Play all audios:
ABSTRACT The importance of dopamine (DA) neurotransmission is emphasized by its direct implication in several neurological and psychiatric disorders. The DA transporter (DAT), target of
psychostimulant drugs, is the key protein that regulates spatial and temporal activity of DA in the synaptic cleft via the rapid reuptake of DA into the presynaptic terminal. There is strong
evidence suggesting that DAT-interacting proteins may have a role in its function and regulation. Performing a two-hybrid screening, we identified snapin, a SNARE-associated protein
implicated in synaptic transmission, as a new binding partner of the carboxyl terminal of DAT. Our data show that snapin is a direct partner and regulator of DAT. First, we determined the
domains required for this interaction in both proteins and characterized the DAT-snapin interface by generating a 3D model. Using different approaches, we demonstrated that (i) snapin is
expressed _in vivo_ in dopaminergic neurons along with DAT; (ii) both proteins colocalize in cultured cells and brain and, (iii) DAT and snapin are present in the same protein complex.
Moreover, by functional studies we showed that snapin produces a significant decrease in DAT uptake activity. Finally, snapin downregulation in mice produces an increase in DAT levels and
transport activity, hence increasing DA concentration and locomotor response to amphetamine. In conclusion, snapin/DAT interaction represents a direct link between exocytotic and reuptake
mechanisms and is a potential target for DA transmission modulation. SIMILAR CONTENT BEING VIEWED BY OTHERS DOPAMINERGIC RIC GTPASE ACTIVITY IMPACTS AMPHETAMINE SENSITIVITY AND SLEEP QUALITY
IN A DOPAMINE TRANSPORTER-DEPENDENT MANNER IN _DROSOPHILA MELANOGASTER_ Article 01 September 2021 MTOR REGULATES COCAINE-INDUCED BEHAVIOURAL SENSITIZATION THROUGH THE SYNDIG1–GLUA2
INTERACTION IN THE NUCLEUS ACCUMBENS Article 14 September 2021 ANTIPSYCHOTIC DRUG EFFICACY CORRELATES WITH THE MODULATION OF D1 RATHER THAN D2 RECEPTOR-EXPRESSING STRIATAL PROJECTION NEURONS
Article 13 July 2023 INTRODUCTION Dopaminergic neurons, located primarily in the midbrain, project into various cortical and subcortical regions regulating sensory-motor and cognitive
functions. The importance of dopamine (DA) neurotransmission is emphasized by its direct implication in neurological and psychiatric disorders, such as Parkinson’s disease, dystonia,
schizophrenia, attention deficit/hyperactivity disorder, Tourette syndrome, and drug addiction (German et al, 2015; Grace, 2016; Martins et al, 2017). These disorders share DA transmission
dysfunction as a common pathological mechanism. The key to understand and reverse the pathophysiology of these disorders is to identify molecular and cellular mechanisms that shape the
kinetics of DA concentration at synapses (Fon and Edwards, 2001; Lin et al, 2011). The DA transporter (DAT) is the key protein that removes DA from the synaptic cleft via an ionic
gradient-dependent reuptake mechanism (Giros and Caron, 1993). By rapidly clearing DA from the extracellular space, DAT regulates the availability of DA in both time and space. The
importance of this reuptake process is sustained by the profound consequences of its blockade using psychostimulant drugs or genetic loss of function (Giros et al, 1996). Indeed, DAT
knockout mice display severe behavioral and neurochemical changes, including spontaneous hyperlocomotion, increased DA receptor activation, and paradoxical responses to psychostimulants
(Gainetdinov et al, 1999; Giros et al, 1996; Jones et al, 1998). In light of the accumulating evidence suggesting that DAT-interacting proteins may have a role in its function and regulation
(Eriksen et al, 2010), our team performed a yeast two-hybrid (Y2H) screening using the DAT carboxy terminus (DAT-CT) to discover new DAT-associated proteins. In the present study, we report
snapin as a new DAT-interacting protein. Snapin (15 kDa) was first identified as a SNARE-associated protein that binds to SNAP25 and is involved in membrane fusion events (Ilardi et al,
1999). It is ubiquitously expressed and binds SNAP-23, which supports that snapin may control general SNARE-mediated fusion events (Buxton et al, 2003). Snapin participates in synchronizing
synaptic vesicle fusion and neurotransmitter release (Pan et al, 2009; Tian et al, 2005). Besides, it coordinates dynein-driven retrograde transport of endosomes and as such, snapin
deficiency impairs the delivery of endocytosed material to the endolysosomal system for degradation (Cai et al, 2010; Lee et al, 2012). This role of snapin in endolysosomal transport and
sorting has also been applied to the recycling of synaptic vesicles (Di Giovanni and Sheng, 2015). Here we show that snapin is expressed in dopaminergic neurons along with DAT, and that both
proteins colocalize in cultured cells and brain. We determined the interaction domains of both proteins, created a 3D model of the complex, and established that snapin modulates DAT uptake
activity. Finally, using viral technique to modify gene expression, we demonstrated the relevance of DAT regulation by snapin _in vivo_. MATERIALS AND METHODS YEAST TWO-HYBRID Y2H screening
was performed by Hybrigenics (France) and experiments were performed as previously described (De Gois et al, 2015). DOUBLE LABELING FLUORESCENT _IN-SITU_ HYBRIDIZATION Hybridization was
performed as described (Viereckel et al, 2016). Frozen brain sections of C57BL/6J mice (16 μm) were used. Probes (cRNA) against DAT (nucleotides 1153–2020; GenBank AccNum: NM_012694.2) and
snapin (nucleotides 102–1382; GenBank AccNum: NM_133854.3) were generated by _in vitro_ transcription with digogexigenin-dUTP and fluorescein-dUTP, respectively. Slides were scanned with the
Nanozoomer Digital Pathology 2.0 high throughput, its fluorescence unit option (L11600-05), and the NanoZoomer’s 3-CCD time delay integration camera (Hamamatsu Photonics). Resolution of
0.23 μm/pixel (× 40) (or 0.46 μm/pixel (× 20)) was used. IMMUNOCYTOCHEMISTRY Forty-eight hours after transfection, cells were rinsed with PBS then fixed on ice with 4% paraformaldehyde for
10 min. Cells were rinsed three times for 10 min with PBS and incubated 30 min at room temperature with blocking buffer (0.2% Triton X-100 and 3% normal goat serum in PBS). Animals were
perfused with 4% paraformaldehyde in PBS and brains were cut in 40 μm-thick slices on a vibrating microtome (Leica Biosystems, VT1000S). Primary antibodies anti-DAT (Chemicon, 1:1000) and
anti-Snapin (Synaptic Systems, 1:1000) were incubated overnight at 4 °C. After three washes for 10 min with PBS, secondary antibodies coupled to Alexa 488 or 555 (Life Technologies, 1:2000)
were applied for 1 h at room temperature. Nuclei were labeled using DAPI (Sigma-Aldrich, 1:20 000). Glass coverslips were mounted on a slide with Fluoromount-G (Clinisciences). All images
were acquired with a × 63 oil-immersion objective on confocal laser-scanning microscope (Leica TCS SP5 with LCS Leica software) using a _z_-stack of 0.5 μm intervals. PROXIMITY LIGATION
ASSAY Experiments were performed according to the manufacturer’s instructions with the following modifications: proximity ligation assay (PLA) probes incubation was for 2 h, ligation was
performed for 45 min, and amplification step was extended for 2 h with a concentration of polymerase of 1/60 (all steps at 37 °C). BON cultured cells were plated into eight-well LabTek
slides (Dutscher). Blocking (1 h at room temperature) and primary antibody (overnight at 4 °C) incubations were performed in a 3% bovine serum albumin and 0.2% Triton X-100 solution. Rabbit
anti-Snapin (Synaptic Systems) and rat anti-DAT (Chemicon) were diluted (1:1000) in the blocking solution, as well as the anti-rabbit (+) PLA probe (1:5) along with an anti-rat (−) probe
(1:100). Anti-rat PLA probes were generated according to the manufacturer’s instructions using the Duolink Probemaker (Olink Bioscience). Goat anti-rat IgGs (Santa Cruz) were used as
control. Slides were mounted in Fluoromont-G. Images were acquired as above. BIOTINYLATION Biotinylation was performed using the Cell Surface Protein Isolation Kit (Pierce) according to the
manufacturer’s protocol, with 0.5 mg of sulfo-NHS-SS-biotin for 0.5 mg of synaptosomes. WESTERN BLOT ANALYSIS Cells were rinsed with PBS, collected on ice by scraping in cold PBS containing
antiproteases, sonicated, and centrifuged at 2000 _g_ for 10 min at 4 °C. Protein concentration of the post-nuclear supernatant was determined with a Bradford assay (Bio-Rad). Proteins were
size fractionated on a 10% precast Bis-Tris polyacrylamide gel (Life Technologies) and electrophoretically transferred to a nitrocellulose membrane (GE Healthcare) using standard protocols.
Membranes were blocked for 1 h at room temperature (in 5% non-fat dry milk in PBS/Tween 0.1%), then incubated overnight at 4 °C with primary antibodies directed against DAT (Chemicon,
1:1000), snapin (Synaptic Systems 1:1000), Myc (Clontech, 1:250), glyceraldehyde 3-phosphate dehydrogenase (Calbiochem, 1:5000), or actin (Sigma, 1:5000). Fluorescent secondary antibodies
were detected and quantified using the Odyssey infrared imaging system (LI-COR Biosciences). For DAT, horseradish peroxidase-conjugated secondary antibodies were detected with enhanced
chemiluminescence using an ImageQuant LAS 4000 Imager. STRIATAL SYNAPTOSOMES Mouse or rat brains were collected in ice-cooled dishes. Striata were homogenized in ice-cold 0.32 M sucrose
using a Teflon-glass homogenizer, centrifuged at 1000 _g_ for 10 min at 4 °C. The resulting supernatant was centrifuged at 12 000 _g_ for 15 min. Pellet was washed in 0.32 M sucrose and
finally suspended in the appropriate buffer. DA UPTAKE ON SYNAPTOSOMES Uptake were performed as described (De Gois et al, 2015) with some modifications. A MultiScreen HTS vacuum manifold
with 96-well plates (Merck Millipore) were used with 10–15 μg of striatal synaptosomes. Thirty nanomolar of 3H-DA (Perkin Elmer) was used with increasing concentration of unlabeled substrate
(70–2970 nM) for the characterization of DAT kinetic parameters. VIRAL MANIPULATION AND ANIMAL SURGERY Snapin shRNA (mouse) lentiviral particles (sc-45546-V, Santa Cruz Biotechnology, USA)
contain 3-5 expression constructs each encoding target-specific 19–25 nucleotides (plus hairpin) shRNA designed to knockdown snapin gene expression. Control shRNA lentiviral particles
(sc-108080) encode a scrambled shRNA sequence. For stereotaxic delivery of the viruses, mice were anesthetized with a ketamine/xylazine mixture (100/10 mg/kg, i.p.) and then given bilateral
microinjections (1.0 μl per side over 10 min) into the substantia nigra _pars compacta_ (SNc) (anteroposterior, −3.1 mm; lateral, +1.7 mm; dorsoventral, −4.4 mm below dura), using a 32-gauge
Hamilton syringe angled at 7°. LOCOMOTOR ACTIVITY Mice were introduced into a circular corridor (4.5 cm width, 17 cm external diameter) crossed by four infrared beams (1.5 cm above the
base) placed at every 90° (Imetronic, Pessac). Locomotor activity was scored when animals interrupted two successive beams and thus had traveled one-quarter of the circular corridor.
Spontaneous activity was recorded for 60 min. Mice were first injected with saline and 60 min later with amphetamine (3 mg/kg). Locomotor responses were recorded for an additional 120 min.
Additional information about these methods and other methods are available in the Supplementary Information. RESULTS INTERACTION OF DAT AND SNAPIN IN THE Y2H SYSTEM AND _IN SILICO_ To search
for DAT-interacting proteins, a Y2H screening was performed using a rat brain library and the intracellular DAT-CT as bait. From several million transformants screened, 16 positive clones
were found to encode sequences of the open reading frame of snapin (411 bp). All clones contained the central large contig 43–364 bp. To rule out the possibility of a false positive
interaction, we tested several control constructs in Y2H. Only transformants bearing the bait plasmid pB6-DAT-CT and the prey plasmid pP6-Snapin were positive for the β-galactosidase and the
histidine selection (Supplementary Figure S1a). We also examined the specificity of this interaction by determining the ability of snapin to bind to the norepinephrine transporter (NET), a
closely related SLC6a family member. We found that snapin also interacted with the intracellular NET-CT (Supplementary Figure S1a). To determine which regions of DAT are involved in the
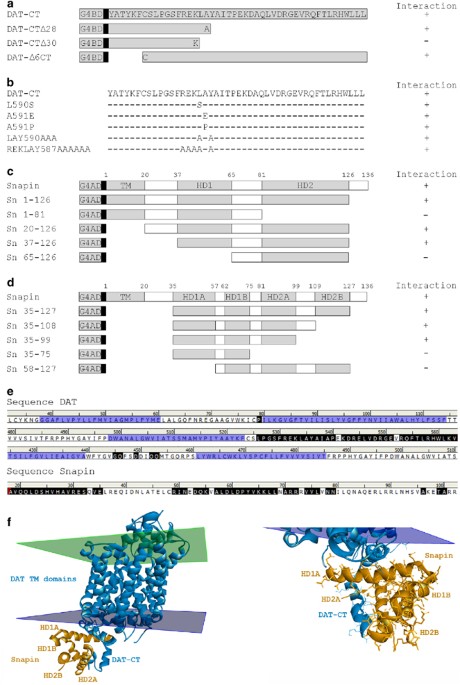
formation of the DAT/snapin complex, we assessed the ability of various portions of DAT-CT to interact with snapin in Y2H. As illustrated in Figure 1a, DAT/snapin complex formation depends
on twelve residues of DAT-CT (Cys580 to Ala591). Indeed, deletion of the last 28 or the first 6 amino acids of DAT-CT does not interfere in snapin interaction with DAT, whereas the deletion
of the last 30 residues of DAT-CT results in the loss of this interaction. To more precisely define the critical amino acids required, we inserted mutations in these twelve residues of
DAT-CT (Figure 1b). However, single or several multiple mutations in this sequence (or the entire DAT-CT, Supplementary Figure S1b) had no effect on DAT/snapin interaction, thus suggesting
that a particular combination of several amino acids within DAT-CT is necessary for this interaction. Next, we studied which regions of snapin are involved in the DAT/snapin complex. Two
helical domains (HDs) have been predicted in snapin (Buxton et al, 2003): HD1 residues 37–65 and HD2 residues 81–126. We observed that deletion mutants of snapin lacking either HD1 or HD2 do
not bind to DAT-CT (Figure 2a and Supplementary Figure S2a). This suggested that both HD are necessary for the interaction, and the smallest positive contig was 37–126. Based on the
recently crystallized hemoglobin of Cormorant (PDB 3WR1), we generated a new prediction for snapin 3D structure. According to our model, each putative HD of snapin would be subdivided in
two, resulting in a total of four HD. We thus generated additional mutants based on this new structure and found that the second part of the HD2 (HD2B) is not required for DAT/snapin
interaction (Figure 1d and Supplementary Figure S1d). This helped us further restrict the smallest positive contig to residues 35–99. We also evaluated point mutations of Ser-50 and Cys-66
in snapin, which have been reported to modulate protein phosphorylation, structure, and stability (Chheda et al, 2001; Navarro et al, 2012). We found out that snapin S50A, mimicking the
unphosphorylated protein, no longer interacted with DAT, whereas S50D, emulating the phosphorylated state, still did (data not shown). To further characterize the binding interface between
DAT-CT and snapin, we generated a three-dimensional model (Figure 1e). The primary step in the generation of a homology model is the sequence alignment between the protein of interest and a
crystallized protein. We previously created an homology model for DAT (De Gois et al, 2015) that we refined following publications of two new DAT crystallized structure (Penmatsa et al,
2015; Wang et al, 2015). For snapin, the model was based on the hemoglobin of the Cormorant. After model generation (50 models per protein) and selection (energy-based), we processed the
docking of the best homology model of snapin to that of DAT-CT, which was used as a receptor. We generated ~2000 poses clustered according to their distances and interaction energies. The
most representative clusters were analyzed and highlighted the interaction on a specific portion of DAT-CT. We selected the best pose in these clusters according to the quality of the
complex structure (Figure 1e and Supplementary Figure S1e). The results confirmed that snapin interacts with DAT-CT. More precisely, around half of the residues in snapin (from 36 to 118,
mainly located in HD1A, HD1B and HD2A) participate in the interaction (Figure 1d). As for DAT, the 3D model showed that almost the entire DAT-CT (from L582 to the end) is involved in the
interaction with snapin (Figure 1d). This suggests that even if only some residues in the Cys580–Ala591 sequence are essential for the direct interaction, many other amino acids may
participate in stabilizing the complex. COEXPRESSION AND COLOCALIZATION OF DAT AND SNAPIN _IN VITRO_ AND _IN VIVO_ We investigated whether DAT and snapin transcripts are coexpressed in
neurons using double-labeling fluorescent _in-situ_ hybridization (FISH). As previously described (De Gois et al, 2015; Giros et al, 1991), DAT expression is restricted to the SNc and the
ventral tegmental area (VTA), whereas snapin expression profile is more ubiquitous (Figure 2a and Supplementary Figure S2a). At high magnification, we observed that snapin and DAT
transcripts are coexpressed in neurons of the SNc and VTA (Figure 2a). We next examined whether these two proteins colocalized in mammalian cells by coexpressing snapin (mouse) and DAT in
BON cells that possess the machinery for a proper expression of active transporters (Tran et al, 2004). As expected, the DAT protein is detected at the plasma membrane with some labeling in
the cytoplasm, whereas snapin displayed a widespread cytoplamic expression. Interestingly, snapin colocalizes with DAT, particularly in cell processes (Figure 2b). Furthermore, we
established that endogenous DAT and snapin proteins colocalize in dopaminergic nigrostriatal fibres _in vivo_ in the mouse (Figure 2c). To demonstrate that DAT and snapin belong to the same
protein complex we used PLAs to show the physical proximity of DAT and snapin in cells and mouse brain neurons. The PLA assays showed a strong punctate signal in cells coexpressing snapin
and DAT, and in DA neurons in the striatum of wild-type mice. Conversely, no PLA signal was detected in DAT knockout animals or in regions where DAT is not expressed (Figures 2d and e, and
Supplementary Figure S2). Moreover, we performed a Myc-tagged DAT immunoprecipitation resulting in the co-precipitation of snapin, only when both proteins are coexpressed in BON cells
(Figure 3a). In addition, GST-pulldown assays showed that GST-snapin precipitated DAT from striatal synaptosomes of wild-type mice (Figure 3b). As controls, we showed that GST-snapin also
precipitated its well-established partner Snap25, but that DAT was not co-precipitated when using DAT knockout mice or GST alone. At last, immunoprecipitation assays in mouse brain confirmed
this complex formation _in vivo_, as DAT and snapin selectively co-immunoprecipitated from striatal synaptosomes (Supplementary Figure S3c). Overall, these data establish the physical
interaction between DAT and snapin. EFFECT OF SNAPIN ON DAT UPTAKE ACTIVITY _IN VITRO_ To identify whether this direct interaction may have any functional relevance, we tested the effect of
snapin expression on DAT activity in cotransfected cells. Snapin overexpression dramatically decreased DAT-mediated DA uptake by 40% of the maximal velocity (_V_max), with no changes in the
_K_M value (_V_max: 100.0±2.5% for DAT, 63.1±1.9% for DAT+Sn; _K_m (nM): 2167±194 for DAT, 1866±214 for DAT+Sn; different curve for each data set, F(2,349)=156.0, _p_<0.0001; with
differences in the _V_max, F(1,349)=128.1, _p_<0.0001) (Figure 3c). The downregulation of DA uptake was specifically mediated by snapin overexpression, as this effect was abolished when
cells were cotransfected with a siRNA targeting the exogenous snapin (F(2,172)= 0.6625, _p_=0.5168, NS; Figure 3d). FUNCTIONAL CONSEQUENCES OF THE DAT/SNAPIN INTERACTION _IN VIVO_ To test
the functional consequences of decreased snapin expression _in vivo_, we injected a lentivirus expressing shRNA targeting snapin or control in the SNc and evaluated the consequences of such
manipulations over DAT expression in the striatal DA terminals ascending from the SNc. As a preliminary step, we checked _in vitro_ that the siRNA sequences, which were used to produce the
lentivirus, completely silenced the expression of snapin in transfected cells, without altering the expression of other proteins (Supplementary Figure S3). _In vivo_, snapin shRNA injection
in the SNc resulted in a marked 23% knockdown of snapin levels in the striatum (Supplementary Figure S3). This may correspond to a strong donwregulation of the snapin located on the striatal
terminals projecting from the SNc (at least 50%), whereas snapin in the striatal neuronal cell bodies are not affected. Snapin shRNA injected-mice showed a dramatic increase (40–70%) in DAT
expression in the striatal terminals, both at the membrane and intracellular levels. As shown in Figure 4a, western blot and biotinylation studies revealed an increase in DAT levels in the
total (*_p_=0.0211), biotinylated membranous (*_p_=0.0211) and non-biotinylated intracellular fractions (*_p_=0.0441). Electron microscopy analysis confirmed this augmentation in DAT levels
in the striatal terminals, although the difference reached significance only in the intracellular DAT located in the terminals (*_p_=0.0454; Figure 4b). Accordingly, snapin downregulation
produced a significant increase in DAT-mediated DA uptake (~ +40% of the _V_max, with no changes in the affinity) in striatal synaptosomes of these mice (different curve for each data set,
F(2,124)=8.491, _p_=0.0003; with differences in the _V_max, F(1,124)=9.297, _p_=0,0028; Figure 4c). As DAT activity in striatum has a major role in locomotion and psychostimulant
sensitivity, we studied the behavioral consequences of manipulating snapin on amphetamine-induced locomotor activity. Snapin shRNA injected mice presented a considerably increased locomotor
response to amphetamine (3 mg/kg), with no differences in basal activity (FD[1,348]=10.96, _p_=0.0162; Ft[58,348]=33.78, _p_<0.0001; Fi[58,348]=2.658, _p_<0.0001; Figure 5a). No
changes were observed in the stereotypies produced by amphetamine at this dose, which could have hampered locomotion (_p_=0.6616, NS; Figure 5b). Given that amphetamine does not only inhibit
DAT, but can also directly trigger DA release through reversal of the DAT uptake activity, these behavioral results agree with the higher DAT levels observed. This increase in
amphetamine-triggered locomotor activity is highly correlated to the strong augmentation (+60 %) in extracellular levels of DA after amphetamine in snapin shRNA animals, as directly measured
by microdialysis performed on freely moving animals (FD[1,88]=7.617, _p_=0.0247; Ft[11,88]=71.43, _p_<0.0001; Fi[11,88]=5.468, _p_<0.0001; Figure 5c), which showed no difference in
the basal DA levels (_p_=0.9410, NS; Figure 5d). Moreover, amphetamine effect was absent when snapin shRNA was injected in SNc of DAT knockout mice (Supplementary Figure S4). In contrast,
when the same experiments were performed with cocaine (10 mg/kg), a ‘pure’ DAT blocker with no releasing activity, we did not observe any significant difference between animals, either in
the cocaine-induced locomotor activity (FD[1,312]=4.182, _p_=0.0617; Ft[24,312]=69.72, _p_<0.0001; Fi[24,312]=0.5888, _p_=0.9395) or cocaine-induced extracellular DA levels
(FD[1,66]=0.1010, _p_=0.7614; Ft[11,66]=13.89, _p_<0.0001; Fi[11,66]=0.4462, _p_=0.9287; Figures 5e and f). Finally, to make sure that this was not a ‘_per se_’ effect of snapin
downregulation following shRNA administration, we assessed that the K+-evoked vesicular DA release was unchanged in snapin shRNA-injected mice (FD[1,120]=0.063, _p_=0.8060; Ft[10,120]=13.21,
_p_<0.0001; Fi[10,120=0.1488, _p_=0.9988; Figure 5g). DISCUSSION Herein, we characterized snapin as a novel DAT partner and regulator. We showed that DAT and snapin colocalize and
directly interact in cells and neurons. We also established that snapin downregulates DAT expression and uptake activity and demonstrated the relevance of this regulation in mice. ROLE OF
SNAPIN IN DAT REGULATION One of the recent roles attributed to snapin is the coordination of endolysosomal retrograde transport and sorting, which has also been applied to the recycling of
synaptic vesicles (Cai et al, 2010; Di Giovanni and Sheng, 2015). As snapin deficiency impairs the delivery of endocytosed material to the endolysosomal system for degradation (Cai et al,
2010; Di Giovanni and Sheng, 2015), snapin downregulation could increase DAT levels by reducing its degradation. Given that we report a direct interaction between snapin and DAT, this could
suggest that the formation of snapin/DAT complex is necessary for targeting the recycling DAT-containing vesicles towards the endocytic pathways. Another possible mechanism for the
regulation of DAT by snapin could involve TorsinA, which regulates DAT trafficking (Torres et al, 2004) and also interacts with snapin, having a role in regulated exocytosis (Granata et al,
2008, 2011). At last, vesicle endo/exocytose implicates various SNARE and related proteins (Südhof, 2013), some of which interact with snapin, such as Snap25 (Ilardi et al, 1999) and
synaptotagmin (Chheda et al, 2001), or with DAT such as Syntaxin1A (Binda et al, 2008; Carvelli et al, 2008; Lee et al, 2004) and Synpatogyrin-3 (Egaña et al, 2009). The formation of
snapin/DAT could thus contribute to an increased endocytosis or decreased export of DAT-containing vesicles. However, it remains unknown whether the formation of DAT/snapin complexes is
constitutive or regulated by neural activity, and whether it occurs at a specific stage in the transporter life cycle. We show that DAT/snapin complex formation depends on twelve amino acids
in the carboxyl terminal of DAT (Cys580–Ala591). It has already been suggested that the central motif FREKLAYAIA (residues 587–596) in hDAT is essential for the constitutive internalization
of the transporter (Holton et al, 2005). Importantly, this motif overlaps with the domain we found necessary for the interaction with snapin. This suggests that snapin might regulate the
internalization of DAT through this domain. However, Holton et al (2005) showed that point mutations in several residues in the FREKLAYAIA motif (L591A, Y593A and I595A in hDAT, being L590,
Y592 and I594 in rat) still expressed a similar distribution to that of wild-type DAT. Accordingly, we observed that point mutations are not sufficient to interrupt the interaction
suggesting that a particular combination of several amino acids within this region is necessary for the interaction. In addition, Miranda et al (2004) found that alanine substitution of K590
in hDAT (K589 in rat) resulted in a strong intracellular localization, produced by a significant delay, not a complete blockade, of the delivery of DAT to the plasma membrane because of its
retention in the endoplasmic reticulum. This is in agreement with our results of single or multiple mutations of K589, which still interact with snapin. At last, another study showed that
progressive deletions in DAT-CT produced a progressive decrease in transport activity and export to the membrane (Torres et al, 2003). More precisely, a DAT-CT mutant stopping at L591
(corresponding to our DAT-ΔCT30) exhibited less than 1% of wild-type uptake function, and a mutant stopping at S582 completely abolished transporter function (Torres et al, 2003). This
suggests that mutants that cannot bind snapin are not correctly exported to the membrane, again reinforcing the hypothesis that snapin has a role in DAT trafficking. Other studies
identifying the molecular determinants regulating transporter trafficking and interactions with other proteins have focused on domains in DAT-CT others than the one identified in our study:
the PDZ domain (Rickhag et al, 2013; Torres et al, 2001), residues 612–617 flanking the PDZ domain (Bjerggaard et al, 2004; Fog et al, 2006), and the initial residues before C580 (Carneiro
et al, 2002; De Gois et al, 2015). NEW INSIGHTS INTO THE STRUCTURE OF SNAPIN Another interesting aspect of our work is that we provided new insights into the structure of snapin. Two HD were
initially predicted in snapin (Buxton et al, 2003). A posterior bioinformatic study that modeled the potential coiled-coil structure of snapin also proposed two helicodial domains composed
of similar series of residues (Gowthaman et al, 2006). We could notice that secondary structure prediction of these types of structure remained challenging. Indeed, various prediction tools
such as DSC (King and Sternberg, 1996), Predictprotein (Yachdav et al, 2014), or YASPIN (Lin et al, 2005) failed to accurately predict the secondary structure of putative crystallized
templates (pdb: 3WR1_A;15MI; 3DHR). Based on this observation, we selected the best template based on sequence similarity and identity. Thus, we generated a new prediction of the 3D
structure for snapin, based on the recently crystallized hemoglobin (PDB code: 3WR1), which has higher sequence homology and identity with snapin (Identity 25%; Similarity: 50% over 92AA).
According to our model, each of the putative HD of snapin would be separated in two, resulting in a total of four HD. With mutants based on this new structure and we observed that the second
part of the HD2 is not required for the interaction with DAT, which could be considered as a validation of the new structure. THE FUNCTIONAL INTERACTION BETWEEN SNAPIN AND DAT
DIFFERENTIALLY MODULATES PSYCHOSTIMULANT ACTIONS We provide strong evidence for the relevance of DAT regulation by snapin _in vivo_: snapin downregulation in mice increases DAT levels and
activity, hence increasing DA concentration and locomotor response to amphetamine. It is striking to see that the phenotype induced by decreasing the snapin/DAT interaction fully
recapitulates the one observed in animals overexpressing DAT (Calipari et al, 2013, 2015; Salahpour et al, 2008). Indeed, animals overexpressing DAT display a threefold increase in the
amount of DA released by amphetamine, compared with controls, that correlates with a threefold increase in protein expression of total and membrane DAT. Behaviorally, these mice also present
a marked increase in locomotor responses to amphetamine compared with WT animals (Salahpour et al, 2008). Interestingly, DAT blockers such as cocaine, GBR12909 and methylphenidate induced
similar locomotor activities in these mice and WT animals (Salahpour et al, 2008). It has also been shown that transgenic animals overexpressing DAT display enhanced neurochemical potency
and reinforcing effects of amphetamine, but not DAT blockers (Calipari et al, 2013, 2015). The same findings have been reported in a rat model of methylphenidate self-administration, which
causes elevations in DAT levels; these rats show enhanced potency for amphetamine on DA responses and drug seeking behaviors, without altered cocaine effects (Calipari et al, 2013, 2015). We
observed the same dissimilarity in mice with snapin downregulation. These mice showed increased DA concentration and locomotor response after amphetamine administration but displayed no
significant differences in cocaine-triggered responses. It thus seems that the effects of amphetamine at the dopaminergic terminal are dependent and directly proportional to the levels of
DAT, while the effects of blockers such as cocaine are substantially less susceptible to DAT overexpression. To conclude, our data demonstrate that the newly identified snapin/DAT
interaction has an important relevance _in vivo_: snapin downregulation directly increases DAT expression and activity at the membrane. This functional interaction represents a novel and
direct link between exocytotic and reuptake organizational clusters that may have a direct impact on our understanding of synaptic functions in the normal brain and synaptic deficits in
various psychiatric disorders. Thus, this novel snapin/DAT complex represents a potential target for DA transmission modulation, essential to many neuropsychiatric diseases. FUNDING AND
DISCLOSURE AME was recipient of a postdoctoral fellowship from Fondation pour la Recherche Médicale (2011–2012) and from the Basque Government (2013–2015). This work was funded by Canadian
Institutes of Health Research (to BG), Schizo Oui Association, and the Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM, Spain. The authors
declare no conflict of interest. REFERENCES * Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU _et al_ (2008). Syntaxin 1A interaction with the dopamine transporter promotes
amphetamine-induced dopamine efflux. _Mol Pharmacol_ 74: 1101–1108. Article CAS PubMed Google Scholar * Bjerggaard C, Fog JU, Hastrup H, Madsen K, Loland CJ, Javitch JA _et al_ (2004).
Surface targeting of the dopamine transporter involves discrete epitopes in the distal C terminus but does not require canonical PDZ domain interactions. _J Neurosci_ 24: 7024–7036. Article
CAS PubMed PubMed Central Google Scholar * Buxton P, Zhang XM, Walsh B, Sriratana A, Schenberg I, Manickam E _et al_ (2003). Identification and characterization of Snapin as a
ubiquitously expressed SNARE-binding protein that interacts with SNAP23 in non-neuronal cells. _Biochem J_ 375: 433–440. Article CAS PubMed PubMed Central Google Scholar * Cai Q, Lu L,
Tian JH, Zhu YB, Qiao H, Sheng ZH (2010). Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. _Neuron_ 68: 73–86. Article CAS
PubMed PubMed Central Google Scholar * Calipari ES, Ferris MJ, Salahpour A, Caron MG, Jones SR (2013). Methylphenidate amplifies the potency and reinforcing effects of amphetamines by
increasing dopamine transporter expression. _Nat Commun_ 4: 2720. Article PubMed Google Scholar * Calipari ES, Ferris MJ, Siciliano CA, Jones SR (2015). Differential influence of dopamine
transport rate on the potencies of cocaine, amphetamine, and methylphenidate. _ACS Chem Neurosci_ 6: 155–162. Article CAS PubMed Google Scholar * Carneiro AM, Ingram SL, Beaulieu J-M,
Sweeney A, Amara SG, Thomas SM _et al_ (2002). The multiple LIM domain-containing adaptor protein Hic-5 synaptically colocalizes and interacts with the dopamine transporter. _J Neurosci_ 22:
7045–7054. Article CAS PubMed PubMed Central Google Scholar * Carvelli L, Blakely RD, DeFelice LJ (2008). Dopamine transporter/syntaxin 1A interactions regulate transporter channel
activity and dopaminergic synaptic transmission. _Proc Natl Acad Sci USA_ 105: 14192–14197. Article CAS PubMed PubMed Central Google Scholar * Chheda MG, Ashery U, Thakur P, Rettig J,
Sheng ZH (2001). Phosphorylation of Snapin by PKA modulates its interaction with the SNARE complex. _Nat Cell Biol_ 3: 331–338. Article CAS PubMed Google Scholar * De Gois S, Slama P,
Pietrancosta N, Erdozain AM, Louis F, Bouvrais-Veret C _et al_ (2015). Ctr9, a protein in the transcription complex Paf1, regulates dopamine transporter activity at the plasma membrane. _J
Biol Chem_ 290: 17848–17862. Article CAS PubMed PubMed Central Google Scholar * Di Giovanni J, Sheng Z-H (2015). Regulation of synaptic activity by snapin-mediated endolysosomal
transport and sorting. _EMBO J_ 34: 2059–2077. Article CAS PubMed PubMed Central Google Scholar * Egaña LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S _et al_ (2009).
Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. _J Neurosci_ 29: 4592–4604. Article PubMed PubMed Central Google
Scholar * Eriksen J, Jorgensen TN, Gether U (2010). Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. _J Neurochem_
113: 27–41. Article CAS PubMed Google Scholar * Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N _et al_ (2006). Calmodulin kinase II interacts with the dopamine transporter C
terminus to regulate amphetamine-induced reverse transport. _Neuron_ 51: 417–429. Article CAS PubMed Google Scholar * Fon EA, Edwards RH (2001). Molecular mechanisms of neurotransmitter
release. _Muscle Nerve_ 24: 581–601. Article CAS PubMed Google Scholar * Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG (1999). Role of serotonin in the paradoxical
calming effect of psychostimulants on hyperactivity. _Science_ 283: 397–401. Article CAS PubMed Google Scholar * German CL, Baladi MG, McFadden LM, Hanson GR, Fleckenstein AE (2015).
Regulation of the dopamine and vesicular monoamine transporters: pharmacological targets and implications for disease. _Pharmacol Rev_ 67: 1005–1024. Article CAS PubMed PubMed Central
Google Scholar * Giros B, Caron MG (1993). Molecular characterization of the dopamine transporter. _Trends Pharmacol Sci_ 14: 43–49. Article CAS PubMed Google Scholar * Giros B, El
Mestikawy S, Bertrand L, Caron MG (1991). Cloning and functional characterization of a cocaine-sensitive dopamine transporter. _FEBS Lett_ 295: 149–154. Article CAS PubMed Google Scholar
* Giros B, Jaber M, Jones SR, Wightman RM, Caron MG (1996). Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. _Nature_ 379: 606–612.
Article CAS PubMed Google Scholar * Gowthaman R, Silvester AJ, Saranya K, Kanya KS, Archana NR (2006). Modeling of the potential coiled-coil structure of snapin protein and its
interaction with SNARE complex. _Bioinformation_ 1: 269–275. Article PubMed PubMed Central Google Scholar * Grace AA (2016). Dysregulation of the dopamine system in the pathophysiology
of schizophrenia and depression. _Nat Rev Neurosci_ 17: 524–532. Article CAS PubMed PubMed Central Google Scholar * Granata A, Koo SJ, Haucke V, Schiavo G, Warner TT (2011). CSN complex
controls the stability of selected synaptic proteins via a torsinA-dependent process. _EMBO J_ 30: 181–193. Article CAS PubMed Google Scholar * Granata A, Watson R, Collinson LM,
Schiavo G, Warner TT (2008). The dystonia-associated protein torsinA modulates synaptic vesicle recycling. _J Biol Chem_ 283: 7568–7579. Article CAS PubMed Google Scholar * Holton KL,
Loder MK, Melikian HE (2005). Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. _Nat Neurosci_ 8: 881–888. Article
CAS PubMed PubMed Central Google Scholar * Ilardi JM, Mochida S, Sheng ZH (1999). Snapin: a SNARE-associated protein implicated in synaptic transmission. _Nat Neurosci_ 2: 119–124.
Article CAS PubMed Google Scholar * Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG (1998). Profound neuronal plasticity in response to inactivation of the dopamine
transporter. _Proc Natl Acad Sci USA_ 95: 4029–4034. Article CAS PubMed PubMed Central Google Scholar * King RD, Sternberg MJ (1996). Identification and application of the concepts
important for accurate and reliable protein secondary structure prediction. _Protein Sci_ 5: 2298–2310. Article CAS PubMed PubMed Central Google Scholar * Lee HH, Nemecek D, Schindler
C, Smith WJ, Ghirlando R, Steven AC _et al_ (2012). Assembly and architecture of biogenesis of lysosome-related organelles complex-1 (BLOC-1). _J Biol Chem_ 287: 5882–5890. Article CAS
PubMed Google Scholar * Lee K-H, Kim M-Y, Kim D-H, Lee Y-S (2004). Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter.
_Neurochem Res_ 29: 1405–1409. Article CAS PubMed Google Scholar * Lin K, Simossis VA, Taylor WR, Heringa J (2005). A simple and fast secondary structure prediction method using hidden
neural networks. _Bioinformatics_ 21: 152–159. Article CAS PubMed Google Scholar * Lin Z, Canales JJ, Björgvinsson T, Thomsen M, Qu H, Liu Q-R _et al_ (2011). Monoamine transporters:
vulnerable and vital doorkeepers. _Prog Mol Biol Transl Sci_ 98: 1–46. Article CAS PubMed PubMed Central Google Scholar * Martins D, Mehta MA, Prata D (2017). The “highs and lows” of
the human brain on dopaminergics: Evidence from neuropharmacology. _Neurosci Biobehav Rev_ 80: 351–371. Article CAS PubMed Google Scholar * Miranda M, Sorkina T, Grammatopoulos TN,
Zawada WM, Sorkin A (2004). Multiple molecular determinants in the carboxyl terminus regulate dopamine transporter export from endoplasmic reticulum. _J Biol Chem_ 279: 30760–30770. Article
CAS PubMed Google Scholar * Navarro A, Encinar JA, López-Méndez B, Aguado-Llera D, Prieto J, Gómez J _et al_ (2012). Mutation of Ser-50 and Cys-66 in Snapin modulates protein structure
and stability. _Biochemistry_ 51: 3470–3484. Article CAS PubMed Google Scholar * Pan PY, Tian JH, Sheng ZH (2009). Snapin facilitates the synchronization of synaptic vesicle fusion.
_Neuron_ 61: 412–424. Article CAS PubMed PubMed Central Google Scholar * Penmatsa A, Wang KH, Gouaux E (2015). X-ray structures of _Drosophila_ dopamine transporter in complex with
nisoxetine and reboxetine. _Nat Struct Mol Biol_ 22: 506–508. Article CAS PubMed PubMed Central Google Scholar * Rickhag M, Hansen FH, Sørensen G, Strandfelt KN, Andresen B, Gotfryd K
_et al_ (2013). A C-terminal PDZ domain-binding sequence is required for striatal distribution of the dopamine transporter. _Nat Commun_ 4: 1580. Article PubMed Google Scholar * Salahpour
A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E _et al_ (2008). Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. _Proc
Natl Acad Sci USA_ 105: 4405–4410. Article CAS PubMed PubMed Central Google Scholar * Südhof TC (2013). Neurotransmitter release: the last millisecond in the life of a synaptic vesicle.
_Neuron_ 80: 675–690. Article PubMed Google Scholar * Tian JH, Wu ZX, Unzicker M, Lu L, Cai Q, Li C _et al_ (2005). The role of Snapin in neurosecretion: snapin knock-out mice exhibit
impaired calcium-dependent exocytosis of large dense-core vesicles in chromaffin cells. _J Neurosci_ 25: 10546–10555. Article CAS PubMed PubMed Central Google Scholar * Torres GE,
Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao W-D _et al_ (2003). Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains
important for the functional expression of the transporter. _J Biol Chem_ 278: 2731–2739. Article CAS PubMed Google Scholar * Torres GE, Sweeney AL, Beaulieu J-M, Shashidharan P, Caron
MG (2004). Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated DeltaE-torsinA mutant. _Proc Natl Acad Sci USA_ 101:
15650–15655. Article CAS PubMed PubMed Central Google Scholar * Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI _et al_ (2001). Functional interaction between monoamine plasma
membrane transporters and the synaptic PDZ domain-containing protein PICK1. _Neuron_ 30: 121–134. Article CAS PubMed Google Scholar * Tran VS, Marion-Audibert A-M, Karatekin E, Huet S,
Cribier S, Guillaumie K _et al_ (2004). Serotonin secretion by human carcinoid BON cells. _Ann N Y Acad Sci_ 1014: 179–188. Article CAS PubMed Google Scholar * Viereckel T, Dumas S,
Smith-Anttila CJA, Vlcek B, Bimpisidis Z, Lagerström MC _et al_ (2016). Midbrain gene screening identifies a new mesoaccumbal glutamatergic pathway and a marker for dopamine cells
neuroprotected in Parkinson’s disease. _Sci Rep_ 6: 35203. Article CAS PubMed PubMed Central Google Scholar * Wang KH, Penmatsa A, Gouaux E (2015). Neurotransmitter and psychostimulant
recognition by the dopamine transporter. _Nature_ 521: 322–327. Article CAS PubMed PubMed Central Google Scholar * Yachdav G, Kloppmann E, Kajan L, Hecht M, Goldberg T, Hamp T _et al_
(2014). PredictProtein—an open resource for online prediction of protein structural and functional features. _Nucleic Acids Res_ 42: W337–W343. Article CAS PubMed PubMed Central Google
Scholar Download references ACKNOWLEDGEMENTS We thank David Godefroy (Plateform of Institut de la Vision, Paris, France) for his technical help in acquiring the scans of the FISH. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Sorbonne Universités, UPMC Univ Paris 06, INSERM, CNRS, Neurosciences Paris Seine—Institut de Biologie Paris Seine (NPS—IBPS), Paris, France Amaia M
Erdozain, Stéphanie De Gois, Véronique Bernard, Victor Gorgievski, Carlos E Macedo, Peter Vanhoutte, Eleni T Tzavara, Vincent Vialou & Bruno Giros * Department of Pharmacology,
University of the Basque Country UPV/EHU, Leloa, Spain Amaia M Erdozain, Jorge E Ortega & J Javier Meana * Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid,
Spain Amaia M Erdozain, Jorge E Ortega & J Javier Meana * Department of Psychiatry, Douglas Hospital Research Center, McGill University, Montreal, QC, Canada Victor Gorgievski &
Bruno Giros * CNRS UMR 8601 and Université Paris Descartes, Paris, France Nicolas Pietrancosta * Oramacell, Paris, France Sylvie Dumas Authors * Amaia M Erdozain View author publications You
can also search for this author inPubMed Google Scholar * Stéphanie De Gois View author publications You can also search for this author inPubMed Google Scholar * Véronique Bernard View
author publications You can also search for this author inPubMed Google Scholar * Victor Gorgievski View author publications You can also search for this author inPubMed Google Scholar *
Nicolas Pietrancosta View author publications You can also search for this author inPubMed Google Scholar * Sylvie Dumas View author publications You can also search for this author inPubMed
Google Scholar * Carlos E Macedo View author publications You can also search for this author inPubMed Google Scholar * Peter Vanhoutte View author publications You can also search for this
author inPubMed Google Scholar * Jorge E Ortega View author publications You can also search for this author inPubMed Google Scholar * J Javier Meana View author publications You can also
search for this author inPubMed Google Scholar * Eleni T Tzavara View author publications You can also search for this author inPubMed Google Scholar * Vincent Vialou View author
publications You can also search for this author inPubMed Google Scholar * Bruno Giros View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING
AUTHOR Correspondence to Bruno Giros. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Neuropsychopharmacology website SUPPLEMENTARY INFORMATION SUPPLEMENTARY
MATERIAL (PDF 1525 KB) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 RIGHTS
AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Erdozain, A., De Gois, S., Bernard, V. _et al._ Structural and Functional Characterization of the Interaction of
Snapin with the Dopamine Transporter: Differential Modulation of Psychostimulant Actions. _Neuropsychopharmacol._ 43, 1041–1051 (2018). https://doi.org/10.1038/npp.2017.217 Download
citation * Received: 12 April 2017 * Revised: 06 September 2017 * Accepted: 08 September 2017 * Published: 14 September 2017 * Issue Date: April 2018 * DOI:
https://doi.org/10.1038/npp.2017.217 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative