Play all audios:
KEY POINTS * Multiple connexins are expressed in musculoskeletal tissues, including in joints * Gap-junctional intercellular communication contributes to interconnected cell syncytium, which
connect various cell types within joints * Connexin dysfunction might contribute to joint disease * Emerging data suggest that connexins might be novel targets for treating joint disease
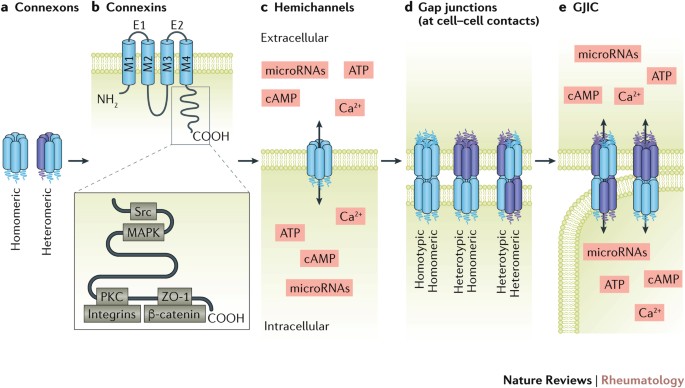
ABSTRACT Connexons form the basis of hemichannels and gap junctions. They are composed of six tetraspan proteins called connexins. Connexons can function as individual hemichannels,
releasing cytosolic factors (such as ATP) into the pericellular environment. Alternatively, two hemichannel connexons from neighbouring cells can come together to form gap junctions,
membrane-spanning channels that facilitate cell–cell communication by enabling signalling molecules of approximately 1 kDa to pass from one cell to an adjacent cell. Connexins are expressed
in joint tissues including bone, cartilage, skeletal muscle and the synovium. Indicative of their importance as gap junction components, connexins are also known as gap junction proteins,
but individual connexin proteins are gaining recognition for their channel-independent roles, which include scaffolding and signalling functions. Considerable evidence indicates that
connexons contribute to the function of bone and muscle, but less is known about the function of connexons in other joint tissues. However, the implication that connexins and gap junctional
channels might be involved in joint disease, including age-related bone loss, osteoarthritis and rheumatoid arthritis, emphasizes the need for further research into these areas and
highlights the therapeutic potential of connexins. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more
Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS PANNEXINS IN THE MUSCULOSKELETAL SYSTEM: NEW TARGETS FOR DEVELOPMENT AND DISEASE PROGRESSION Article Open access 06 May 2024
ION CHANNELS IN OSTEOARTHRITIS: EMERGING ROLES AND POTENTIAL TARGETS Article 09 August 2024 CARTILAGE CALCIFICATION IN OSTEOARTHRITIS: MECHANISMS AND CLINICAL RELEVANCE Article 12 December
2022 REFERENCES * Genetos, D. C., Kephart, C. J., Zhang, Y., Yellowley, C. E. & Donahue, H. J. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from
MLO-Y4 osteocytes. _J. Cell. Physiol._ 212, 207–214 (2007). Article CAS PubMed PubMed Central Google Scholar * Jiang, J. X. & Cherian, P. P. Hemichannels formed by connexin 43 play
an important role in the release of prostaglandin E(2) by osteocytes in response to mechanical strain. _Cell Commun. Adhes._ 10, 259–264 (2003). CAS PubMed Google Scholar * Donahue, H.
Gap junctions and biophysical regulation of bone cell differentiation. _Bone_ 26, 417–422 (2000). Article CAS PubMed Google Scholar * Thi, M. M., Islam, S., Suadicani, S. O. & Spray,
D. C. Connexin 43 and pannexin 1 channels in osteoblasts: who is the “hemichannel”? _J. Membr. Biol._ 245, 401–409 (2012). Article CAS PubMed PubMed Central Google Scholar * Willecke,
K. _ et al_. Structural and functional diversity of connexin genes in the mouse and human genome. _Biol. Chem._ 383, 725–737 (2002). Article CAS PubMed Google Scholar * Beyer, E. C.,
Paul, D. L. & Goodenough, D. A. Connexin 43: a protein from rat heart homologous to a gap junction protein from liver. _J. Cell Biol._ 105, 2621–2629 (1987). Article CAS PubMed Google
Scholar * Kumar, N. M. & Gilula, N. B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. _J. Cell Biol._ 103, 767–776 (1986). Article CAS
PubMed Google Scholar * Toyofuku, T. _ et al_. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. _J. Biol. Chem._ 273, 12725–12731 (1998). Article
CAS PubMed Google Scholar * Plotkin, L. I. & Bellido, T. Bisphosphonate-induced, hemichannel-mediated, anti-apoptosis through the Src/ERK pathway: a gap junction-independent action of
connexin 43. _Cell Commun. Adhes._ 8, 377–382 (2001). Article CAS PubMed Google Scholar * Plotkin, L. I., Manolagas, S. C. & Bellido, T. Transduction of cell survival signals by
connexin-43 hemichannels. _J. Biol. Chem._ 277, 8648–8657 (2002). Article CAS PubMed Google Scholar * Stains, J. P. & Civitelli, R. Connexins in the skeleton. _Semin. Cell Dev.
Biol._ 50, 31–39 (2016). Article CAS PubMed Google Scholar * Loiselle, A. E., Jiang, J. X. & Donahue, H. J. Gap junction and hemichannel functions in osteocytes. _Bone_ 54, 205–212
(2013). Article CAS PubMed Google Scholar * Kumar, N. M. & Gilula, N. B. The gap junction communication channel. _Cell_ 84, 381–388 (1996). Article CAS PubMed Google Scholar *
Martinez, A. D., Hayrapetyan, V., Moreno, A. P. & Beyer, E. C. Connexin 43 and connexin 45 form heteromeric gap junction channels in which individual components determine permeability
and regulation. _Circ. Res._ 90, 1100–1107 (2002). Article CAS PubMed Google Scholar * Dallas, S. L., Prideaux, M. & Bonewald, L. F. The osteocyte: an endocrine cell and more.
_Endocr. Rev._ 34, 658–690 (2013). Article CAS PubMed PubMed Central Google Scholar * Li, Z., Zhou, Z., Saunders, M. M. & Donahue, H. J. Modulation of connexin 43 alters expression
of osteoblastic differentiation markers. _Am. J. Physiol. Cell Physiol._ 290, C1248–C1255 (2006). Article CAS PubMed Google Scholar * Grellier, M., Bareille, R., Bourget, C. &
Amedee, J. Responsiveness of human bone marrow stromal cells to shear stress. _J. Tissue Eng. Regen Med._ 3, 302–309 (2009). Article CAS PubMed Google Scholar * Stains, J. P. &
Civitelli, R. Gap junctions in skeletal development and function. _Biochim. Biophys. Acta_ 1719, 69–81 (2005). Article CAS PubMed Google Scholar * Krüger, O. _ et al_. Defective vascular
development in connexin 45-deficient mice. _Development_ 127, 4179–4193 (2000). PubMed Google Scholar * Chaible, L. M., Sanches, D. S., Cogliati, B., Mennecier, G. & Dagli, M. L. Z.
Delayed osteoblastic differentiation and bone development in Cx43 knockout mice. _Toxicol. Pathol._ 39, 1046–1055 (2011). Article CAS PubMed Google Scholar * Koval, M., Harley, J., Hick,
E. & Steinberg, T. Connexin 46 is retained as monomers in a _trans_-Golgi compartment of osteoblastic cells. _J. Cell Biol._ 137, 847–857 (1997). Article CAS PubMed PubMed Central
Google Scholar * Pacheco-Costa, R. _ et al_. High bone mass in mice lacking Cx37 because of defective osteoclast differentiation. _J. Biol. Chem._ 289, 8508–8520 (2014). Article CAS
PubMed PubMed Central Google Scholar * Paic, F. _ et al_. Identification of differentially expressed genes between osteoblasts and osteocytes. _Bone_ 45, 682–692 (2009). Article CAS
PubMed PubMed Central Google Scholar * Reaume, A. G. _ et al_. Cardiac malformation in neonatal mice lacking connexin 43. _Science_ 267, 1831–1834 (1995). Article CAS PubMed Google
Scholar * Lecanda, F. _ et al_. Connexin 43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. _J. Cell Biol._ 151, 931–944 (2000). Article CAS
PubMed PubMed Central Google Scholar * Chung, D. J. _ et al_. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of
connexin 43. _J. Cell Sci._ 119, 4187–4198 (2006). Article CAS PubMed Google Scholar * Lima, F., Niger, C., Hebert, C. & Stains, J. P. Connexin 43 potentiates osteoblast
responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism. _Mol. Biol. Cell_ 20, 2697–2708 (2009). Article CAS PubMed PubMed Central Google
Scholar * Otto, F. _ et al_. _Cbfa1_, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. _Cell_ 89, 765–771 (1997).
Article CAS PubMed Google Scholar * Buo, A. M., Tomlinson, R. E., Eidelman, E. R., Chason, M. & Stains, J. P. Connexin 43 and Runx2 interact to affect cortical bone geometry,
skeletal development, and osteoblast and osteoclast function. _J. Bone Miner. Res._ 32, 1727–1738 (2017). Article CAS PubMed PubMed Central Google Scholar * Paznekas, W. A. _ et al_.
Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. _Am. J. Hum. Genet._ 72, 408–418 (2003). Article CAS PubMed Google Scholar * Paznekas, W. A.
_ et al_. _GJA1_ mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. _Hum. Mutat._ 30, 724–733 (2009). Article CAS PubMed Google
Scholar * Laird, D. W. Syndromic and non-syndromic disease-linked Cx43 mutations. _FEBS Lett._ 588, 1339–1348 (2014). Article CAS PubMed Google Scholar * Chiba, H. _ et al_.
Relationship between the expression of the gap junction protein and osteoblast phenotype in a human osteoblastic cell line during cell proliferation. _Cell Struct. Funct._ 18, 419–426
(1993). Article CAS PubMed Google Scholar * Schiller, P. C., Roos, B. A. & Howard, G. A. Parathyroid hormone up-regulation of connexin 43 gene expression in osteoblasts depends on
cell phenotype. _J. Bone Miner. Res._ 12, 2005–2013 (1997). Article CAS PubMed Google Scholar * Lecanda, F. _ et al_. Gap junctional communication modulates gene expression in
osteoblastic cells. _Mol. Biol. Cell_ 9, 2249–2258 (1998). Article CAS PubMed PubMed Central Google Scholar * Watkins, M. _ et al_. Osteoblast connexin 43 modulates skeletal
architecture by regulating both arms of bone remodeling. _Mol. Biol. Cell_ 22, 1240–1251 (2011). Article CAS PubMed PubMed Central Google Scholar * Bivi, N. _ et al_. Cell autonomous
requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. _J. Bone Miner. Res._ 27, 374–389 (2011). Article CAS Google
Scholar * Grimston, S. K., Watkins, M. P., Brodt, M. D., Silva, M. J. & Civitelli, R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional
connexin 43 deficient mice. _PLoS ONE_ 7, e44222 (2012). Article CAS PubMed PubMed Central Google Scholar * Ilvesaro, J., Tavi, P. & Tuukkanen, J. Connexin-mimetic peptide Gap 27
decreases osteoclastic activity. _BMC Musculoskelet Disord._ 2, 10 (2001). Article CAS PubMed PubMed Central Google Scholar * Ilvesaro, J., Vaananen, K. & Tuukkanen, J.
Bone-resorbing osteoclasts contain gap-junctional connexin-43. _J. Bone Miner. Res._ 15, 919–926 (2000). Article CAS PubMed Google Scholar * Ransjö, M., Sahli, J. & Lie, A.
Expression of connexin 43 mRNA in microisolated murine osteoclasts and regulation of bone resorption _in vitro_ by gap junction inhibitors. 303, 1179–1185 (2003). * Jeansonne, B. G., Feagin,
F. F., McMinn, R. W., Shoemaker, R. L. & Rehm, W. S. Cell-to-cell communication of osteoblasts. _J. Dent. Res._ 58, 1415–1423 (1979). Article CAS PubMed Google Scholar * Yellowley,
C. E., Li, Z., Zhou, Z., Jacobs, C. R. & Donahue, H. J. Functional gap junctions between osteocytic and osteoblastic cells. _J. Bone Miner. Res._ 15, 209–217 (2000). Article CAS PubMed
Google Scholar * Rawlinson, S., Pitsillides, A. & Lanyon, L. Involvement of different ion channels in osteoblasts' and osteocytes' early response to mechanical strain.
_Bone_ 19, 609–614 (1996). Article CAS PubMed Google Scholar * Duncan, R. L. & Turner, C. H. Mechanotransduction and the functional response of bone to mechanical strain. _Calcif.
Tissue Int._ 57, 344–358 (1995). Article CAS PubMed Google Scholar * Ziambaras, K., Lecanda, F., Steinberg, T. H. & Civitelli, R. Cyclic stretch enhances gap junctional communication
between osteoblastic cells. _J. Bone Miner. Res._ 13, 218–228 (1998). Article CAS PubMed Google Scholar * Alford, A. I., Jacobs, C. R. & Donahue, H. J. Oscillating fluid flow
regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. _Bone_ 33, 64–70 (2003). Article CAS PubMed Google Scholar * Cherian, P. P. _
et al_. Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. _J. Biol. Chem._ 278, 43146–43156 (2003). Article
CAS PubMed Google Scholar * Cheng, B. _ et al_. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. _J. Bone Miner. Res._ 16, 249–259
(2001). Article CAS PubMed Google Scholar * Thi, M., Kojima, T., Cowin, S., Weinbaum, S. & Spray, D. Fluid shear stress remodels expression and function of junctional proteins in
cultured bone cells. _Am. J. Physiol. Cell Physiol._ 284, C389–C403 (2003). Article CAS PubMed Google Scholar * Li, X. _ et al_. Connexin 43 is a potential regulator in fluid shear
stress-induced signal transduction in osteocytes. _J. Orthop. Res._ 31, 1959–1965 (2013). Article CAS PubMed Google Scholar * Saunders, M. M. _ et al_. Gap junctions and fluid flow
response in MC3T3-E1 cells. _Am. J. Physiol. Cell Physiol._ 281, C1917–C1925 (2001). Article CAS PubMed Google Scholar * Schirrmacher, K. & Bingmann, D. Effects of vitamin D3,
17β-estradiol, vasoactive intestinal peptide, and glutamate on electric coupling between rat osteoblast-like cells _in vitro_. _Bone_ 23, 521–526 (1998). Article CAS PubMed Google Scholar
* Taylor, A. F. _ et al_. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. _Am. J. Physiol. Cell Physiol._ 292, C545–C552 (2007). Article CAS PubMed
Google Scholar * Jørgensen, N. R. _ et al_. Human osteoblastic cells propagate intercellular calcium signals by two different mechanisms. _J. Bone Miner. Res._ 15, 1024–1032 (2000). Article
PubMed Google Scholar * Bonewald, L. F. The amazing osteocyte. _J. Bone Miner. Res._ 26, 229–238 (2011). Article CAS PubMed Google Scholar * Batra, N. _ et al_. Mechanical
stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. _Proc. Natl Acad. Sci. USA_ 109, 3359–3364 (2012). Article PubMed Google Scholar * Civitelli, R. Cell-cell
communication in the osteoblast/osteocyte lineage. _Arch. Biochem. Biophys._ 473, 188–192 (2008). Article CAS PubMed PubMed Central Google Scholar * Grimston, S. K., Brodt, M. D.,
Silva, M. J. & Civitelli, R. Attenuated response to _in vivo_ mechanical loading in mice with conditional osteoblast ablation of the connexin 43 gene (_Gja1_). _J. Bone Miner. Res._ 23,
879–886 (2008). Article PubMed PubMed Central Google Scholar * Zhang, Y. _ et al_. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone.
_PLoS ONE_ 6, e23516 (2011). Article CAS PubMed PubMed Central Google Scholar * Bivi, N. _ et al_. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical
force in mice. _J. Orthop. Res._ 31, 1075–1081 (2013). Article CAS PubMed Google Scholar * Lloyd, S. A., Loiselle, A. E., Zhang, Y. & Donahue, H. J. Connexin 43 deficiency
desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. _Bone_ 57, 76–83 (2013). Article CAS PubMed PubMed Central Google Scholar *
Grimston, S. K. _ et al_. Connexin 43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. _J. Bone Miner. Res._ 26, 2151–2160 (2011). Article CAS PubMed
PubMed Central Google Scholar * Robling, A. G. _ et al_. Mechanical stimulation of bone _in vivo_ reduces osteocyte expression of Sost/sclerostin. _J. Biol. Chem._ 283, 5866–5875 (2008).
Article CAS PubMed Google Scholar * Tu, X. _ et al_. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. _Bone_ 50, 209–217
(2011). Article CAS PubMed PubMed Central Google Scholar * Oyamada, M., Takebe, K. & Oyamada, Y. Regulation of connexin expression by transcription factors and epigenetic
mechanisms. _Biochim. Biophys. Acta_ 1828, 118–133 (2013). Article CAS PubMed Google Scholar * Robinson, J. A. _ et al_. Wnt/β-catenin signaling is a normal physiological response to
mechanical loading in bone. _J. Biol. Chem._ 281, 31720–31728 (2006). Article CAS PubMed Google Scholar * Xu, H. _ et al_. Connexin 43 channels are essential for normal bone structure
and osteocyte viability. _J. Bone Miner. Res._ 30, 550–562 (2015). CAS PubMed Central Google Scholar * Somoza, R. A., Welter, J. F., Correa, D. & Caplan, A. I. Chondrogenic
differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. _Tissue Eng. Part B Rev._ 20, 596–608 (2014). Article PubMed PubMed Central Google Scholar * Eiberger,
J. _ et al_. Expression pattern and functional characterization of connexin 29 in transgenic mice. _Glia_ 53, 601–611 (2006). Article PubMed Google Scholar * Ralphs, J. R. _ et al_.
Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. _J. Anat._ 193, 215–222 (1998). Article CAS PubMed
PubMed Central Google Scholar * Bruehlmann, S. B., Rattner, J. B., Matyas, J. R. & Duncan, N. A. Regional variations in the cellular matrix of the annulus fibrosus of the
intervertebral disc. _J. Anat._ 201, 159–171 (2002). Article PubMed PubMed Central Google Scholar * Hellio Le Graverand, M. P. _ et al_. Formation and phenotype of cell clusters in
osteoarthritic meniscus. _Arthritis Rheum._ 44, 1808–1818 (2001). Article CAS PubMed Google Scholar * Gruber, H. E., Ma, D., Hanley, E. N., Ingram, J. & Yamaguchi, D. T. Morphologic
and molecular evidence for gap junctions and connexin 43 and 45 expression in annulus fibrosus cells from the human intervertebral disc. _J. Orthop. Res._ 19, 985–989 (2001). Article CAS
PubMed Google Scholar * Knight, M. M., McGlashan, S. R., Garcia, M., Jensen, C. G. & Poole, C. A. Articular chondrocytes express connexin 43 hemichannels and P2 receptors - a putative
mechanoreceptor complex involving the primary cilium? _J. Anat._ 214, 275–283 (2009). Article CAS PubMed PubMed Central Google Scholar * Mayan, M. D. _ et al_. Human articular
chondrocytes express multiple gap junction proteins: differential expression of connexins in normal and osteoarthritic cartilage. _Am. J. Pathol._ 182, 1337–1346 (2013). Article CAS PubMed
PubMed Central Google Scholar * Schwab, W., Hofer, A. & Kasper, M. Immunohistochemical distribution of connexin 43 in the cartilage of rats and mice. _Histochem. J._ 30, 413–419
(1998). Article CAS PubMed Google Scholar * Donahue, H. J. _ et al_. Chondrocytes isolated from mature articular cartilage retain the capacity to form functional gap junctions. _J. Bone
Miner. Res._ 10, 1359–1364 (1995). Article CAS PubMed Google Scholar * Mayan, M. D. _ et al_. Articular chondrocyte network mediated by gap junctions: role in metabolic cartilage
homeostasis. _Ann. Rheum. Dis._ 74, 275–284 (2015). Article CAS PubMed Google Scholar * D'Andrea, P. & Vittur, F. Propagation of intercellular Ca2+ waves in mechanically
stimulated articular chondrocytes. _FEBS Lett._ 400, 58–64 (1997). Article CAS PubMed Google Scholar * Schrobback, K., Klein, T. J. & Woodfield, T. B. F. The importance of connexin
hemichannels during chondroprogenitor cell differentiation in hydrogel versus microtissue culture models. _Tissue Engineer. Part A_ 21, 1785–1794 (2015). Article CAS Google Scholar *
Loty, S., Foll, C., Forest, N. & Sautier, J. M. Association of enhanced expression of gap junctions with _in vitro_ chondrogenic differentiation of rat nasal septal cartilage-released
cells following their dedifferentiation and redifferentiation. _Arch. Oral Biol._ 45, 843–856 (2000). Article CAS PubMed Google Scholar * Willebrords, J., Maes, M., Crespo Yanguas, S.
& Vinken, M. Inhibitors of connexin and pannexin channels as potential therapeutics. _Pharmacol. Ther._ 180, 144–160 (2017). Article CAS PubMed PubMed Central Google Scholar *
Garcia, M. & Knight, M. M. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. _J. Orthop. Res._ 28, 510–515 (2010). CAS PubMed
Google Scholar * Contreras, J. E. _ et al_. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical
astrocytes in culture. _Proc. Natl Acad. Sci. USA_ 99, 495–500 (2002). Article CAS PubMed Google Scholar * Stout, C., Costantin, J., Naus, C. & Charles, A. Intercellular calcium
signaling in astrocytes via ATP release through connexin hemichannels. _J. Biol. Chem._ 277, 10482–10488 (2002). Article CAS PubMed Google Scholar * Gomes, P., Srinivas, S. P., Van
Driessche, W., Vereecke, J. & Himpens, B. ATP release through connexin hemichannels in corneal endothelial cells. _Invest. Ophthalmol. Vis. Sci._ 46, 1208–1218 (2005). Article PubMed
Google Scholar * Burnstock, G., Arnett, T. R. & Orriss, I. R. Purinergic signalling in the musculoskeletal system. _Purinerg. Signal_ 9, 541–572 (2013). Article CAS Google Scholar *
Graff, R. D., Lazarowski, E. R., Banes, A. J. & Lee, G. M. ATP release by mechanically loaded porcine chondrons in pellet culture. _Arthritis Rheum._ 43, 1571–1579 (2000). Article CAS
PubMed Google Scholar * Zhang, J. _ et al_. Connexin 43 hemichannels mediate small molecule exchange between chondrocytes and matrix in biomechanically-stimulated temporomandibular joint
cartilage. _Osteoarthr. Cartilage_ 22, 822–830 (2014). Article CAS Google Scholar * Groth, H. P. Cellular contacts in the synovial membrane of the cat and the rabbit: an ultrastructural
study. _Cell Tissue Res._ 164, 52541 (1975). Article CAS PubMed Google Scholar * Dryll, A., Lansaman, J., Peltier, A. P. & Ryckewaert, A. Cellular junctions in normal and
inflammatory human synovial-membrane revealed by tannic-acid and freeze-fracture. _Virchows Arch. A Pathol. Anat. Histol._ 386, 293–302 (1980). Article CAS PubMed Google Scholar *
Kolomytkin, O. V. _ et al_. Gap junctions in human synovial cells and tissue. _J. Cell. Physiol._ 184, 110–117 (2000). Article CAS PubMed Google Scholar * Capozzi, I., Tonon, R. &
D'Andrea, P. Ca2+-sensitive phosphoinositide hydrolysis is activated in synovial cells but not in articular chondrocytes. _Biochem. J._ 344, 545–553 (1999). Article CAS PubMed PubMed
Central Google Scholar * D'Andrea, P., Calabrese, A. & Grandolfo, M. Intercellular calcium signalling between chondrocytes and synovial cells in co-culture. _Biochem. J._ 329,
681–687 (1998). Article CAS PubMed PubMed Central Google Scholar * Merrifield, P. A. & Laird, D. W. Connexins in skeletal muscle development and disease. _Semin. Cell Dev. Biol._
50, 67–73 (2016). Article CAS PubMed Google Scholar * von Maltzahn, J., Euwens, C., Willecke, K. & Söhl, G. The novel mouse connexin 39 gene is expressed in developing striated
muscle fibers. _J. Cell Sci._ 117, 5381–5392 (2004). Article CAS PubMed Google Scholar * Belluardo, N., Trovato-Salinaro, A., Mudò, G. & Condorelli, D. F. Expression of the rat
connexin 39 (rCx39) gene in myoblasts and myotubes in developing and regenerating skeletal muscles: an _in situ_ hybridization study. _Cell Tissue Res._ 320, 299–310 (2005). Article CAS
PubMed Google Scholar * von Maltzahn, J., Wulf, V. & Willecke, K. Spatiotemporal expression of connexin 39 and 43 during myoblast differentiation in cultured cells and in the mouse
embryo. _Cell Commun. Adhes._ 13, 55–60 (2006). Article CAS PubMed Google Scholar * von Maltzahn, J., Wulf, V., Matern, G. & Willecke, K. Connexin 39 deficient mice display
accelerated myogenesis and regeneration of skeletal muscle. _Exp. Cell Res._ 317, 1169–1178 (2011). Article CAS PubMed Google Scholar * Dahl, E., Winterhager, E., Traub, O. &
Willecke, K. Expression of gap junction genes, connexin 40 and connexin 43, during fetal mouse development. _Anat. Embryol._ 191, 267–278 (1995). Article CAS PubMed Google Scholar *
Balogh, S., Naus, C. C. & Merrifield, P. A. Expression of gap junctions in cultured rat L6 cells during myogenesis. _Dev. Biol._ 155, 351–360 (1993). Article CAS PubMed Google Scholar
* Proulx, A., Merrifield, P. A. & Naus, C. Blocking gap junctional intercellular communication in myoblasts inhibits myogenin and MRF4 expression. _Dev. Genet._ 20, 133–144 (1997).
Article CAS PubMed Google Scholar * Yamanouchi, K., Yada, E., Ishiguro, N. & Nishihara, M. 18α-glycyrrhetinic acid induces phenotypic changes of skeletal muscle cells to enter
adipogenesis. _Cell. Physiol. Biochem._ 20, 781–790 (2007). Article CAS PubMed Google Scholar * Plotkin, L. I., Davis, H. M., Cisterna, B. A. & Sáez, J. C. Connexins and pannexins in
bone and skeletal muscle. _Curr. Osteoporosis Rep._ 15, 326–334 (2017). Article Google Scholar * Araya, R. _ et al_. Expression of connexins during differentiation and regeneration of
skeletal muscle: functional relevance of connexin 43. _J. Cell Sci._ 118, 27–37 (2005). Article CAS PubMed Google Scholar * Cea, L. A. _ et al_. De novo expression of connexin
hemichannels in denervated fast skeletal muscles leads to atrophy. _Proc. Natl Acad. Sci. USA_ 110, 16229–16234 (2013). Article PubMed Google Scholar * Cea, L. A. _ et al_. Fast skeletal
myofibers of mdx mouse, model of Duchenne muscular dystrophy, express connexin hemichannels that lead to apoptosis. _Cell. Mol. Life Sci._ 73, 2583–2599 (2016). Article CAS PubMed Google
Scholar * Muramatsu, T. _ et al_. Reduction of connexin 43 expression in aged human dental pulp. _Int. Endod J._ 37, 814–818 (2004). Article CAS PubMed Google Scholar * Yeh, H. I. _ et
al_. Age-related alteration of gap junction distribution and connexin expression in rat aortic endothelium. _J. Histochem. Cytochem._ 48, 1377–1389 (2000). Article CAS PubMed Google
Scholar * Xie, H. Q. & Hu, V. W. Modulation of gap junctions in senescent endothelial cells. _Exp. Cell Res._ 214, 172–176 (1994). Article CAS PubMed Google Scholar * Boengler, K.,
Heusch, G. & Schulz, R. Connexin 43 and ischemic preconditioning: effects of age and disease. _Exp. Gerontol._ 41, 485–488 (2006). Article CAS PubMed Google Scholar * Genetos, D. C.,
Zhou, Z., Li, Z. & Donahue, H. J. Age-related changes in gap junctional intercellular communication in osteoblastic cells. _J. Orthop. Res._ 30, 1979–1984 (2012). Article CAS PubMed
PubMed Central Google Scholar * Kastner, M. _ et al_. Complex interventions can increase osteoporosis investigations and treatment: a systematic review and meta-analysis. _Osteoporos Int._
7, 407 (2017). Google Scholar * Schiller, P. C., D'Ippolito, G., Balkan, W., Roos, B. A. & Howard, G. A. Gap-junctional communication mediates parathyroid hormone stimulation of
mineralization in osteoblastic cultures. _Bone_ 28, 38–44 (2001). Article CAS PubMed Google Scholar * Plotkin, L. I. _ et al_. Connexin 43 is required for the anti-apoptotic effect of
bisphosphonates on osteocytes and osteoblasts _in vivo_. _J. Bone Miner. Res._ 23, 1712–1721 (2008). Article CAS PubMed PubMed Central Google Scholar * Loiselle, A. E. _ et al_.
Specific biomimetic hydroxyapatite nanotopographies enhance osteoblastic differentiation and bone graft osteointegration. _Tissue Eng. Part A_ 19, 1704–1712 (2013). Article CAS PubMed
PubMed Central Google Scholar * Lloyd, S. A., Loiselle, A. E., Zhang, Y. & Donahue, H. J. Shifting paradigms on the role of connexin 43 in the skeletal response to mechanical load. _J.
Bone Miner. Res._ 29, 275–286 (2014). Article CAS PubMed PubMed Central Google Scholar * Malemud, C. J. Biologic basis of osteoarthritis: state of the evidence. _Curr. Opin. Rheumatol_
27, 289–294 (2015). Article CAS PubMed PubMed Central Google Scholar * Goldring, M. B. & Goldring, S. R. Osteoarthritis. _J. Cell. Physiol._ 213, 626–634 (2007). Article CAS
PubMed Google Scholar * arcOGEN Consortium _ et al_. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. _Lancet_ 380, 815–823 (2012).
* Hellio Le Graverand, M. P. _ et al_. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. _J. Anat._ 198, 525–535 (2001).
Article CAS PubMed PubMed Central Google Scholar * Miyamoto, Y. _ et al_. A functional polymorphism in the 5′ UTR of GDF5 is associated with susceptibility to osteoarthritis. _Nat.
Genet._ 39, 529–533 (2007). Article CAS PubMed Google Scholar * Chapman, K. _ et al_. A meta-analysis of European and Asian cohorts reveals a global role of a functional SNP in the 5′
UTR of GDF5 with osteoarthritis susceptibility. _Hum. Mol. Genet._ 17, 1497–1504 (2008). Article CAS PubMed Google Scholar * Chatterjee, B. _ et al_. BMP regulation of the mouse connexin
43 promoter in osteoblastic cells and embryos. _Cell Commun. Adhes._ 10, 37–50 (2003). Article CAS PubMed Google Scholar * Coleman, C. M., Loredo, G. A., Lo, C. W. & Tuan, R. S.
Correlation of GDF5 and connexin 43 mRNA expression during embryonic development. _Anat. Rec. A Discov. Mol. Cell Evol. Biol._ 275, 1117–1121 (2003). Article CAS PubMed Google Scholar *
Marino, A. A. _ et al_. Increased intercellular communication through gap junctions may contribute to progression of osteoarthritis. _Clin. Orthop. Relat. Res._ 422, 224–232 (2004). Article
Google Scholar * Giepmans, B. N. G. Role of connexin 43-interacting proteins at gap junctions. _Adv. Cardiol._ 42, 41–56 (2006). Article CAS PubMed Google Scholar * Giepmans, B. N. _
et al_. Gap junction protein connexin-43 interacts directly with microtubules. _Curr. Biol._ 11, 1364–1368 (2001). Article CAS PubMed Google Scholar * Moorby, C. & Patel, M. Dual
functions for connexins: Cx43 regulates growth independently of gap junction formation. _Exp. Cell Res._ 271, 238–248 (2001). Article CAS PubMed Google Scholar * Gago-Fuentes, R. _ et
al_. The C-terminal domain of connexin 43 modulates cartilage structure via chondrocyte phenotypic changes. _Oncotarget_ 7, 73055–73067 (2016). Article PubMed PubMed Central Google
Scholar * Gago-Fuentes, R. _ et al_. Proteomic analysis of connexin 43 reveals novel interactors related to osteoarthritis. _Mol. Cell. Proteom._ 14, 1831–1845 (2015). Article CAS Google
Scholar * Homandberg, G. A., Meyers, R. & Williams, J. M. Intraarticular injection of fibronectin fragments causes severe depletion of cartilage proteoglycans _in vivo_. _J. Rheumatol._
20, 1378–1382 (1993). CAS PubMed Google Scholar * Homandberg, G. A. & Hui, F. Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage
cultured with fibronectin fragments. _Arch. Biochem. Biophys._ 334, 325–331 (1996). Article CAS PubMed Google Scholar * Benito, M. J., Veale, D. J., FitzGerald, O., van den Berg, W. B.
& Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. _Ann. Rheum. Dis._ 64, 1263–1267 (2005). Article CAS PubMed PubMed Central Google Scholar * Bondeson,
J. Are we moving in the right direction with osteoarthritis drug discovery? _Expert Opin. Ther. Targets_ 15, 1355–1368 (2011). Article CAS PubMed Google Scholar * Kolomytkin, O. V. _ et
al_. IL-1β-induced production of metalloproteinases by synovial cells depends on gap junction conductance. _Am. J. Physiol. Cell Physiol._ 282, C1254–C1260 (2002). Article CAS PubMed
Google Scholar * Tonon, R. & D'Andrea, P. Interleukin-1β increases the functional expression of connexin 43 in articular chondrocytes: evidence for a Ca2+-dependent mechanism. _J.
Bone Miner. Res._ 15, 1669–1677 (2000). Article CAS PubMed Google Scholar * Tonon, R. & D'Andrea, P. The functional expression of connexin 43 in articular chondrocytes is
increased by interleukin 1ß: evidence for a Ca2+-dependent mechanism. _Biorheology_ 39, 153–160 (2002). CAS PubMed Google Scholar * Gupta, A. _ et al_. Connexin 43 enhances the expression
of osteoarthritis-associated genes in synovial fibroblasts in culture. _BMC Musculoskelet Disord._ 15, 425 (2014). Article CAS PubMed PubMed Central Google Scholar * Tsuchida, S. _ et
al_. Silencing the expression of connexin 43 decreases inflammation and joint destruction in experimental arthritis. _J. Orthop. Res._ 31, 525–530 (2013). Article CAS PubMed Google
Scholar * Grek, C. L., Rhett, J. M. & Ghatnekar, G. S. Cardiac to cancer: Connecting connexins to clinical opportunity. _FEBS Lett._ 588, 1349–1364 (2014). Article CAS PubMed PubMed
Central Google Scholar * Baklaushev, V. P. _ et al_. Treatment of glioma by cisplatin-loaded nanogels conjugated with monoclonal antibodies against Cx43 and BSAT1. _Drug Delivery_ 22,
276–285 (2014). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The work of the authors is supported by grants from the NIH, National Institute of Arthritis and
Musculoskeletal and Skin Diseases, R01AR068132-17 (to H.J.D.), R01AR 064255–05 (to D.C.G.) and a Virginia Commonwealth University School of Engineering Foundation Endowment (to H.J.D.).
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biomedical Engineering, Virginia Commonwealth University, 601 West Main Street, Richmond, 23284, Virginia, USA Henry J. Donahue *
Department of Anatomy, Physiology and Cell Biology, School of Veterinary Medicine, University of California at Davis, One Shields Avenue, Davis, 95616, California, USA Roy W. Qu & Damian
C. Genetos Authors * Henry J. Donahue View author publications You can also search for this author inPubMed Google Scholar * Roy W. Qu View author publications You can also search for this
author inPubMed Google Scholar * Damian C. Genetos View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors researched the data for the
article, provided substantial contributions to discussions of its content, wrote the article and reviewed and/or edited the manuscript before submission. CORRESPONDING AUTHOR Correspondence
to Henry J. Donahue. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2
POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 GLOSSARY * Cre-lox recombination A site-specific recombinase technology that is used to produce deletions, insertions, translocations
and inversions at specific sites in the DNA of cells. * Anabolic loading Mechanical loading that increases the abundance of bone. * Pannexin channels A family of vertebrate proteins that
predominantly exist as large transmembrane channels connecting the intracellular and extracellular space. * Chondron pellets Groups of chondrocytes and their adjacent pericellular
environment that have been centrifuged to form dense pellets. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Donahue, H., Qu, R. & Genetos, D. Joint
diseases: from connexins to gap junctions. _Nat Rev Rheumatol_ 14, 42–51 (2018). https://doi.org/10.1038/nrrheum.2017.204 Download citation * Published: 19 December 2017 * Issue Date:
January 2018 * DOI: https://doi.org/10.1038/nrrheum.2017.204 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative