Play all audios:
ABSTRACT Photooxidation of multivitamin solutions results in the generation of peroxides. Because peroxides are associated with hepatic steatosis and fibrosis as well as cholestasis, we
questioned whether multivitamins are implicated in hepatic complications of parenteral nutrition. Guinea pig pups were assigned to groups receiving intravenously either total parenteral
nutrition, photo-protected or not, or a control solution (5% dextrose + 0.45% NaCl) supplemented with either a) multivitamins; b) photo-protected multivitamins; c) multivitamins without
riboflavin; or d) peroxides (H2O2, tert-butylhydroperoxide). After 4 d, liver was sampled for histology and isoprostane-F2α levels, a marker of radical attack. Multivitamins as well as total
parenteral nutrition were associated with steatosis (scored 0–4), the severity of which was reduced (_p_ < 0.05) by photo protection. Although H2O2 is the major peroxide contaminating
multivitamins, it did not induce steatosis scores different than the controls. Compared with controls, hepatic isoprostane-F2α content increased in animals infused with H2O2 (_p_ < 0.05),
but not in those infused with Multi-12 pediatric multivitamins or total parenteral nutrition. Results suggest that peroxides and/or free radicals are not mediators of the induction of
steatosis observed with infusion of photo-exposed multivitamins, as there was no correspondence between histologic findings and hepatic levels of isoprostanes. It is suspected that a
component of the multivitamin solution becomes hepato-toxic after photo-exposure, as indicated by the protective effect observed when withdrawing riboflavin. Photo-oxidation of multivitamins
might be the common link between reports involving amino acids, lipids, and light exposure in the ethiology of hepatic complications of parenteral nutrition. SIMILAR CONTENT BEING VIEWED BY
OTHERS INTESTINAL FAILURE-ASSOCIATED LIVER DISEASE MODEL: A REDUCED PHYTOSTEROL INTRAVENOUS LIPID EMULSION PREVENTS LIVER INJURY Article 27 November 2024 HARNESSING ALGAE OIL AS A
SUSTAINABLE DHA SOURCE FOR PARENTERAL NUTRITION IN VEGAN PATIENTS Article Open access 27 May 2025 A MEDIUM-CHAIN FATTY ACID ANALOGUE PREVENTS ENDOTOXIN LIVER INJURY IN A MURINE MODEL Article
Open access 20 April 2025 MAIN Exposure of TPN solutions to light is linked to alterations in hepatobiliary function and histology in a perfused liver model (1) as well as _in vivo_(2). In
the presence of light, the parenteral multivitamin preparation is the site of reactions between oxygen and electron donors, resulting in the generation of peroxides. Hydrogen peroxide
(H2O2), which is regarded as a key element in the oxygen toxicity of the cell, represents over 80% of peroxides generated in multivitamins (3). Organic peroxides have also been reported to
contaminate solutions of parenteral nutrition (3–6). In parenterally fed neonates, the exposure of multivitamins to daylight is associated with an infused peroxide load and its urinary
excretion (7). Peroxides have been linked to hepatic steatosis (8) and fibrosis (9), as well as cholestasis (10). Multivitamins and peroxides induce the same oxidant response in the lungs of
guinea pig pups (11). The relative liver weight of these animals was 40% higher than controls (12), and histology revealed the presence of hepatic steatosis. We hypothesize that
multivitamins might account for part of the role of light in TPN-related liver complications. Because of the catalytic role of photo-excited riboflavin (13), protection of TPN solutions from
exposure to light results in a decreased generation of peroxides and a reduction in their rate of infusion (3). As H2O2 is a weak oxidant, it can accumulate to high concentrations owing to
its stability, or it can also be converted into reactive species (14) in the presence of transition metals (15, 16) such as Fe2+ and Cu+_via_ the Fenton/Haber-Weiss reactions. Furthermore,
the antioxidant components of the parenteral multivitamins would be expected to protect against the free radicals derived from peroxides generated in TPN solutions (12). The objective of
this study was to evaluate the role of multivitamins in TPN-related liver complications as documented by histology and to separate the effect of light exposure from that of peroxides and/or
free radicals. METHODS ANIMAL MODEL. Under ketamine/xylazine, 3-d-old guinea pig pups (Charles River, Montreal, Quebec, Canada) were fitted with a 0.4-mm polyurethane catheter (Luther
Medical Products, Tustin, CA, U.S.A.) in the external jugular vein and exteriorized in the scapular region. The catheter was connected to a flow-through system permitting mobility of the
animals, which were housed separately in plastic boxes with wire mesh bottoms. The animals were kept in a thermo-controlled environment with a 12-h light/dark cycle, and they were fed
exclusively intravenously at 240 mL/kg/d. After 4 d, the animals were killed and the liver was divided into aliquots with samples kept in formol for histology or minced and stored at −80°C
until analysis of triglycerides and isoprostanes, an index of radical attack (17). The study was performed in accordance with the guidelines of the institutional animal committee. This
animal model was chosen because of our previous unpublished observation of hepatic steatosis in guinea pig pups. To verify whether this steatosis could be prevented by photoprotection, the
same experimental conditions had to be replicated. PROTOCOLS. To separate the effect of multivitamins and light, animals were infused with one of the following regimens containing a
multivitamin solution at a concentration similar to the one used in neonatal parenteral nutrition. Photoprotection was achieved by preparing the solutions in darkness and covering the
infusion set with opaque material and using photo-protected intravenous tubing (13) provided graciously by Baxter Canada. _MVP + light:_ 1% (vol/vol) MVP (Sabex, Boucherville, Quebec,
Canada) unprotected from ambient light, diluted in the control solution (50 g/L dextrose + 4.5 g/L NaCl + 1 unit heparin/mL); 5 mL of MVP contains the following: vitamin A, 2300 IU; vitamin
D, 400 IU; α-tocopherol, 7 IU; vitamin K, 200 μg; ascorbate, 80 mg; thiamine, 1.2 mg; riboflavin, 1.4 mg; pyridoxine, 1.0 mg; pantothenate, 5 mg; folate, 0.14 mg; cyanocobalamin, 1 μg;
nicotinamide, 17 μg; biotin, 20 μg; and the additives mannitol and polysorbates as emulsifiers. _MVP_ −_light:_ 1% (vol/vol) MVP photo-protected, diluted in the control solution. _TPN +
light:_ Two solutions unprotected from ambient light were infused in a “piggy-backed” set-up mixed close to the infusion site: a) control + 9.6 g/kg/d amino acids (Travasol, Baxter Canada,
Mississauga, Ontario, Canada) + 2% MVP at 120 mL/kg/d; b) control + 9.6 g/kg/d amino acids + 7.6 g/kg/d lipids (Intralipid 20%, Pharmacia, Peapack, NJ, U.S.A.) at 120 mL/kg/d; for a final
concentration (at 240 mL/kg/d) of 1% MVP + 4.8 g/kg/d amino acids + 3.8 g/kg/d lipids. The composition of the TPN regimen was derived from recommendations for studies in adult guinea pigs
(18). _TPN_ (−) _light:_ The same TPN preparation protected from ambient light. To evaluate whether the effects observed with MVP and TPN were related to peroxides and/or free radicals,
other groups of animals were infused with one of the following solutions: _C (control):_ 50 g/L dextrose + 4.5 g/L NaCl + 1 unit heparin/mL. _H_2_O_2: control + 200 or 500 μM H2O2 (Aldrich
Chemical, Milwaukee, WI, U.S.) (208 ± 15 and 500 ± 20 μM measured). _TBH:_ control + 50 μM TBH (Aldrich Chemical) (47 ± 1 measured). To isolate the role of riboflavin and to determine
whether the effects of multivitamins were specific to MVP, other groups of animals were infused with one of the following regimens: _C:_ same as above _MVP + light:_ as described above in
protocol 1. _MVP + light without riboflavin:_ 1% MVP prepared by Sabex without riboflavin, unprotected from ambient light and diluted in the control solution. _MVI + light:_ 1% (vol/vol) MVI
(Aventis, Strasbourg, France) unprotected from ambient light and diluted in the control solution; 5 mL solution reconstituted from powder contains the following: vitamin A, 2300 IU; vitamin
D, 400 IU; α-tocopherol, 7 IU; vitamin K, 200 μg; ascorbate, 80 mg; thiamine, 1.2 mg; riboflavin, 1.4 mg; pyridoxine, 1.0 mg; pantothenate, 5 mg; folate, 0.14 mg; cyanocobalamin, 1 μg;
nicotinamide, 17 mg; biotin, 20 μg; and the additives BHA, BHT, and mannitol, as well as polysorbates as emulsifiers. _CVI + light:_ 1% (vol/vol) Cernevit (Baxter Canada) unprotected from
ambient light and diluted in the control solution; the 5 mL solution contains vitamin A, 3500 IU; vitamin D, 220 IU; α-tocopherol, 11.2 IU; ascorbate, 125 mg; thiamine, 3.51 mg; riboflavin,
4.14 mg; pyridoxine, 4.53 mg; pantothenate, 17.25 mg; folate, 414 μg; cyanocobalamin, 6 μg; nicotinamide, 46 mg; biotin, 69 μg; and the additives glycocoll, glycocholic acid, and soy bean
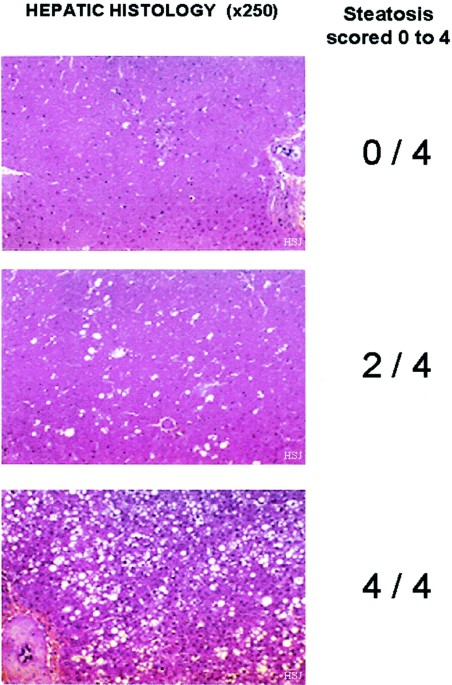
lecithin. HISTOLOGY. After staining with hematoxylin-eosin, hepatic histology was performed, and the severity of steatosis scored 0–4 (Fig. 1) by a pathologist (P.B.) and a hepatologist
(F.A.) unaware of the intravenous regimen received. The scores indicated the following: 0 = normal hepatic structure; 1 = presence of visible fat vacuoles in single, isolated hepatocytes; 2
= focal fatty changes of heterogeneous lobular distribution; 3 = diffuse fatty changes with rare “fat-free” zones; 4 = diffused fatty changes, without “fat-free” zones. ANALYTICAL
PROCEDURES. Peroxides were measured with the xylenol orange technique (19), as described previously. This chemical assay, which measures H2O2 as well as organic peroxides (19, 20) was
validated for TPN solutions using an enzymatic assay (21). The mean peroxide levels for three samples were MVI = 295 ± 8 μM; CVI = 358 ± 28 μM; MVP = 198 ± 14 μM; MVP photo-protected = 131 ±
10 μM; and MVP without riboflavin = 129 ± 3 μM. Isoprostane F2α was measured using a commercial enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, U.S.A.). Liver triglyceride levels
were measured enzymatically using commercial kits (Roche Diagnostics, Laval, Quebec, Canada). STATISTICAL ANALYSIS. All results are presented as mean ± SEM. Results for steatosis were
analyzed by Mann-Whitney test for nonparametric data. Anthropometric characteristics of the animals (Table 1) and hepatic isoprostane F2α were compared by ANOVA after transformation (natural
logarithm) to ensure homoscedasticity as determined by Bartlett's chi-square. The level of significance was set at _p_ < 0.05. RESULTS There was no difference in the initial body
weight of the animals within the various protocols (Table 1). In the first protocol, photo-protection was associated with a significantly lower (_p_ < 0.05) relative liver weight
(percentage of body weight). Animals receiving photo-exposed multivitamin solutions infused as an admixture to C or to TPN presented varying degrees of hepatic steatosis. In cases of hepatic
steatosis scored 2/4, the macrovesicular fatty changes were mainly observed in lobular zones 3 and 2. In specimens evaluated as showing scores of 3 to 4/4, nodules of fat-laden hepatocytes
gathered into a circumscribed nodule. Whatever the steatosis score recorded, no inflammatory infiltration or enlargement of portal tracts were observed. Multivitamins as well as TPN were
associated with steatosis (scored 0–4), the severity of which was reduced (_p_ < 0.05) by photo-protection (Fig. 2_A_). Compared with MVP, the apparently lower score of hepatic steatosis
observed in animals receiving TPN regimens did not reach statistical significance. These histologic findings were not accompanied by corresponding changes in hepatic isoprostane levels (Fig.
2_B_). Animals receiving photo-protected solutions had significantly lower isoprostane levels (_p_ < 0.01). However, compared with MVP, those animals receiving TPN exhibited
significantly higher (_p_ < 0.01) hepatic levels of this eicosanoid marker of radical attack. Figure 3, _A_ and _B_, illustrates that the oxidant load induced by peroxides generated in
light-exposed multivitamins or TPN did not account for the induction of hepatic steatosis. H2O2 infused at concentrations found in MVP or TPN (3) did not induce a score of steatosis
significantly higher than the controls (Fig. 3_A_), which exhibited a lower score (_p_ < 0.01) than animals receiving MVP exposed to light (Fig. 4). Results obtained with TBH infused at a
concentration compatible with levels of organic peroxides detected in MVI (3) were similar to C and H2O2. The results presented in Figure 3_B_ support the contention that the infused
peroxides carried an oxidant load. Indeed, the levels of hepatic isoprostanes found in animals receiving H2O2 were significantly higher than controls (Fig. 3_B_) and between 50% and 100%
higher than in animals receiving multivitamin solutions inducing a score of steatosis >2 (Fig. 2_B_). When exposed to ambient light, the different multivitamin solutions evaluated in this
study (MVP, MVI, CVI) induced a similar score of hepatic steatosis, which was significantly higher (_p_ < 0.01) than the score recorded in C or in the animals receiving MVP without
riboflavin exposed to light (Fig. 4). The latter two groups exhibited a similar low score of hepatic steatosis. Hepatic steatosis was confirmed by documenting higher (_p_ < 0.05) liver
triglyceride levels (0.81 ± 0.32 _versus_ 0.23 ± 0.07 mg/mg protein) in a subgroup of animals with overt steatosis (score 2–4: 3.1 ± 0.3, _n_ = 6) compared with those with no or little
steatosis (score 0 or 1: 0.7 ± 0.1, _n_ = 13). DISCUSSION Steatosis is a hepatic micro- or macrovesicular fat accumulation associated with metabolic diseases, diabetes, obesity (22), drugs
and toxins (23), surgical procedures, and parenteral nutrition (21), as well as nonalcoholic steato-hepatitis, in which case an oxidant stress is thought to play a pathogenic role (24).
TPN-related hepatic dysfunctions (25) have been linked to oxidant stress (26, 27) as well as genetic (28, 29), nutritional, environmental, and inflammatory factors (23). More specifically,
the following nutritional factors have been associated with steatosis and/or cholestasis: enteral starvation (30, 31), an amino acid imbalance (32, 33), photo-oxidized products of amino
acids (11, 12, 34), an excessive infusion of glucose (35) or lipids (36), a carbohydrate to nitrogen imbalance (37, 38), and the source of infused lipids (39). The results of the present
study suggest that TPN, when exposed to light, induces hepatic steatosis. The fact that this effect of light is linked to MVP points toward an oxidant stress (11, 40). The ethiology of this
steatosis might be explained by infused oxidants increasing lipogenesis, or decreasing lipid transport (2) and oxidative metabolism (41, 42). However, the results suggest that peroxides
and/or free radical are not mediators of the induction of steatosis observed with infusion of photo-exposed multivitamins, as there was no correspondence between histologic findings and
hepatic levels of isoprostanes. Starvation might account for the hepatic steatosis observed when glucose is the only source of energy provided with MVP. TPN prevented only partially the
steatosis induced by MVP. This effect of TPN could be explained by the lipid emulsion acting either as a shield protecting the multivitamins against the effect of light or as a substrate
improving the nutritional status. Since the effect of light was also observed with TPN, the later explanation is more likely. Among the nutritional factors contributing to steatosis, the
carbohydrate to nitrogen imbalance might be implicated. However, light protection reduced the risk of steatosis even in animals receiving only dextrose + MVP. In rats, diets without choline
are used to induce steatosis (43) and in humans, steatosis is corrected by giving choline (44). Choline is an important component of phosphocholine, which is involved in lipid transport.
Therefore, the 99 mg% phosphocholine provided to the guinea pigs by the lipid emulsion might account for this observation. Various sources of multivitamins (MVP, MVI, and CVI) were found to
induce the same score of steatosis despite differences in vitamin concentrations, in additives (polysorbates, BHT, BHA, mannitol), in means of conservation (powder _versus_ liquid form) or
in levels of H2O2. This is further evidence that infused H2O2 is not implicated in this common hepatic complication of TPN. Although TBH was not found to be associated with steatosis, this
does not preclude a possible role for further organic peroxides generated by photo-oxidation. It is suspected that a component of the multivitamin solution becomes hepato-toxic after light
exposure as indicated by the protective effect observed when withdrawing riboflavin. Apart from the generation of peroxides, photo-excited riboflavin also produces strongly oxidizing
triplets. A direct effect of activated riboflavin on the induction of steatosis cannot be excluded. Photo-oxidation of multivitamins might be the common link between reports involving amino
acids (34), lipids (39), and light exposure (1, 2) in the ethiology of hepatic complications of parenteral nutrition. Multivitamins and H2O2 induced similar responses on markers of oxidation
measured in the lungs (11, 40), whereas in the liver they induced separate effects on hepatic isoprostane levels as well as on steatosis. After their infusion, multivitamins and peroxides
will transit through the lungs before reaching the liver. This could explain in part why the responses elicited in these tissues are different. However, this discrepancy does not result from
peroxides being consumed before reaching the liver, as they did cause a marked increase in hepatic isoprostane levels. It is questionable whether our observations made in a 4-d infusion
model can be extrapolated to subjects receiving complete TPN for a longer time, or to patients with low antiperoxide defenses such as premature infants (22). The development of hepatic
steatosis observed in the present study could be related specifically to the animal model. Guinea pig tissues other than the brain do not use much glucose, and they do not respond to insulin
by increasing glucose uptake. It is therefore important to recognize that interspecies differences make extrapolations to the clinical situation hazardous (21). In summary, multivitamins
exposed to light induce hepatic steatosis. This complication of TPN is not related to the level of hydrogen peroxide or to a radical attack. A photosensitive component of the multivitamin
solution is suspected. In spite of its peroxide load, MVP carries antioxidant properties (12) as documented by the 3-fold lower hepatic isoprostane levels observed with photo-protected MVP
(Fig. 2_B_) compared with C (Fig. 3_B_). Because free radicals are produced in response to various sources of oxidant stress, improving free radical scavenging properties of MVP by
photo-protection (Fig. 2_B_) may in itself be of biologic significance. ABBREVIATIONS * C: control * CVI: Cernevit multivitamins * MVP: Multi-12 pediatric multivitamins * MVI: pediatric
multivitamins * TBH: tert-butylhydroperoxide * TPN: total parenteral nutrition REFERENCES * Shattuck KE, Bhatia J, Grinnell C, Rassin DK 1995 The effect of light exposure on the _in vitro_
hepatic response to an amino acid-vitamin solution. _JPEN J Parenteral Enteral Nutr_ 19: 398–402 Article CAS Google Scholar * Bhatia J, Moslen T, Haque AK, McCleery R, Rassin DK 1993
Total parenteral nutrition-associated alterations in hepatobiliary function and histology in rats: is light exposure a clue?. _Pediatr Res_ 33: 487–492 Article CAS Google Scholar * Lavoie
JC, Bélanger S, Spalinger M, Chessex P 1997 Admixture of a multivitamin preparation to parenteral nutrition: the major contributor to _in vitro_ generation of peroxides. _Pediatrics_ 99:
E61–E70 Article Google Scholar * Laborie S, Lavoie JC, Chessex P 1998 Paradoxical role of ascorbic acid and riboflavin in solutions of total parenteral nutrition: implications in
photo-induced peroxide generation. _Pediatr Res_ 43: 601–606 Article CAS Google Scholar * Helbock HJ, Motchnick PA, Ames BN 1993 Toxic hydroperoxides in intravenous lipid emulsions used
in preterm infants. _Pediatrics_ 91: 83–88 CAS PubMed Google Scholar * Neuzil J, Darlow BA, Inder TE, Sluis KB, Winterbourn CC, Stocker R 1995 Oxidation of parenteral lipid emulsion by
ambiant and phototherapy lights: potential toxicity of routine parenteral feeding. _J Pediatr_ 126: 785–790 Article CAS Google Scholar * Laborie S, Lavoie JC, Chessex P 2000 Increased
urinary peroxides in newborn infants receiving parenteral nutrition exposed to light. _J Pediatr_ 136: 628–632 Article CAS Google Scholar * May JM, de Haën C 1979 The insulin-like effect
of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. _J Biol Chem_ 24: 9017–9021 Google Scholar * Greenwel P, Dominguez-Rosales JA, Mavi G, Rivas-Estilla M, Rojkin M 2000
Hydrogen peroxide: a link between acetaldehyde-elicited α(1) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. _Hepatology_ 31: 109–116 Article CAS Google
Scholar * Panozzo MP, Basso D, Balint L, Biasin MR, Bonvicini P, Metus P, Infantolino D, Plebani M 1995 Altered lipid peroxidation/glutathione ratio in experimental extrahepatic
cholestasis. _Clin Exp Pharm Physiol_ 22: 266–271 Article CAS Google Scholar * Lavoie JC, Laborie S, Rouleau T, Spalinger M, Chessex P 2000 Peroxide-like response in lungs of newborn
guinea pigs following the parenteral infusion of a multivitamin preparation. _Biochem Pharmacol_ 60: 1297–1303 Article CAS Google Scholar * Chessex P, Lavoie JC, Laborie S, Rouleau T 2001
Parenteral multivitamin supplementation induces oxidant as well as antioxidant responses in the liver of newborn guinea pigs. _J Pediatr Gastroenterol Nutr_ 32: 316–321 Article CAS Google
Scholar * Laborie S, Lavoie JC, Pineault M, Chessex P 1999 Protecting solutions of parenteral nutrition from peroxidation. _JPEN J Parenteral Enteral Nutr_ 23: 104–108 Article CAS Google
Scholar * Sasaki K, Bannai S, Makino N 1998 Kinetics of hydrogen peroxide elimination by human umbilical vein endothelial cells in culture. _Biochim Biophys Acta_ 1380: 275–288 Article
CAS Google Scholar * Moison RMW, Palinckx JJS, Roest M, Houdkamp E, Berger HM 1993 Induction of lipid peroxidation of pulmonary surfactant by plasma of preterm babies. _Lancet_ 341: 79–82
Article CAS Google Scholar * Lavoie JC, Chessex P 1997 Bound iron admixture prevents the spontaneous generation of peroxides in total parenteral nutrition solutions. _J Pediatr
Gastroenterol Nutr_ 25: 307–311 Article CAS Google Scholar * Roberts LJ, Morrow JD 2000 Measurement of F(2)-isoprostanes as an index of oxidative stress _in vivo_. _Free Radic Biol Med_
28: 505–513 Article CAS Google Scholar * Lu CJH, Redmond D, Baggs RB, Schnechter A, Gasiewicz TA 1986 Growth and hepatic composition in the guinea-pig after long term parenteral
alimentation. _Am J Physiol_ 251: R388–R397 CAS PubMed Google Scholar * Jiang ZY, Woollard ACS, Wolff SP 1991 Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of
xylenol orange. Comparison with the TBA assay and an iodometric method. _Lipids_ 26: 853–856 Article CAS Google Scholar * Lavoie JC, Lachance C, Chessex P 1994 Antiperoxyde activity of
sodium metabisulfite: a double-edged sword. _Biochem Pharmacol_ 47: 871–876 Article CAS Google Scholar * Quigley EMM, Arsh MN, Shaffer JL, Markin RS 1993 Hepatobiliary complications of
total parenteral nutrition. _Gastroenterology_ 104: 286–301 Article CAS Google Scholar * Vajro P, Franzese A, Valeiro G, Iannucci M, Aragione N 2000 Lack of efficacy of ursodeoxycholic
acid for the treatment of liver abnormalities in obese children. _J Pediatr_ 136: 739–745 Article CAS Google Scholar * Trauner M, Fickert P, Stauber R 1999 Inflammation induced
cholestasis. _J Gastroenterol Hepatol_ 14: 946–959 Article CAS Google Scholar * Day C, James P 1998 Steatohepatitis: a tale of two “hits”?. _Gastroenterology_ 114: 842–845 Article CAS
Google Scholar * Teitelbaum D 1997 Parenteral nutrition-associated cholestasis. _Curr Opin Pediatr_ 9: 270–275 Article CAS Google Scholar * Bolder U, Ton-Nu HT, Schteingart CD, Frick E,
Hofmann AF 1997 Hepatocyte transport of bile acids and organic anions in endotoxemic rats: impaired uptake and secretion. _Gastroenterology_ 112: 214–225 Article CAS Google Scholar *
Sokol RJ, Taylor SF, Devereaux MW, Khandwala R, Sondheimer NJ, Shikes RH, Mierau G 1996 Hepatic oxidant injury and glutathione depletion during total parenteral nutrition in weanling rats.
_Am J Physiol_ 270: G691–G700 CAS PubMed Google Scholar * Balistreri WF 1991 Fetal and neonatal bile acid synthesis and metabolism—clinical implications. _J Inherit Metab Dis_ 14: 459–477
Article CAS Google Scholar * Suchy FJ, Sippel CJ, Ananthanarayanan M 1997 Bile acid transport across the hepatocyte canalicular membrane. _FASEB J_ 11: 199–205 Article CAS Google
Scholar * Lucas A, Bloom SR, Aynsley-Green A 1986 Gut hormones and ‘minimal enteral feeding.'. _Acta Paediatr Scand_ 75: 719–723 Article CAS Google Scholar * Jones RS, Grossman MI
1971 The choleretic effects of glucacon and secretin in the dog. _Gastroenterology_ 60: 64–68 CAS PubMed Google Scholar * Moss RL, Haynes AL, Patuszyn A, Glew RH 1999 Methionine infusion
reproduces liver injury of parenteral nutrition cholestasis. _Pediatr Res_ 45: 664–668 Article CAS Google Scholar * Belli DC, Fournier LA, Lepage G, Yousef I, Weber AM, Turchweber B, Roy
CC 1987 Total parenteral nutrition-associated cholestasis in rats: comparison of different amino acid mixtures. _JPEN J Parenteral Enteral Nutr_ 11: 67–73 Article CAS Google Scholar *
Bathia J, Mims LC, Rorsel RA 1982 The effect of phototherapy on amino acid solutions containing multivitamins. _J Pediatr_ 96: 284–286 Google Scholar * Lowry SF, Brennan MF 1979 Abnormal
liver function during parenteral nutrition: relation to infusion excess. _J Surg Res_ 26: 300–307 Article Google Scholar * Allardyce DB 1982 Cholestasis caused by lipid emulsions. _Surg
Gynecol Obstet_ 154: 641–647 CAS PubMed Google Scholar * Keim NL 1987 Nutritional effects of hepatic steatosis induced by parenteral nutrition in rats. _JPEN J Parenteral Enteral Nutr_ 1:
18–22 Article Google Scholar * Ikeda Y, Soda S, Okada A, Kawashima Y 1979 Are hepatomegaly and jaundice attributable to “glucose overload”?. _Acta Chir Scand suppl_ 494: 170–172 CAS
Google Scholar * Van Aerde J, Duerksen DR, Gramlich L, Medings JB, Chan G, Thomson ABR, Clandinin MT 1999 Intravenous fish oil emulsion attenuates total parenteral nutrition-induced
choestatsis in newborn piglets. _Pediatr Res_ 45: 202–208 Article CAS Google Scholar * Lavoie JC, Rouleau T, Gagnon C, Chessex P 2002 Photoprotection prevents TPN-induced procollagen mRNA
in newborn guinea pigs. _Free Radic Biol Med_ 33: 512–520 Article CAS Google Scholar * Fromenty B, Pessayre D 1995 Inhibition of mitochondrial beta-oxidation as a mechanism of
hepatotoxicity. _Pharmacol Ther_ 67: 101–154 Article CAS Google Scholar * Abernathy F, Pacht E 1995 Alteration of adenosine triphosphate and other nucleotides after sublethal oxidant
injury to rat type II alveolar epithelial cells. _Am J Med Sci_ 309: 140–145 Article CAS Google Scholar * Grattagliano I, Vendemiale G, Caraceni P, Domenicali M, Nardo B, Cavallari A,
Trevisani F, Bernardi M, Altomare E 2000 Starvation impairs antioxidant defence in fatty livers of rats fed a choline-deficient diet. _J Nutr_ 130: 2131–2136 Article CAS Google Scholar *
Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, Gornbein J, Ament ME 1995 Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed
with intravenous choline supplementation. _Hepatology_ 22: 1399–1403 CAS PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics,
Perinatal Service and Research Center, Hôpital Ste-Justine, University of Montreal, Montreal, Quebec, Canada Philippe Chessex, Jean-Claude Lavoie, Thérèse Rouleau, Pierre Brochu, Patrick
St-Louis, Emile Lévy & Fernando Alvarez Authors * Philippe Chessex View author publications You can also search for this author inPubMed Google Scholar * Jean-Claude Lavoie View author
publications You can also search for this author inPubMed Google Scholar * Thérèse Rouleau View author publications You can also search for this author inPubMed Google Scholar * Pierre
Brochu View author publications You can also search for this author inPubMed Google Scholar * Patrick St-Louis View author publications You can also search for this author inPubMed Google
Scholar * Emile Lévy View author publications You can also search for this author inPubMed Google Scholar * Fernando Alvarez View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Jean-Claude Lavoie. ADDITIONAL INFORMATION Supported in part by a grant from Sabex (Boucherville, Québec, Canada) and by
institutional funding from the Research Centre of Hospital Sainte-Justine. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chessex, P., Lavoie, JC.,
Rouleau, T. _et al._ Photooxidation of Parenteral Multivitamins Induces Hepatic Steatosis in a Neonatal Guinea Pig Model of Intravenous Nutrition. _Pediatr Res_ 52, 958–963 (2002).
https://doi.org/10.1203/00006450-200212000-00023 Download citation * Received: 03 July 2001 * Accepted: 17 July 2002 * Issue Date: 01 December 2002 * DOI:
https://doi.org/10.1203/00006450-200212000-00023 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative