Play all audios:
ABSTRACT Linking neural microcircuit function to emergent properties of the mammalian brain requires fine-scale manipulation and measurement of neural activity during behavior, where each
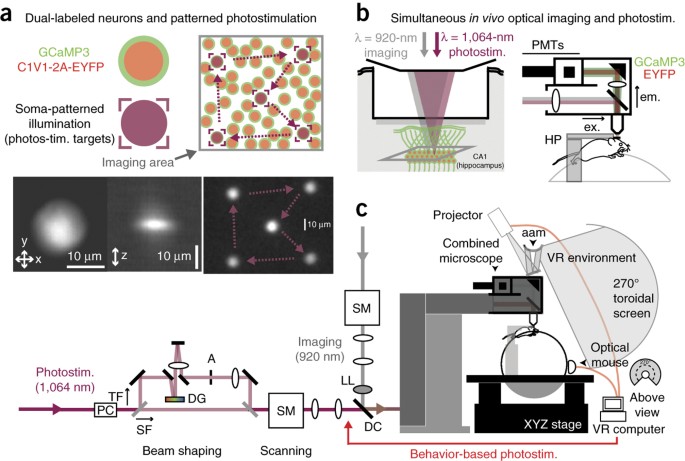
neuron's coding and dynamics can be characterized. We developed an optical method for simultaneous cellular-resolution stimulation and large-scale recording of neuronal activity in
behaving mice. Dual-wavelength two-photon excitation allowed largely independent functional imaging with a green fluorescent calcium sensor (GCaMP3, λ = 920 ± 6 nm) and single-neuron
photostimulation with a red-shifted optogenetic probe (C1V1, λ = 1,064 ± 6 nm) in neurons coexpressing the two proteins. We manipulated task-modulated activity in individual hippocampal CA1
place cells during spatial navigation in a virtual reality environment, mimicking natural place-field activity, or 'biasing', to reveal subthreshold dynamics. Notably, manipulating
single place-cell activity also affected activity in small groups of other place cells that were active around the same time in the task, suggesting a functional role for local place cell
interactions in shaping firing fields. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through
your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant
access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions *
Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ALL-OPTICAL INTERROGATION OF NEURAL CIRCUITS IN BEHAVING MICE Article 27 April 2022 HIGH-SPEED LOW-LIGHT IN
VIVO TWO-PHOTON VOLTAGE IMAGING OF LARGE NEURONAL POPULATIONS Article 27 March 2023 ULTRAFAST LIGHT TARGETING FOR HIGH-THROUGHPUT PRECISE CONTROL OF NEURONAL NETWORKS Article Open access 05
April 2023 REFERENCES * Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. _Science_ 248, 73–76 (1990). CAS PubMed Google Scholar * Greenberg,
D.S., Houweling, A.R. & Kerr, J.N. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. _Nat. Neurosci._ 11, 749–751 (2008). CAS PubMed Google Scholar *
Dombeck, D.A., Harvey, C.D., Tian, L., Looger, L.L. & Tank, D.W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. _Nat. Neurosci._ 13,
1433–1440 (2010). CAS PubMed PubMed Central Google Scholar * Petreanu, L. et al. Activity in motor-sensory projections reveals distributed coding in somatosensation. _Nature_ 489,
299–303 (2012). Article CAS PubMed PubMed Central Google Scholar * Adamantidis, A.R., Zhang, F., Aravanis, A.M., Deisseroth, K. & de Lecea, L. Neural substrates of awakening probed
with optogenetic control of hypocretin neurons. _Nature_ 450, 420–424 (2007). CAS PubMed PubMed Central Google Scholar * Tye, K.M. et al. Amygdala circuitry mediating reversible and
bidirectional control of anxiety. _Nature_ 471, 358–362 (2011). CAS PubMed PubMed Central Google Scholar * Carter, M.E. et al. Tuning arousal with optogenetic modulation of locus
coeruleus neurons. _Nat. Neurosci._ 13, 1526–1533 (2010). CAS PubMed PubMed Central Google Scholar * Akerboom, J. et al. Genetically encoded calcium indicators for multi-color neural
activity imaging and combination with optogenetics. _Front. Mol. Neurosci._ 6, 2 (2013). CAS PubMed PubMed Central Google Scholar * Chang, Y.F., Arai, Y. & Nagai, T. Optogenetic
activation during detector “dead time” enables compatible real-time fluorescence imaging. _Neurosci. Res._ 73, 341–347 (2012). PubMed Google Scholar * Husson, S.J. et al. Optogenetic
analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors. _Curr. Biol._ 22, 743–752 (2012). CAS PubMed PubMed Central Google Scholar * Lin,
J.Y., Lin, M.Z., Steinbach, P. & Tsien, R.Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. _Biophys. J._ 96, 1803–1814 (2009). CAS
PubMed PubMed Central Google Scholar * Wilson, N.R., Runyan, C.A., Wang, F.L. & Sur, M. Division and subtraction by distinct cortical inhibitory networks _in vivo_. _Nature_ 488,
343–348 (2012). CAS PubMed PubMed Central Google Scholar * Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. _Nature_ 446, 633–639 (2007). CAS PubMed Google
Scholar * Guo, Z.V., Hart, A.C. & Ramanathan, S. Optical interrogation of neural circuits in _Caenorhabditis elegans_. _Nat. Methods_ 6, 891–896 (2009). CAS PubMed PubMed Central
Google Scholar * Rickgauer, J.P. & Tank, D.W. Two-photon excitation of channelrhodopsin-2 at saturation. _Proc. Natl. Acad. Sci. USA_ 106, 15025–15030 (2009). CAS PubMed PubMed
Central Google Scholar * Andrasfalvy, B.K., Zemelman, B.V., Tang, J. & Vaziri, A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. _Proc. Natl. Acad.
Sci. USA_ 107, 11981–11986 (2010). CAS PubMed PubMed Central Google Scholar * Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. _Nat. Methods_ 7, 848–854
(2010). CAS PubMed PubMed Central Google Scholar * Prakash, R. et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. _Nat. Methods_ 9, 1171–1179
(2012). CAS PubMed PubMed Central Google Scholar * Packer, A.M. et al. Two-photon optogenetics of dendritic spines and neural circuits. _Nat. Methods_ 9, 1202–1205 (2012). CAS PubMed
PubMed Central Google Scholar * Yaroslavsky, A.N. et al. Optical properties of selected native and coagulated human brain tissues _in vitro_ in the visible and near infrared spectral
range. _Phys. Med. Biol._ 47, 2059–2073 (2002). CAS PubMed Google Scholar * Oheim, M., Beaurepaire, E., Chaigneau, E., Mertz, J. & Charpak, S. Two-photon microscopy in brain tissue:
parameters influencing the imaging depth. _J. Neurosci. Methods_ 111, 29–37 (2001). CAS PubMed Google Scholar * Tian, L. et al. Imaging neural activity in worms, flies and mice with
improved GCaMP calcium indicators. _Nat. Methods_ 6, 875–881 (2009). CAS PubMed PubMed Central Google Scholar * Yizhar, O. et al. Neocortical excitation/inhibition balance in information
processing and social dysfunction. _Nature_ 477, 171–178 (2011). CAS PubMed PubMed Central Google Scholar * Oron, D., Tal, E. & Silberberg, Y. Scanningless depth-resolved
microscopy. _Opt. Express_ 13, 1468–1476 (2005). PubMed Google Scholar * Zhu, G., van Howe, J., Durst, M., Zipfel, W. & Xu, C. Simultaneous spatial and temporal focusing of femtosecond
pulses. _Opt. Express_ 13, 2153–2159 (2005). PubMed Google Scholar * Zimmermann, T., Rietdorf, J. & Pepperkok, R. Spectral imaging and its applications in live cell microscopy. _FEBS
Lett._ 546, 87–92 (2003). CAS PubMed Google Scholar * Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. _Nat.
Methods_ 9, 159–172 (2012). CAS Google Scholar * Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2–assisted circuit mapping of long-range callosal projections.
_Nat. Neurosci._ 10, 663–668 (2007). CAS PubMed Google Scholar * Aronov, D. & Tank, D.W. Engagement of the neural circuits underlying 2D spatial navigation in a rodent virtual reality
system. _Neuron_ 84, 442–456 (2014). CAS PubMed PubMed Central Google Scholar * Harvey, C.D., Collman, F., Dombeck, D.A. & Tank, D.W. Intracellular dynamics of hippocampal place
cells during virtual navigation. _Nature_ 461, 941–946 (2009). CAS PubMed PubMed Central Google Scholar * Lee, D., Lin, B.J. & Lee, A.K. Hippocampal place fields emerge upon
single-cell manipulation of excitability during behavior. _Science_ 337, 849–853 (2012). CAS PubMed Google Scholar * Fenton, A.A. & Muller, R.U. Place cell discharge is extremely
variable during individual passes of the rat through the firing field. _Proc. Natl. Acad. Sci. USA_ 95, 3182–3187 (1998). CAS PubMed PubMed Central Google Scholar * Ziv, Y. et al.
Long-term dynamics of CA1 hippocampal place codes. _Nat. Neurosci._ 16, 264–266 (2013). CAS PubMed PubMed Central Google Scholar * Kaifosh, P., Lovett-Barron, M., Turi, G.F., Reardon,
T.R. & Losonczy, A. Septo-hippocampal GABAergic signaling across multiple modalities in awake mice. _Nat. Neurosci._ 16, 1182–1184 (2013). CAS PubMed Google Scholar * Lovett-Barron,
M. et al. Dendritic inhibition in the hippocampus supports fear learning. _Science_ 343, 857–863 (2014). CAS PubMed PubMed Central Google Scholar * Chen, T.W. et al. Ultrasensitive
fluorescent proteins for imaging neuronal activity. _Nature_ 499, 295–300 (2013). Article CAS PubMed PubMed Central Google Scholar * St-Pierre, F. et al. High-fidelity optical reporting
of neuronal electrical activity with an ultrafast fluorescent voltage sensor. _Nat. Neurosci._ 17, 884–889 (2014). CAS PubMed PubMed Central Google Scholar * Grienberger, C., Chen, X.
& Konnerth, A. NMDA receptor-dependent multidendrite Ca2+ spikes required for hippocampal burst firing in vivo. _Neuron_ 81, 1274–1281 (2014). CAS PubMed Google Scholar *
Papagiakoumou, E. et al. Functional patterned multiphoton excitation deep inside scattering tissue. _Nat. Photonics_ 7, 274–278 (2013). CAS Google Scholar * Losonczy, A., Zemelman, B.V.,
Vaziri, A. & Magee, J.C. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. _Nat. Neurosci._ 13, 967–972 (2010). CAS PubMed PubMed Central
Google Scholar * Dana, H. et al. _Thy1_-GCaMP6 transgenic mice for neuronal population imaging _in vivo_. _PLoS ONE_ 9, e108697 (2014). PubMed PubMed Central Google Scholar * Amaral,
D.G. & Witter, M.P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. _Neuroscience_ 31, 571–591 (1989). CAS PubMed Google Scholar *
Deuchars, J. & Thomson, A.M. CA1 pyramid-pyramid connections in rat hippocampus in vitro: dual intracellular recordings with biocytin filling. _Neuroscience_ 74, 1009–1018 (1996). CAS
PubMed Google Scholar * Marshall, L. et al. Hippocampal pyramidal cell-interneuron spike transmission is frequency dependent and responsible for place modulation of interneuron discharge.
_J. Neurosci._ 22, RC197 (2002). PubMed PubMed Central Google Scholar * Galarreta, M. & Hestrin, S. Spike transmission and synchrony detection in networks of GABAergic interneurons.
_Science_ 292, 2295–2299 (2001). CAS PubMed Google Scholar * Jonas, P., Bischofberger, J., Fricker, D. & Miles, R. Interneuron diversity series: fast in, fast out–temporal and spatial
signal processing in hippocampal interneurons. _Trends Neurosci._ 27, 30–40 (2004). CAS PubMed Google Scholar * Freund, T.F. & Buzsaki, G. Interneurons of the hippocampus.
_Hippocampus_ 6, 347–470 (1996). CAS PubMed Google Scholar * Ko, H. et al. Functional specificity of local synaptic connections in neocortical networks. _Nature_ 473, 87–91 (2011). CAS
PubMed PubMed Central Google Scholar * Royer, S. et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. _Nat. Neurosci._ 15, 769–775
(2012). CAS PubMed PubMed Central Google Scholar * Briggman, K.L., Helmstaedter, M. & Denk, W. Wiring specificity in the direction-selectivity circuit of the retina. _Nature_ 471,
183–188 (2011). CAS PubMed Google Scholar * Vaziri, A. & Emiliani, V. Reshaping the optical dimension in optogenetics. _Curr. Opin. Neurobiol._ 22, 128–137 (2012). CAS PubMed Google
Scholar * Dana, H. & Shoham, S. Numerical evaluation of temporal focusing characteristics in transparent and scattering media. _Opt. Express_ 19, 4937–4948 (2011). PubMed Google
Scholar * Grewe, B.F., Voigt, F.F., van't Hoff, M. & Helmchen, F. Fast two-layer two-photon imaging of neuronal cell populations using an electrically tunable lens. _Biomed. Opt.
Express_ 2, 2035–2046 (2011). CAS PubMed PubMed Central Google Scholar * Pologruto, T.A., Sabatini, B.L. & Svoboda, K. ScanImage: flexible software for operating laser scanning
microscopes. _Biomed. Eng. Online_ 2, 13 (2003). PubMed PubMed Central Google Scholar * Domnisoru, C., Kinkhabwala, A.A. & Tank, D.W. Membrane potential dynamics of grid cells.
_Nature_ 495, 199–204 (2013). CAS PubMed PubMed Central Google Scholar * Harvey, C.D., Coen, P. & Tank, D.W. Choice-specific sequences in parietal cortex during a virtual-navigation
decision task. _Nature_ 484, 62–68 (2012). CAS PubMed PubMed Central Google Scholar * Zimmermann, T., Rietdorf, J., Girod, A., Georget, V. & Pepperkok, R. Spectral imaging and linear
un-mixing enables improved FRET efficiency with a novel GFP2-YFP FRET pair. _FEBS Lett._ 531, 245–249 (2002). CAS PubMed Google Scholar * Miri, A., Daie, K., Burdine, R.D., Aksay, E.
& Tank, D.W. Regression-based identification of behavior-encoding neurons during large-scale optical imaging of neural activity at cellular resolution. _J. Neurophysiol._ 105, 964–980
(2011). PubMed Google Scholar * Mukamel, E.A., Nimmerjahn, A. & Schnitzer, M.J. Automated analysis of cellular signals from large-scale calcium imaging data. _Neuron_ 63, 747–760
(2009). CAS PubMed PubMed Central Google Scholar * Dombeck, D.A., Graziano, M.S. & Tank, D.W. Functional clustering of neurons in motor cortex determined by cellular resolution
imaging in awake behaving mice. _J. Neurosci._ 29, 13751–13760 (2009). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank D. Kim and C. Guo
(Genetically Encoded Neuronal Indicator and Effector Project, Janelia Research Campus) for transgenic mice, D. Aronov for VR software, B. Scott for discussions, and C. Domnisoru, A. Miri, F.
Collman and S. Wang for comments on the manuscript. This work was supported by the US National Institutes of Health (R01-MH083686; P50-GM071508) and a National Science Foundation Graduate
Research Fellowship to J.P.R. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Princeton Neuroscience Institute, Princeton University, Princeton, New Jersey, USA John Peter Rickgauer &
David W Tank * Bezos Center for Neural Circuit Dynamics, Princeton University, Princeton, New Jersey, USA John Peter Rickgauer & David W Tank * Lewis-Sigler Institute for Integrative
Genomics, Princeton University, Princeton, New Jersey, USA John Peter Rickgauer & David W Tank * Department of Molecular Biology, Princeton University, Princeton, New Jersey, USA John
Peter Rickgauer & David W Tank * Department of Bioengineering, Stanford University, Stanford, California, USA Karl Deisseroth * CNC Program, Stanford University, Stanford, California,
USA Karl Deisseroth * Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California, USA Karl Deisseroth * Howard Hughes Medical Institute, Stanford University,
Stanford, California, USA Karl Deisseroth Authors * John Peter Rickgauer View author publications You can also search for this author inPubMed Google Scholar * Karl Deisseroth View author
publications You can also search for this author inPubMed Google Scholar * David W Tank View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
J.P.R. and D.W.T. designed the study. K.D. contributed reagents. J.P.R. and D.W.T. performed the experiments. J.P.R. analyzed data with strategy and methods contributions from D.W.T. J.P.R.
and D.W.T. wrote the paper with comments from K.D. CORRESPONDING AUTHOR Correspondence to David W Tank. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial
interests. INTEGRATED SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 SPECTRAL PROPERTIES OF MOLECULES, LASER SOURCES, AND OPTICS USED IN THIS APPROACH. A. Visible-wavelength regime. Shown
are: fluorescence emission spectra for EGFP and EYFP (obtained from the Tsien Lab website, University of California, San Diego); the single-photon excitation spectrum for C1V1(E122T/E162T)
(adapted from Yizhar _et al_., _Nature_ 477, 171-178 [2011]); transmission curves for the dichroic (dark line) and emission filters (shaded areas) used in two-channel fluorescence detection
(filter part numbers indicated); and the transmission curve for the long-pass laser-blocking filter (blue line; curves from Semrock). The 473 nm laser line used in single-photon excitation
experiments is also indicated (dashed blue line). Each curve is normalized to its own peak value. B. Infrared-wavelength regime. Two-photon action cross section for GCaMP3 (green) and
relative C1V1 photocurrent response amplitudes (see inset) sampled at two infrared TPE center wavelengths (λ=900 nm and λ=1050 nm). Inset: sample intracellular photocurrents from illuminated
HEK293T cells expressing C1V1; peak squared-intensity values were similar (2.76x1054 γ2/cm4-s2 and 1.68x1054 γ2/cm4-s2 at 900 nm and 1050 nm; assuming a fixed output temporal pulse-width).
The GCaMP3 action cross-section was measured using fluorescence excited by focused low-power illumination (regime of quadratic power dependence) of a purified GCaMP3.3 sample (37 μM
concentration in 20 mM MOPS, 100 mM KCl, 2.7 mM K2CaEGTA, at pH 7.4; R. Sun and S. S.-H. Wang, Princeton), normalized at each wavelength using side-by-side measurements of a reference
fluorophore (20 µM fluorescein in water, pH 11; see Albota, M. A., Xu, C. & Webb, W., _Appl. Opt_. 37, 7352–7356 [1998]). C1V1 wavelength-sensitivity was evaluated at two spectral bands
(λ=900 nm and 1050 nm), using whole-cell electrode recordings at constant voltage (-50 mV) in HEK293T cells transiently expressing the pLenti-CaMKIIa-C1V1(E162T)-TS-EYFP construct with
focused scanning methods and an apparatus described previously (Rickgauer and Tank, _PNAS_ 106, 15025-15030 [2009]). SUPPLEMENTARY FIGURE 2 SCHEMATIC FOR EITHER SINGLE-PHOTON OR TWO-PHOTON
EXCITATION (TPE) PHOTOSTIMULATION AND TPE IMAGING. A. Position of optics used to introduce the SPE source into the TPE microscope head. Abbreviations: AM, alignment mirror; FT, focusing
telescope; SP, short-pass filter; DC, dichroic filter; LP, long-pass filter; PMTs, photomultiplier tubes. B. TPE images (acquired at 920 nm) of a volume in a fluorescent plastic slide after
bleaching neighboring areas using SPE (473 nm) and TPE (1064 nm, spatial focusing path; SF in Fig. 1, main text). Image intensity is inverted. Images are shown at the 1064 nm focal plane
(upper) and as an _xz_ projection of a through-focus series (lower). SUPPLEMENTARY FIGURE 3 TPE STIMULATION EVOKES GCAMP3 TRANSIENTS CONSISTENT WITH ACTION POTENTIALS (APS) AND
OPSIN-MEDIATED DEPOLARIZATION IN AWAKE MICE. A. GCaMP3 _ΔF/F_ values vs. stimulation pulse number. Somatic _ΔF/F_ values for 6 neurons stimulated at 5, 10, and 20 Hz (16 ms per pulse),
measured 500 ms after pulse-train onset (values for each cell are normalized to the peak response; average of 3-7 trials per data point). The monotonic relationship between _ΔF/F_ and
stimulation pulse number is consistent with a regime in which _ΔF/F_ values also scale approximately linearly with AP number (assuming 1 AP per pulse; Tian _et al_., _Nat. Methods_ 6,
875-881 [2009]). _Inset_: sample traces from one neuron stimulated with 10 pulses at 5, 10, and 20 Hz (each trace is a 5-trial average). Colored underlines indicate the corresponding stim.
train period. Dashed line indicates the time at which values _ΔF/F_ values were measured (500 ms after stim. onset). B. Histogram of measured GCaMP3 fluorescence transient half-decay times
following offset of a photostimulation epoch (τ1/2 calculated from single-exponential decay fits). Following stim. offset, transients evoked in cells returned to resting levels with
off-kinetics (τ1/2 = 375+/-196 ms; mean +/- s.d.) in the range observed _in vivo_ during trains of electrically stimulated APs (τ1/2 = 384+/-76 ms for 10 APs; Tian _et al_., _Nat. Methods_
6, 875-881 [2009]). C. Peak GCaMP3 transient amplitude during raster-scanning photostimulation of a cell using the spatial focusing path (SF in Fig. 1, main text) shown for different TPE
raster-scan periods, which varied by changing the number of lines in a raster-scan, and which were repeated over an interval of 512 ms. The dashed line indicates the approximate C1V1(t/t)
inactivation time-constant (τoff = 40-50 ms; Mattis _et al_., _Nat. Methods_ 9, 159-172 [2012]; Prakash _et al_., _Nat. Methods_ 9, 1171-1179 [2012]). Faster-scanning photostimulation trials
(Ts< τoff) produced larger-amplitude responses than slower-scanning trials (Ts> τoff; n=31 target cells; values for each cell normalized by maximum amplitude in that cell). This
relationship is a signature of membrane depolarization mediated by scanning recruitment of opsin probes (Rickgauer and Tank, _PNAS_ 106, 15025-15030 [2009]; Prakash _et al_., _Nat. Methods_
9, 1171-1179 [2012]; Packer _et al_., _Nat. Methods_ 9, 1202-1205 [2012]). _Inset_: Exemplary _ΔF/F_ traces from one cell illustrating this relationship (colors indicate scan periods of
same-color dots in panel; bars indicate s.d.). SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–3 (PDF 277 kb) SUPPLEMENTARY METHODS CHECKLIST (PDF 350 kb)
RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Rickgauer, J., Deisseroth, K. & Tank, D. Simultaneous cellular-resolution optical perturbation and
imaging of place cell firing fields. _Nat Neurosci_ 17, 1816–1824 (2014). https://doi.org/10.1038/nn.3866 Download citation * Received: 16 July 2014 * Accepted: 15 October 2014 * Published:
17 November 2014 * Issue Date: December 2014 * DOI: https://doi.org/10.1038/nn.3866 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative