Scholar * Vanden Bush, T.J. & Bishop, G.A. TLR7 and CD40 cooperate in IL-6 production via enhanced JNK and AP-1
activation. _Eur. J. Immunol._ 38, 400–409 (2008). Article CAS Google Scholar * Wilker, P.R. et al. Transcription factor Mef2c is required for B cell proliferation and survival after
antigen receptor stimulation. _Nat. Immunol._ 9, 603–612 (2008). Article CAS Google Scholar * Khiem, D., Cyster, J.G., Schwarz, J.J. & Black, B.L. A p38 MAPK-MEF2C pathway regulates
B-cell proliferation. _Proc. Natl. Acad. Sci. USA_ 105, 17067–17072 (2008). Article CAS Google Scholar * Sosic, D., Richardson, J.A., Yu, K., Ornitz, D.M. & Olson, E.N. Twist
regulates cytokine gene expression through a negative feedback loop that represses NF-κB activity. _Cell_ 112, 169–180 (2003). Article CAS Google Scholar * Niesner, U. et al.
Autoregulation of Th1-mediated inflammation by twist1. _J. Exp. Med._ 205, 1889–1901 (2008). Article CAS Google Scholar * Bubier, J.A. et al. A critical role for IL-21 receptor signaling
in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. _Proc. Natl. Acad. Sci. USA_ 106, 1518–1523 (2009). Article CAS Google Scholar * Sasaki, Y. et al. Canonical NF-κB
activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. _Immunity_ 24, 729–739 (2006). Article CAS Google Scholar *
Brien, G., Trescol-Biemont, M.C. & Bonnefoy-Berard, N. Downregulation of Bfl-1 protein expression sensitizes malignant B cells to apoptosis. _Oncogene_ 26, 5828–5832 (2007). Article
CAS Google Scholar * de Brouwer, A.P., van Bokhoven, H. & Kremer, H. Comparison of 12 reference genes for normalization of gene expression levels in Epstein-Barr virus-transformed
lymphoblastoid cell lines and fibroblasts. _Mol. Diagn. Ther._ 10, 197–204 (2006). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank H. Mitchison and G. Hinkal for
critical reading of the manuscript; C. Delprat and G. Salles for scientific discussions; the laboratory of A. Puisieux (INSERM U590) for anti-Twist-1 as well as the Twist-1- and
Twist-2-related vectors; and C. Bella for cell sorting. Supported by INSERM, UCB Lyon 1, the Arthritis Fondation Courtin (A.D.), the Association pour la Recherche sur le Cancer and the Ligue
Contre le Cancer (07 and 26). AUTHOR INFORMATION Author notes * Alexandre Belot and Jérémy Bastid: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Université de
Lyon, Lyon, France Agnès Doreau, Alexandre Belot, Jérémy Bastid, Benjamin Riche, Marie-Claude Trescol-Biemont, Bruno Ranchin, Nicole Fabien, Pierre Cochat, Claire Pouteil-Noble, Pierre
Trolliet, Isabelle Durieu, Jacques Tebib, Berhouz Kassai, Stéphane Ansieau, Alain Puisieux & Nathalie Bonnefoy-Bérard * Institut National de la Santé et de la Recherche Médicale (INSERM)
U851, Lyon, France Agnès Doreau, Marie-Claude Trescol-Biemont, Nicole Fabien & Nathalie Bonnefoy-Bérard * Université Lyon 1, Institut Fédératif de Recherche 128, Lyon, France Agnès
Doreau, Marie-Claude Trescol-Biemont & Nathalie Bonnefoy-Bérard * Centre de Référence des Maladies Rénales Rares, Service de Néphrologie et Rhumatologie Pédiatriques, Hôpital Femme Mère
Enfant, Bron, France Alexandre Belot, Bruno Ranchin & Pierre Cochat * Centre National de la Recherche Scientifique, UMR5239, Oullins, France Alexandre Belot * INSERM U590, Lyon, France
Jérémy Bastid, Stéphane Ansieau & Alain Puisieux * Centre Léon Bérard, Fédération Nationale des Centres de Lutte Contre le Cancer, Lyon, France Jérémy Bastid, Stéphane Ansieau &
Alain Puisieux * Service de Biostatistique, Lyon, France Benjamin Riche * Service d'Immunologie, Hospices Civils de Lyon, Centre Hospitalier Lyon-Sud, Lyon, France Nicole Fabien *
INSERM U820, Lyon, France Pierre Cochat * Service de Néphrologie, Lyon, France Claire Pouteil-Noble & Pierre Trolliet * Service de Médecine Interne, Lyon, France Isabelle Durieu *
Service de Rhumatologie, Hospices Civils de Lyon, Centre Hospitalier Lyon-Sud, Lyon, France Jacques Tebib * Service de Pharmacologie Clinique, Hôpital L.-Pradel, INSERM CIC201, Epidémiologie
Pharmacologie Investigation Clinique Information Médicale Mère-Enfant, Centre National de la Recherche Scientifique Unité Mixte de Recherche 5558, Centre Hospitalier Universitaire de Lyon,
Lyon, France Berhouz Kassai * Service d'Immunologie, Centre Hospitalier Universitaire de Montpellier, Faculté de Médecine; Université Montpellier 1, Jean-François Eliaou * Université
Montpellier 1, Montpellier, France Jean-François Eliaou Authors * Agnès Doreau View author publications You can also search for this author inPubMed Google Scholar * Alexandre Belot View
author publications You can also search for this author inPubMed Google Scholar * Jérémy Bastid View author publications You can also search for this author inPubMed Google Scholar *
Benjamin Riche View author publications You can also search for this author inPubMed Google Scholar * Marie-Claude Trescol-Biemont View author publications You can also search for this
author inPubMed Google Scholar * Bruno Ranchin View author publications You can also search for this author inPubMed Google Scholar * Nicole Fabien View author publications You can also
search for this author inPubMed Google Scholar * Pierre Cochat View author publications You can also search for this author inPubMed Google Scholar * Claire Pouteil-Noble View author
publications You can also search for this author inPubMed Google Scholar * Pierre Trolliet View author publications You can also search for this author inPubMed Google Scholar * Isabelle
Durieu View author publications You can also search for this author inPubMed Google Scholar * Jacques Tebib View author publications You can also search for this author inPubMed Google
Scholar * Berhouz Kassai View author publications You can also search for this author inPubMed Google Scholar * Stéphane Ansieau View author publications You can also search for this author
inPubMed Google Scholar * Alain Puisieux View author publications You can also search for this author inPubMed Google Scholar * Jean-François Eliaou View author publications You can also
search for this author inPubMed Google Scholar * Nathalie Bonnefoy-Bérard View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.B.-B. designed
and supervised the study and wrote the manuscript; A.D. did all experiments presented in Figures 1,2,3,4,5,6 and Supplementary Figures 1,2,3,4,5,6; A.B. did statistical analysis and
collected clinical data in Supplementary Tables 1 and 2; J.B. helped design the Twist-related experiments and wrote the manuscript; M.-C.T.-B. analyzed _BCL2A1_ expression in B cells from
patients with SLE and healthy volunteers; B. Riche did statistical analysis; B. Ranchin, N.F., P.C., C.P.-N., P.T., I.D, J.T. and B.K. recruited patients; and S.A., A.P. and J.-F.E.
contributed to the design and interpretation of experiments and to the editing of the manuscript. CORRESPONDING AUTHOR Correspondence to Nathalie Bonnefoy-Bérard. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–6, Tables 1–2 and Supplementary Methods (PDF 2734 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Doreau, A., Belot, A., Bastid, J. _et al._ Interleukin 17 acts in synergy with B cell–activating factor to influence B cell biology and the pathophysiology of systemic lupus
erythematosus. _Nat Immunol_ 10, 778–785 (2009). https://doi.org/10.1038/ni.1741 Download citation * Received: 05 March 2009 * Accepted: 16 April 2009 * Published: 31 May 2009 * Issue Date:
July 2009 * DOI: https://doi.org/10.1038/ni.1741 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative