Play all audios:
ABSTRACT Assays focusing on emerging biological phenomena in an animal’s life can be performed during embryogenesis. While the embryo of _Caenorhabditis elegans_ has been extensively
studied, its biomechanical properties are largely unknown. Here, we demonstrate that cellular force microscopy (CFM), a recently developed technique that combines micro-indentation with high
resolution force sensing approaching that of atomic force microscopy, can be successfully applied to _C. elegans_ embryos. We performed, for the first time, a quantitative study of the
mechanical properties of the eggshell of living _C. elegans_ embryos and demonstrate the capability of the system to detect alterations of its mechanical parameters and shell defects upon
chemical treatments. In addition to investigating natural eggshells, we applied different eggshell treatments, i.e., exposure to sodium hypochlorite and chitinase solutions, respectively,
that selectively modified the multilayer eggshell structure, in order to evaluate the impact of the different layers on the mechanical integrity of the embryo. Finite element method
simulations based on a simple embryo model were used to extract characteristic eggshell parameters from the experimental micro-indentation force-displacement curves. We found a strong
correlation between the severity of the chemical treatment and the rigidity of the shell. Furthermore, our results showed, in contrast to previous assumptions, that short bleach treatments
not only selectively remove the outermost vitelline layer of the eggshell, but also significantly degenerate the underlying chitin layer, which is primarily responsible for the mechanical
stability of the egg. SIMILAR CONTENT BEING VIEWED BY OTHERS 3D MECHANICAL CHARACTERIZATION OF SINGLE CELLS AND SMALL ORGANISMS USING ACOUSTIC MANIPULATION AND FORCE MICROSCOPY Article Open
access 10 May 2021 THE ARCHITECTURE AND OPERATING MECHANISM OF A CNIDARIAN STINGING ORGANELLE Article Open access 17 June 2022 A SPIRAL MICROFLUIDIC DEVICE FOR RAPID SORTING, TRAPPING, AND
LONG-TERM LIVE IMAGING OF _CAENORHABDITIS ELEGANS_ EMBRYOS Article Open access 21 February 2023 INTRODUCTION The nematode _Caenorhabditis elegans_ is an important model for biomedical
research. The mechanisms of several human diseases, including drug-target interactions, may be studied using this organism since many human disease genes and disease pathways have been
conserved between _C. elegans_ and humans1,2,3. The _C. elegans_ embryo is also an interesting model system to study biological processes at a very early stage, e.g., the formation of
functional neuronal networks4 and fundamental cellular mechanisms, in particular asymmetric cell division5. The development of new therapeutic drug agents can benefit from the use of _C.
elegans_ as well6. The eggshell of its embryo has a direct impact on many early developmental events7. This 300–400 nm thick multilayer composite structure provides physical support and
protection of the developing organism from external cues, prevents small molecules from permeating it, and blocks polyspermy8. For several decades, the eggshell of a nematode was thought to
consist of three main layers9,10. Recently two additional inner layers have been identified through electron micrographs and by diagnostic biochemical treatments. The first of these layers
was referred to as either the embryonic layer, perivitelline space, or extra-embryonic matrix7,8,11,12. The innermost layer is called the permeability barrier layer, as it appears to be
responsible for preventing small molecules from permeating12. Here, we focus on the three main morphologically distinct strata of the eggshell, i.e., an outermost thin vitelline layer (VL),
a thicker middle chitin layer (CL), and an underlying chondroitin proteoglycan layer (CPGL)8. Each of these layers has a specific function. The VL mediates sperm-oocyte binding, CL provides
the structural strength of the eggshell, and the CPGL prevents cytoplasm membrane adhesion to the CL and is required to assemble the inner permeability barrier12. Although one of the main
functions of the eggshell is to mechanically protect the embryo, its biomechanical properties are largely unknown. Accurate sensing of changes in its mechanical integrity could offer a new
way to identify genes that may up- or downregulate the synthesis of specific shell layers. To date, such biomechanical alterations have only been observed qualitatively by eye, e.g., by the
loss of the ovoid egg shape, or by evaluating egg breakage rates while exiting the uterus8. In this work, we present an extensive and quantitative micro-indentation study of living _C.
elegans_ embryos using cellular force microscopy (CFM) to extract the biomechanical properties of the eggshell. The CFM is a microrobotic platform for mechanical stimulation and
characterization of living entities of different sizes, ranging from individual cells and tissues to full organs13,14. Compared to other mechanical characterization tools used for biological
samples, such as atomic force microscopy15,16,17, CFM offers the possibility to apply multi-scale loads in the nN and mN range with significantly higher displacement, i.e., indentation
depths in the range of several micrometers with nm-resolution. Previously, CFM has been successfully used for the characterization of the cell wall of growing pollen tubes18,19,20,21,22,23.
CFM is a particularly suitable tool for the mechanical characterization of _C. elegans_ embryos, as it allows substantial bending and stretching of the hard eggshell with high spatial and
force resolution. To demonstrate the versatility of our method, we applied different chemical treatments to the embryos that are known to affect the eggshell multilayer structure. Embryos
were exposed to sodium hypochlorite (NaOCl), a chemical used to obtain age-synchronized _C. elegans_ worm populations, which selectively removes the VL8,24,25, and to chitinase, an enzyme
that digests the CL. Our micro-indentation study revealed that these shell-degrading treatments have a drastic effect on the mechanical integrity of the eggshell. We established a
biomechanical finite element (FEM) model of the embryo, enabling accurate fitting of the experimental force–displacement curves in order to estimate the Young’s modulus of the eggshell
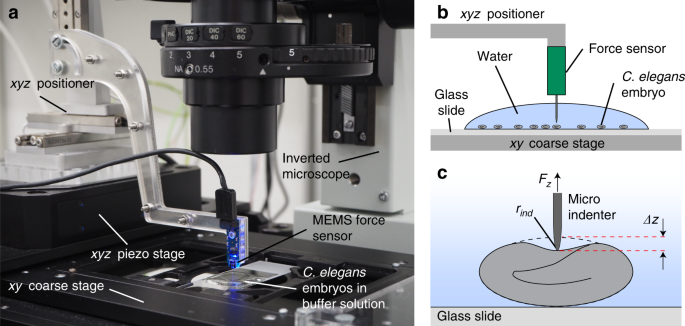
layers under different conditions. RESULTS CFM MICRO-INDENTATION PROTOCOL Micro-indentation assays were performed by means of a custom CFM setup20,21 that was integrated with an optical
microscope for high-resolution imaging (Fig. 1a). Nematode embryos in egg buffer were first spotted on a standard microscopy slide, which was inserted into the sample holder of the inverted
microscope. After alignment of the sensor tip above the center position and in proximity to the eggshell of a selected embryo, a series of consecutive loading cycles was performed (Fig. 1b).
A micro-indentation measurement consists in controlling the indentation depth _Δz_ of the sensing microprobe via the _xyz_ piezo stage while simultaneously measuring the force _F__z_
generated by the eggshell deformation (Fig. 1c). As a reference for measuring the indentation depth _Δz_, the _z_ coordinate of the initial shell contact of the sensor tip during loading,
determined at a force threshold level of 0.1 µN, was used. The resulting force–displacement curves _F__z_(_Δz_) were used to extract several mechanical parameters of eggshells subjected to
different chemical treatment protocols. Once a preset maximal force _F_max value is reached (loading curve), indentation is stopped and the indenter is automatically retracted. Multiple
successive measurements with gradually increasing _F_max values were carried out up to _F__z_punct, corresponding to the force level, where irreversible eggshell puncture occurred, typically
ranging from ∼5 µN to ∼70 µN depending on the egg treatment. A baseline drift due to viscous friction of water surrounding the sensor tip was taken into account by subtracting a linear fit
of the _F__z_(_Δz_) curve during sensor tip approach of the eggshell. Video sequences were recorded during indentation to monitor the morphological changes of the embryos and ImageJ was used
for quantification of the parameters. MORPHOLOGICAL CHANGES UPON INDENTATION FOR DIFFERENT EGGSHELL TREATMENTS We performed indentation assays on untreated embryos and embryos exposed to
three different conditions (2 min bleach without/with subsequent chitinase treatment, and 5 min bleach, respectively). The chemical treatments affect the structure and mechanical properties
of the eggshell membrane, as is schematically indicated in Fig. 2a. The untreated eggshell comprises the full trilaminar structure, consisting of the VL, CL, and CPGL. According to the
literature, treatment with bleach solution selectively removes the VL8. However, our results show that this treatment also attacks and degrades the CL even after a short incubation time (see
Discussion). Chitinase treatment is applied after a bleach treatment to strip the CL away from the embryo. Furthermore, measurements were carried out using eggs at different embryonic
development stages, i.e., in the gastrulation, bean, and twitching stages, respectively. The gastrulation stage corresponds to the first stage of freshly laid embryos (∼2.5 to ∼6 h after
fertilization), followed by the bean stage (∼6 h to ∼9 h after fertilization), and the twitching stage prior to hatching, during which the embryo starts moving (∼9 h to ∼13 h after
fertilization)26. In a first approach, we experimentally quantified the deformation of the spheroid-shaped embryos during indentation by analysis of microscopy bright field images for all
conditions. For increasing indentation depth _Δz_, eggs may elongate by _Δl_ and widen by _Δw_ with respect to the initial unloaded shape as is schematically illustrated in Fig. 2b (length
_l_ and width _w_, typically ≈50 µm and ≈35 µm, respectively). Figure 2c shows representative high-resolution bright field images of a bleach-treated embryo in the twitching stage, in the
relaxed and in a poked state with increased egg width. The embryos, which can be observed through the transparent eggshell, exhibit normal motility after treatment and during indentation.
Values for _Δl_ and _Δw_ were determined at a preset indentation force value _F__z_ = 5.0 µN for untreated and bleach-treated samples. Scatter plots for all conditions are presented in Fig.
2d. A small elongation _Δl_ = 0.3 ± 0.4 µm along the major axis of the spheroid was found for untreated eggs, which was only slightly higher for bleach-treated eggshells. As expected,
widening _Δw_ of the prolate spheroid was more pronounced upon indentation, i.e., _Δw_ = 1.1 ± 0.7 µm for untreated samples and about twice that value for bleached embryos. The duration of
bleach treatment did not affect this parameter. Nevertheless, elongation and widening of the embryos was always small compared to the indentation depth (_Δz_ ≈ 10–15 µm). Chitinase-treated
shells are significantly less resistant to mechanical stress and eggshell puncture occurred already at _F__z_ ≤ 1 µN (see Fig. 3b), thus _Δl_ and _Δw_ values for these samples had to be
evaluated at a one order of magnitude lower force value (_F__z_ = 0.5 µN). No measurable deformation was observed for untreated or bleach-treated eggs in this case. Furthermore, we
calculated the ellipticity of the individual samples (ratio _l/w_) in relaxed and poked conditions. The results are displayed in Fig. 2e for all conditions. Mean _l/w_ values remain in the
range between 1.6 and 1.8 for untreated and bleached conditions. Interestingly, chitinase-treated eggs tend to shorten, i.e., to adopt a more spherical shape (_Δl_ = −0.6 ± 2.4 µm), even if
the variation of the measured values is large. Conversely, the widening _Δw_ of chitinase-treated eggs was stronger than for untreated samples (_Δw_ = 5.2 ± 2.4 µm). In the relaxed
condition, chitinase-treated eggs are more circular than untreated or bleached eggs (_l/w_ = 1.5), and in the poked condition, eggs become even rounder (_l/w_ = 1.3). Figure 2d, e also
provide information about the measurements performed at different embryonic development stages, however, calculated mean values did not show a significant dependence on embryonic stages. A
video sequence illustrating the morphological changes of the 4 treated conditions was added to the electronic supplementary information (Vid. S1). Detailed values are summarized in Table S1.
EXPERIMENTAL INDENTATION CURVE ANALYSIS Figure 3a shows a set of representative force-indentation curves _F__z__(Δz)_ for untreated, as well as for bleach- and chitinase-treated embryos,
respectively. A video sequence in the electronic supplementary information (Vid. S2) illustrates the indentation experiments and shows the shell puncturing. _F__z__(Δz)_ curves for all
conditions showed a nonlinear force response, indicating a general trend of increasing effective eggshell stiffness _k(Δz)_ for stronger indentation. As _k(Δz)_, corresponding to the slope
of _F__z__(Δz)_ at a point _Δz_, is a parameter that is directly extracted from the experimental curve, its value is determined not only by the egg sample properties, but also by the entire
indenter system. Figure 3a also illustrates that the mechanical resistance of an embryo with respect to puncture strongly weakens for increasingly shell-degrading treatments. Shell puncture
events, corresponding to an abrupt drop in the _F__z__(Δz)_ curves, are marked as black dotted circles. The shell puncture force _F__z_punct was defined as the maximum force _F__z_ that the
shell could sustain before rupture. For stronger eggshell treatments _F__z__(Δz)_ curves become flatter, i.e._, k(Δz)_, as well as the effective stiffness at the shell puncture point
_k(F__z_punct_)_, decreased. _k(F__z_punct_)_ values were extracted from the experimental data for all conditions and larval stages and are indicated in Tab. S1. After the sharp drop of
_F__z_ at the shell puncture point, the curves proceed at non-zero force levels, most likely due to residual friction forces of the shell and the embryo body on the indenter tip. Notably,
the slope of the _F__z__(Δz)_ curves for chitinase-treated eggs is much smaller compared to the other treated and untreated eggs, indicating that the shell itself is generating only a very
weak resistance to the indenter in this case and that most of the chitin which provides the mechanical stability has been dissolved. Figure 3b summarizes experimental _F__z_punct values and
the corresponding tip indentation depth _Δz_punct, i.e., the maximal depth by which the sensor tip may deform the eggshell before irreversible rupture occurred. Data points for the different
treatments and embryonic stages are shown. The scatter plot indicates that _F__z_punct mean values clearly diminished with increasing eggshell degradation, i.e., by a factor of ∼2.9 after
bleach treatment for 2 min and by a factor of ∼7.2 after bleach treatment for 5 min with respect to untreated shells (_F__z_punct = 49 ± 13 µN). Chitinase treatment after 2 min bleach
resulted in a substantial decay of the puncture force, i.e., by a factor of ∼98, compared to untreated embryos. The critical tip indentation depth _Δz_punct was 14 ± 3 µm for untreated eggs
and somewhat less after bleach treatment (11 ± 4 µm and 10 ± 4 µm after treatment for 2 min or 5 min, respectively). Chitinase exposure did only slightly affect this parameter with respect
to bleach treatment (_Δz_punct = 10 ± 4 µm). To deduce potential mechanical changes of the eggshell in different embryonic stages, mean values were calculated for each stage. We did not,
however, observe a clear variation of the shell puncture resistance with respect to the larval development stage. Detailed results for these parameters are presented in Table S1. MODEL FOR
FEM SIMULATIONS OF EMBRYO INDENTATION To extract the mechanical properties of the embryo eggshell itself from experimental _F__z__(Δz)_ measurements, parameters such as shape and thickness
of the shell, as well as the micro-indenter tip size and shape, must be considered. The complexity of the problem makes analytical calculations difficult. Hence, we applied the finite
element method (FEM) to model _C. elegans_ egg indentation and to calculate _F__z__(Δz)_ loading curves numerically. For this purpose, we model the elastic force component related to
membrane deformation and the force generated by the internal egg pressure build-up _p_int upon indentation. Comparing simulated and experimental data allowed the Young’s moduli _E_shell of
the eggshells to be determined for different conditions. Our simulations are based on an isotropic linear elastic material model, which is in general a good assumption for small material
deformations. This approach requires only two independent eggshell material parameters, i.e., _E_shell and the Poisson ratio _ν_shell, to relate stress _σ__ij_ and strain _ε__ij_ components
in three dimensions (_i_, _j_ = _x_, _y_, _z_) (see SI Eq. 1). We assume a value of _ν_shell = 0.3, which is a generally accepted compromise for biomaterials with largely undefined material
properties, but which can be considered as neither fully compressible (_ν_ = 0) nor perfectly incompressible (_ν_ = 0.5). The eggshell geometry was approximated as a prolate spheroid, which
coincides well with the actual _C. elegans_ egg shape. The shell thickness was taken as _t_ = 300 nm, based on TEM images from Olsen et al.12, using uniform material parameters. The egg is
considered to be filled with an incompressible fluid, _i.e_. the internal egg volume is considered constant during indentation. A spherical tungsten indenter with tip radius _r_ind = 1 µm
presses against the embryo from the top, which lies on a glass baseplate. Standard material parameters of tungsten and glass were used for the rigid components of the model setup. A
representative example of the numerical model applied to the embryo is shown in Fig. 4a. The computed stress that builds up in the deformed shell is displayed by a color scheme, assuming an
indentation depth _Δz_ = 15 µm (i.e., the largest value considered for the simulations), a representative shell modulus _E_shell = 0.12 GPa and _p_int = 1.6 × 105 Pa. The highest stress
arises directly under the indenter tip, corresponding to the shell rupture point for _F__z_ > _F__z_punct. Stress in the membrane is negligible at locations more than a few micrometers
away from the indentation point. DETERMINATION OF THE EGG SHELL YOUNG’S MODULI In order to extract the Young’s modulus _E_shell of the _C. elegans_ embryonic eggshell for a specific
condition from the experimental indentation curves, first the internal egg pressure _p_int that builds as a function of the indentation depth _Δz_ was derived by means of our model. _p_int
not only deforms the spheroidal egg along the minor and major axes but is also an important parameter for accurate fitting of the experimental _F__z__(Δz)_ curves, as both, pressure and
elastic membrane forces, contribute to the measured indentation force. The total simulated force _F__z_sim exerted on the micro-indenter by the eggshell was then calculated by integrating
the membrane stress components in _z_ direction, under consideration of the internal pressure force. A parametric sweep for _E_shell values from 5.0 × 106 Pa to 1.8 × 108 Pa (incremented by
_∆E_ = 1.0 × 107 Pa) was used in the simulations to cover the relevant _p_int-_Δz-E_shell and _F__z_sim-_Δz-E_shell spaces. The interior pressure surface _p_int(_Δz, E_shell), shown in Fig.
4b, as well as the simulated indentation force surface _F__z_sim(_Δz, E_shell), plotted in Fig. 4c, were obtained by interpolation using a cubic spline algorithm. The simulated deformations
_Δl_sim _and Δw_sim are shown in Fig. S1. The quality of our model can be evaluated by means of Fig. 5a, where a direct comparison of a representative experimental indentation loading curve
for an untreated embryo, and the corresponding best-fitting computed _F__z_sim(_Δz_) curve (_E_shell = 0.12 GPa) is plotted. The model reproduces well the nonlinear force response to
indentation, and excellent congruence of the two curves over the full _Δz-_range up to the puncture point at _Δz_ ≈ 13 µm can be observed. In order to determine the _E_shell value
corresponding to a particular experimental _F__z_(_Δz_) curve, the best-fitting simulated curve _F__z_sim(_Δz, E_shell) was identified by minimizing the sum of squares of the force value
difference of a simulated trace and the experimental data \(\left( {SS = {\sum} {\left( {F_z^{{\mathrm{sim}}} - F_z} \right)^2} } \right)\), considering the full set of simulated _F__z_sim
curves. This protocol was performed for all recorded indentation curves. The results are displayed in Fig. 5b, where the entire set of experimental curves for all conditions and embryo
stages is superposed to the simulated and interpolated indentation force surface _F__z_sim(_Δz, E_shell). Here, the _F__z_(_Δz_) curves are arranged according to the corresponding extracted
_E_shell values based on our model assumptions (incremented by ∆_E_ = 1.0 × 107 Pa). The scatter plot in Fig. 5c groups all extracted _E_shell values for the different eggshell treatments.
Deterioration of the shell mechanical properties, i.e., decreasing mean _E_shell values, depending on type and duration of the treatment, can be clearly observed. For untreated samples, a
value _E_shell = 0.12 ± 0.03 GPa was found, which decreased considerably, i.e., by a factor of ∼1.3, ∼2.4 and ∼17.2 for 2 min, 5 min bleach and chitinase-treated samples, respectively.
Furthermore, we analyzed the maximal internal pressure _p_maxint characterizing the pressure value just before shell rupture, which could be extracted from the _p_int_-Δz-E_shell space (Fig.
4b) by means of the corresponding maximum indentation depth _Δz_punct (Figs. 3b or 4b) and _E_shell values. Since treated shells have lower mean _E_shell and _Δz_punct values, resulting
_p_intmax values are lower in treated cases. These results are plotted in Fig. 5d for the four eggshell treatment conditions. For untreated embryos a value _p_maxint = 1.2 × 105 Pa was
found, whereas _p_maxint decreased by a factor of ∼2.4, ∼5.2 and ∼32 for 2 min or 5 min bleach-treated, and chitinase-treated samples, respectively. Mean values for _E_shell and _p_maxint
parameters have also been determined for the different embryo developmental stages, but no significant stage dependence was found. Details are summarized in Table S1. DISCUSSION Although
there are various studies looking at the biomechanical properties of adult _C. elegans_ worms27,28,29, the embryonic stage of this nematode has not been mechanically characterized. Here, we
took advantage of the versatility of an integrated CFM-optical microscope setup for measuring the mechanical properties of embryonic eggshells by means of micro-indentation. The system does
not require complicated sample preparation protocols and manipulations. Since the sensor tip can operate in a liquid environment, live embryos can be readily probed in a non-invasive manner
under physiological conditions by simply locating an egg suspension on a glass slide. We demonstrated that our system enables detecting impairment of the embryo eggshells under various
(bio-)chemical conditions. To extract mechanical shell parameters from experimental force–displacement curves _F__z_(_Δz_), a FEM model was used, where the egg in its unloaded state is
considered as a non-pressurized ellipsoidal capsule, undergoing an increasing internal pressure build-up during indentation. Available analytical solutions for ellipsoidal shell structures
are not easily applicable for our assays, as these solutions address only the non-pressurized or highly pressurized state without taking into account possible variations of the internal
pressure as a function of an external parameter, such as the indentation depth _Δz_30,31. Furthermore, the pressure increases during the indentation (since the volume inside the egg stays
constant), which affects the measured force and adds another layer of complexity. However, very good agreement of experimental and simulated indentation curves was observed in Fig. 5a and
revealed that the simple egg model developed in this work was sufficient to reliably extract fundamental biomechanical properties of the C. _elegans_ embryo. Local buckling effects, as for
example measured during the indentation of thick-walled axisymmetric domes, were not observed during the indentation of _C. elegans_ eggs32. Our sample is a thin-walled (_t_ = 300 nm, _w_ =
35 µm) shell structure and filled with an incompressible liquid, which with a pressure increase during the indentation stabilizes the overall convex structure of the egg. The experimental
force–displacement curves recorded during embryo indentation experiments show a gradual increase of the force response until shell puncture. The shell puncture event can be optically
detected by a decrease in egg circumferences, indicating a pressure drop in the shell, and worm death (Vid. S2). Force response of the shell to loading was strongest for untreated eggs and
became weak for bleach + chitinase-treated eggshells. It is generally assumed that the relatively thick intermediate CL mainly determines the mechanical properties and stability of the
eggshell trilayer structure8,33. For example, we found a value of _E_shell = 0.12 GPa for the Young’s modulus of the untreated embryonic eggshell. Interestingly, this value falls into the
range of the Young’s moduli reported for chitin-protein networks measured in the beak of the squid _Dosidicus gigas_, i.e., 0.03–5 GPa, depending on hydration and protein content34. Reported
values for pure chitin structures, such as crystalline chitin in dry state (_E_ = 40 GPa)35 or chitin nanofibers (estimated _E_ = 150 GPa)36, however, are up to three orders of magnitude
higher than the _E_shell value in the present case. For the time being, nothing is known about the exact structure and composition of the CL, nevertheless, our results indicate that chitin
in the _C. elegans_ eggshell is probably present in a mixture with proteins. Moreover, we applied NaOCl (bleach) for selectively removing the outermost VL, and chitinase, which dissolves the
underlying CL, and measured the impact of these treatments on the mechanical integrity of the shell. Since the CL provides the mechanical stability to the egg, bleach treatments should not
significantly modify the recorded _F__z__(Δz)_ curves, and thereby the mechanical shell parameters, with respect to untreated embryos. On the contrary, our experiments revealed a clear
effect of bleach treatment. Even a short treatment drastically changed the mechanical integrity of the embryonic egg, resulting in lower resistance to puncture or lower Young’s moduli
_E_shell compared to untreated embryos. Furthermore, as shown qualitatively in Fig. 3a and quantitatively in Fig. 3b and Fig. 5c, the impact increased for longer incubation times (i.e., 2
min vs 5 min). Our observations indicate that the CL is partially removed or at least weakened under these conditions, a fact that disagrees with previous claims in literature, assuming that
NaOCl only attacks the VL8,24,25. Subsequent treatment with chitinase is expected to completely remove the CL structural backbone of the eggshell, which can indeed be concluded from our
measurements, showing extremely low Young’s moduli and puncture forces in this case. Staining of the CL in _C. elegans_ embryos at different developmental stages, using either
immunogold-labeled chitin-binding domain probe or wheat germ agglutinin labeling, indicated that the CL undergoes modifications during embryo development, but it is unclear how exactly the
CL changes37,38. In our study, we performed extensive measurements at all embryo development stages, nonetheless, we could not determine a significant variation of the mechanical properties
for a given treatment. These findings indicate that the biomolecular modifications occurring in the shell during ex-uterus embryogenesis of wild-type _C. elegans_ do not affect its
mechanical properties. Nevertheless, the capability of the CFM to detect mechanical alterations of the eggshell structure with high sensitivity possibly might be applied in the future to
identify genes that are responsible for up- and downregulating layer synthesis in the shell stratum during embryogenesis. Eggshell synthesis begins at fertilization through modifications of
the extracellular matrix surrounding the oocyte and proceeds sequentially starting from the outer VL. Interestingly, over 100 genes have been identified by RNA interference, whose
suppression generates phenotypes showing an osmotic integrity defect (OID)39,40,41. Thus, a depletion of genes playing a crucial role in the formation of the successive eggshell layers
eventually may lead to the disruption of the inner permeability barrier. Nevertheless, the specific function of most of these genes is largely unknown. Some exceptions are the _chs-1_ gene,
which is likely to be the responsible for chitin synthesis in the CL37, _cbd-1_, encoding a component of the VL, and _cpg-1_, synthesizing chondroitin proteoglycan for the CPGL40.
Furthermore, Carvalho _et al_. identified 310 more gene candidates using bioinformatics that might play a role in eggshell synthesis with 20 of them showing the OID phenotype6. Even, if most
of these genes are not related to the OID phenotype, they could still have a notable impact on eggshell synthesis. Tools that enable assessing the significance of such genes based on other
phenotypic criteria are therefore of interest, especially the micro-indentation technique presented in this paper that accurately probes the biomechanical integrity of the eggshell.
CONCLUSION In this work, we conducted a quantitative study of the mechanical properties of the _C. elegans_ eggshell. The assays were performed by micro-indentation measurements, taking
advantage of a custom CFM setup that was integrated with an optical microscope for high-resolution imaging of the embryos during indentation. We performed micro-indentation assays of _C.
elegans_ embryos in an untreated state and in three modified conditions, i.e., exposure to two different bleach treatments with subsequent chitinase exposure. Application of these protocols
was expected to selectively remove the outermost VL and the underlying CL of the eggshell. We concluded from our experiments, however, that the CL is also affected by the bleach treatment.
Experimental micro-indentation curves revealed that the eggshell elastic force _F__z_ increased in a nonlinear way as a function of the indentation depth _Δz_ for all four conditions.
Mechanical parameters, specifically the Young’s modulus _E_shell of the shell, were extracted from experimental data by means of a simple numerical ellipsoid-indentation model. Our results
clearly indicate that the mechanical integrity of the eggshell is strongly affected by the applied chemical treatments, especially after removal of the CL by successive bleach/chitinase
exposure. With our study, we demonstrate that CFM is an accurate and versatile tool to determine the mechanical properties of natural _C. elegans_ eggshells, as well as of chemically
modified shells. This approach opens the way for more advanced embryo assays in view of correlating mechanical properties with other phenotypic or genetic parameters. MATERIALS AND METHODS
AGE-SYNCHRONIZED _C. ELEGANS_ CULTURE AND EMBRYO HARVEST _C. elegans_ N2 wild-type worms were obtained from the Caenorhabditis Genetics Center (CGC). Standard nematode growth medium (NGM)
agar plates were provided by the EPFL Solution Preparation Facility (EPFL SV-IN). _Escherichia coli_ OP50 and S Basal media were prepared following standard protocols42. An _E. coli_ OP50
bacterial lawn was added to the center of the NGM agar plates, on which worm populations were subsequently grown at room temperature43. Age-synchronized worm populations were obtained by a
worm bleaching protocol (adapted from Stiernagle et al., described in Krenger et al.)42,44, followed by seeding of 500–1000 isolated eggs on fresh NGM plates. The plates were then incubated
for a duration of ∼64 h at room temperature until gravid adult worms were obtained. Freshly laid eggs were then harvested by scraping the surface of the agar plate with a cell scraper
(Sarstedt, Germany) and collecting the sample in an Eppendorf tube. Eggs were separated from adult worms by sedimentation for 30 s and subsequent removal of the egg-containing supernatant.
EGGSHELL TREATMENT PROTOCOLS Eggs were suspended in egg buffer (118 mM NaCl, 48 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 25 mM HEPES, pH 7.3) before applying shell treatments45. Untreated eggs were
taken directly from this suspension. Bleach treatment was performed in a 0.27 M NaOH + 2% NaOCl solution (bleach) for 2 or 5 min, respectively. Egg samples were then washed three times by
repeated centrifugation (1680 rcf for 60 s) and resuspended in egg buffer by vortexing. Chitinase-treated shells were obtained by suspending 2-min bleach-treated eggs in egg buffer
containing 5 mg mL−1 chitinase from _Streptomyces griseus_ (Sigma-Aldrich, Buchs, Switzerland) until the start of the experiments (i.e., for ∼15 min). CELLULAR FORCE MICROSCOPY The system,
including the CFM used for micro-indentation, was shown in Fig. 1a. One of the main features of the CFM is a commercially available MEMS-based capacitive force sensor (FT-S100; FemtoTools
AG, Buchs, Switzerland) to which the micro-indenter is attached, consisting of a tungsten wire with a shaft diameter of 22 μm and a tip radius _r_ind ≤ 1 μm (T-4-22; Picoprobe R by GGB
Industries INC, Naples, FL, USA). A microscopy image of the micro-indenter is shown in Fig. S2. The force sensor encompasses a measurement range of ± 100 µN with a resolution of 15 nN,
recording at a sampling rate of 500 Hz. The working principle of the force sensor is described in detail by Sun et al.46. The force sensor is fixed to the PMMA arm of an _xyz_ positioner
with a travel range of several cm and a closed-loop resolution of 50 nm (a stack of three SLC-2475-S; SmarAct, Oldenburg, Germany). This _xyz_ positioner is used to place the sensor probe in
close proximity to the _C. elegans_ embryos prior to the indentation experiment. Fine control of micro-indentation is performed by means of a piezo flexure-guided nanomanipulation system
(P-563.3CD PIMars; Physik Instrumente GmbH & Co, Karlsruhe, Germany). The piezo stage allows for continuous and high-precision control of the indenter tip position with a spatial
closed-loop resolution of 2 nm in _xyz_ direction and a full travel range of 300 µm. The CFM is mounted on an inverted microscope (IX71; Olympus K.K., Tokyo, Japan) using a ×20 objective
lens (LUCPlanFL N ×20; Olympus K.K., Tokyo) and a CCD camera (Orca-D2; Hamamatsu Photonics K.K., Hamamatsu, Japan) for high-resolution bright field imaging of the embryo samples. The
microscope features a motorized _xy_ stage system (M-687 PILine XY; PI GmbH & Co.) with a large travel range of 100 mm × 75 mm and 0.1 µm resolution. Data acquisition and control was
implemented in LabVIEWTM and executed using a real-time computer with an integrated field programmable gate array (FPGA) (NI cRIO 9024; National Instruments Corporation (NI), Austin, TX,
USA). An analog output module (NI-9022; NI) powers all three axes of the CFM piezo stage, and two analog input modules (NI 9215; NI) monitor the _xyz_ positions as well as the force signal.
The accuracy of the CFM was previously tested using a Si-traceable force-standard (FS-C 15, Si-METRICS, Limbach-Oberfrohna, Germany) that was calibrated and certified by the
Physikalisch-Technische Bundesanstalt (PTB), the National Metrology Institute of Germany20,21. REFERENCES * Kaletta, T. & Hengartner, M. O. Finding function in novel targets: _C.
elegans_ as a model organism. _Nat. Rev. Drug Discov._ 5, 387–399 (2006). Article Google Scholar * Shaye, D. D. & Greenwald, I. OrthoList: a compendium of _C. elegans_ genes with human
orthologs. _PLOS ONE_ 6, e20085 (2011). Article Google Scholar * O’Reilly, L. P., Luke, C. J., Perlmutter, D. H., Silverman, G. A. & Pak, S. C. _C. elegans_ in high-throughput drug
discovery. _Adv. Drug Deliv. Rev._ 69–70, 247–253 (2014). Article Google Scholar * DevoWorm Group & Alicea, B. J. The emergent connectome in _Caenorhabditis elegans_ embryogenesis.
_bioRxiv_ 146035 https://doi.org/10.1101/146035 (2018). * Gönczy, P. Mechanisms of asymmetric cell division: flies and worms pave the way. _Nat. Rev. Mol. Cell Biol._ 9, 355–366 (2008).
Article Google Scholar * Carvalho, A. et al. Acute drug treatment in the early _C. elegans_ embryo. _PLoS ONE_ 6, e24656 (2011). Article Google Scholar * Johnston, W. L. & Dennis, J.
W. The eggshell in the _C. elegans_ oocyte-to-embryo transition. _Genesis_ 50, 333–349 (2012). Article Google Scholar * Stein, K. K. & Golden, A. The _C. elegans_ eggshell. _WormBook_
1–36 https://doi.org/10.1895/wormbook.1.179.1 (2015). * Bird, A. F. & Bird, J. _The Structure of Nematodes_. (Academic Press, 1971). * Wharton, D. Nematode egg-shells. _Parasitology_
81, 447–463 (1980). Article Google Scholar * Benenati, G., Penkov, S., Müller-Reichert, T., Entchev, E. V. & Kurzchalia, T. V. Two cytochrome P450s in _Caenorhabditis elegans_ are
essential for the organization of eggshell, correct execution of meiosis and the polarization of embryo. _Mechanisms Dev._ 126, 382–393 (2009). Article Google Scholar * Olson, S. K.,
Greenan, G., Desai, A., Müller-Reichert, T. & Oegema, K. Hierarchical assembly of the eggshell and permeability barrier in _C. elegans_. _J. Cell Biol._ 198, 731–748 (2012). Article
Google Scholar * Felekis, D. et al. Quantifying growth mechanics of living, growing plant cells in situ using microbotics. _IET Micro Nano Lett._ 6, 311–316 (2011). Article Google Scholar
* Weber, A. et al. Measuring the mechanical properties of plant cells by combining micro-indentation with osmotic treatments. _J. Exp. Bot._ 66, 3229–3241 (2015). Article Google Scholar
* Arfsten, J., Leupold, S., Bradtmöller, C., Kampen, I. & Kwade, A. Atomic force microscopy studies on the nanomechanical properties of _Saccharomyces cerevisiae_. _Colloids Surf. B:
Biointerfaces_ 79, 284–290 (2010). Article Google Scholar * Deng, Y., Sun, M. & Shaevitz, J. W. Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial
cells. _Phys. Rev. Lett._ 107, 158101 (2011). Article Google Scholar * Moreno-Madrid, F. et al. Atomic force microscopy of virus shells. _Biochem. Soc. Trans._ 45, 499–511 (2017). Article
Google Scholar * Routier-Kierzkowska, A.-L. et al. Cellular force microscopy for in vivo measurements of plant tissue mechanics1[W][OA]. _Plant Physiol._ 158, 1514–1522 (2012). Article
Google Scholar * Vogler, H. et al. The pollen tube: a soft shell with a hard core. _Plant J._ 73, 617–627 (2013). Article Google Scholar * Felekis, D. et al. Real-time automated
characterization of 3D morphology and mechanics of developing plant cells. _Int. J. Robot. Res._ 34, 1136–1146 (2015). Article Google Scholar * Burri, J. T. et al. Dual-axis cellular force
microscope for mechanical characterization of living plant cells. _IEEE_ 942–947. https://doi.org/10.1109/COASE.2016.7743504 (2016). * Burri, J. T. et al. Feeling the force: how pollen
tubes deal with obstacles. _N. Phytologist_ 220, 187–195 (2018). Article Google Scholar * Burri, J. T. et al. A microrobotic system for simultaneous measurement of turgor pressure and
cell-wall elasticity of individual growing plant. _Cells IEEE Robot. Autom. Lett._ 4, 641–646 (2019). Article Google Scholar * Schierenberg, E. & Junkersdorf, B. The role of eggshell
and underlying vitelline membrane for normal pattern formation in the early _C. elegans_ embryo. _Rouxs Arch. Dev. Biol._ 202, 10–16 (1992). Article Google Scholar * Rappleye, C. A.,
Paredez, A. R., Smith, C. W., McDonald, K. L. & Aroian, R. V. The coronin-like protein POD-1 is required for anterior–posterior axis formation and cellular architecture in the nematode
_Caenorhabditis elegans_. _Genes Dev._ 13, 2838–2851 (1999). Article Google Scholar * Wood, W. B. Introduction to _C. elegans_ biology. _Cold Spring Harbor Monograph Archive_ 17, 1–16
(1988). Google Scholar * Park, S.-J., Goodman, M. B. & Pruitt, B. L. Analysis of nematode mechanics by piezoresistive displacement clamp. _Proc. Natl Acad. Sci. USA_ 104, 17376–17381
(2007). Article Google Scholar * Doll, J. C. et al. SU-8 force sensing pillar arrays for biological measurements. _Lab Chip_ 9, 1449–1454 (2009). Article Google Scholar * Backholm, M.,
Ryu, W. S. & Dalnoki-Veress, K. Viscoelastic properties of the nematode Caenorhabditis elegans, a self-similar, shear-thinning worm. _Proc. Natl Acad. Sci. USA_ 110, 4528–4533 (2013).
Article Google Scholar * Vella, D., Ajdari, A., Vaziri, A. & Boudaoud, A. Indentation of ellipsoidal and cylindrical elastic shells. _Phys. Rev. Lett._ 109, 144302-1–144302-5 (2012).
Article Google Scholar * Lazarus, A., Florijn, H. C. B. & Reis, P. M. Geometry-induced rigidity in nonspherical pressurized elastic shells. _Phys. Rev. Lett._ 109, 144301 (2012).
Article Google Scholar * Merzendorfer, H. Insect chitin synthases: a review. _J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol._ 176, 1–15 (2006). Article Google Scholar * Madhukar,
A., Perlitz, D., Grigola, M., Gai, D. & Hsia, K. J. Bistable characteristics of thick-walled axisymmetric domes. _J. Solids Struct._ 51, 2590–2597 (2014). Article Google Scholar *
Miserez, A., Schneberk, T., Sun, C., Zok, F. W. & Waite, J. H. The transition from stiff to compliant materials in squid beaks. _Science_ 319, 1816–1819 (2008). Article Google Scholar
* Nishino, T., Matsui, R. & Nakamae, K. Elastic modulus of the crystalline regions of chitin and chitosan. _J. Polym. Sci. Part B: Polym. Phys._ 37, 1191–1196 (1999). Article Google
Scholar * Ifuku, S. & Saimoto, H. Chitin nanofibers: preparations, modifications, and applications. _Nanoscale_ 4, 3308–3318 (2012). Article Google Scholar * Zhang, Y., Foster, J. M.,
Nelson, L. S., Ma, D. & Carlow, C. K. S. The chitin synthase genes chs-1 and chs-2 are essential for _C. elegans_ development and responsible for chitin deposition in the eggshell and
pharynx, respectively. _Dev. Biol._ 285, 330–339 (2005). Article Google Scholar * Bembenek, J. N. et al. Cortical granule exocytosis in C. elegans is regulated by cell cycle components
including separase. _Development_ 134, 3837–3848 (2007). Article Google Scholar * Piano, F. et al. Gene clustering based on RNAi phenotypes of ovary-enriched genes in _C. elegans_. _Curr.
Biol._ 12, 1959–1964 (2002). Article Google Scholar * Sönnichsen, B. et al. Full-genome RNAi profiling of early embryogenesis in _Caenorhabditis elegans_. _Nature_ 434, 462–469 (2005).
Article Google Scholar * Johnston, W. L., Krizus, A. & Dennis, J. W. Eggshell chitin and chitin-interacting proteins prevent polyspermy in _C. elegans_. _Curr. Biol._ 20, 1932–1937
(2010). Article Google Scholar * Stiernagle, T. Maintenance of _C. elegans_, WormBook. _WormBook_ https://doi.org/10.1895/wormbook.1.101.1 (2006). * Brenner, S. The genetics of
_Caenorhabditis elegans_. _Genetics_ 77, 71–94 (1974). Google Scholar * Krenger, R., Lehnert, T. & Gijs, M. A. M. Dynamic microfluidic nanocalorimetry system for measuring
_Caenorhabditis elegans_ metabolic heat. _Lab Chip_ 18, 1641–1651 (2018). Article Google Scholar * Bianchi, L. & Driscoll, M. _Culture of Embryonic C. Elegans Cells for
Electrophysiological and Pharmacological Analyses_. (WormBook, 2006). * Sun, Y., Fry, S. N., Potasek, D. P., Bell, D. J. & Nelson, B. J. Characterizing fruit fly flight behavior using a
microforce sensor with a new comb-drive configuration. _J. Microelectromechanical Syst._ 14, 4–11 (2005). Article Google Scholar Download references ACKNOWLEDGEMENTS This work was
supported by the EU Ideas program (ERC-2012-AdG-320404), the Swiss National Science Foundation (SNSF) grant CR32I3_156724 and the Ecole Polytechnique Fédérale de Lausanne. The authors would
like to thank H. Baris Atakan, H. Vogler and G. Munglani for insightful discussions and U. Grossniklaus for the provided infrastructure during the experiments. AUTHOR INFORMATION Author
notes * These authors contributed equally: Roger Krenger, Jan T. Burri AUTHORS AND AFFILIATIONS * Laboratory of Microsystems, Ecole Polytechnique Fédérale de Lausanne, 1015, Lausanne,
Switzerland Roger Krenger, Thomas Lehnert & Martin A. M. Gijs * Multi-Scale Robotics Laboratory, ETH Zurich, Zürich, 8092, Switzerland Jan T. Burri & Bradley J. Nelson Authors *
Roger Krenger View author publications You can also search for this author inPubMed Google Scholar * Jan T. Burri View author publications You can also search for this author inPubMed Google
Scholar * Thomas Lehnert View author publications You can also search for this author inPubMed Google Scholar * Bradley J. Nelson View author publications You can also search for this
author inPubMed Google Scholar * Martin A. M. Gijs View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.K. and J.T.B conceived the study. R.K.
prepared the samples, analyzed the data, and presented the results. J.T.B. adapted the experimental system and conducted the indentation experiments. R.K., J.T.B. and T.L. wrote the
manuscript. All authors reviewed and commented on the manuscript. CORRESPONDING AUTHORS Correspondence to Bradley J. Nelson or Martin A. M. Gijs. ETHICS DECLARATIONS CONFLICT OF INTEREST The
authors declare that they have no conflict of interest. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION SUPPLEMENTARY VIDEO S1 SUPPLEMENTARY VIDEO S2 RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Krenger, R., Burri, J.T., Lehnert,
T. _et al._ Force microscopy of the _Caenorhabditis elegans_ embryonic eggshell. _Microsyst Nanoeng_ 6, 29 (2020). https://doi.org/10.1038/s41378-020-0137-3 Download citation * Received: 15
February 2019 * Revised: 20 December 2019 * Accepted: 13 February 2020 * Published: 04 May 2020 * DOI: https://doi.org/10.1038/s41378-020-0137-3 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative