Play all audios:
ABSTRACT BACKGROUND We aimed to comprehensively evaluate the immunologic landscape at baseline and upon chemotherapy in cervical cancer. The information should aid ongoing clinical
investigations of checkpoint blockade immunotherapies in this disease setting. METHODS A series of 109 cervical carcinoma patients was retrospectively assayed before and after neoadjuvant
chemotherapy. Tumour-infiltrating immune markers (CD3, CD4, CD8, CD20, CD56, CD68, PD-1, PD-L1) were assessed by immunohistochemistry. RNA sequencing analysis was performed on matched pre-
and post-treatment fresh-frozen tissues. RESULTS At diagnosis, diverse immune cell types including CD20+ B cells, CD3+ T cells, CD56+ natural killer (NK) cells, and CD68+ macrophages were
detected in different proportions of cervical carcinoma. Unsupervised hierarchical clustering evidently showed that CD4+ and CD8+ T cell abundance correlated with PD-L1 expression. Based on
the immune infiltration patterns, the patients could be stratified into four groups with prognostic relevance, namely, ‘immuno-active’, ‘immuno-medial’, ‘immuno-NK’, and ‘immuno-deficient’.
Neoadjuvant chemotherapy was associated with increased CD4, CD8, CD20, and CD56 signals, most prominently in good responders. Transcriptomic data corroborated the improved anticancer
immunity and identified immunosuppressive CD200 upregulation following chemotherapeutic intervention. CONCLUSIONS A subset of cervical cancer harbours active immune microenvironment, and
chemotherapy treatment may further exert locoregional immunostimulation. Immune checkpoint inhibitors as combination or maintenance therapies warrant future exploration in clinic. SIMILAR
CONTENT BEING VIEWED BY OTHERS SINGLE-CELL RNA-SEQUENCING REVEALS RADIOCHEMOTHERAPY-INDUCED INNATE IMMUNE ACTIVATION AND MHC-II UPREGULATION IN CERVICAL CANCER Article Open access 30 January
2023 LINKING TUMOR IMMUNE INFILTRATE AND SYSTEMIC IMMUNE MEDIATORS TO TREATMENT RESPONSE AND PROGNOSIS IN ADVANCED CERVICAL CANCER Article Open access 19 December 2023 CRUCIAL IMMUNOLOGICAL
ROLES OF THE INVASION FRONT IN INNATE AND ADAPTIVE IMMUNITY IN CERVICAL CANCER Article 29 October 2024 BACKGROUND Cervical cancer is a significant cause of women’s mortality with
approximately 569,847 new cases and 311,365 deaths annually worldwide.1 Currently, patients with advanced cervical cancer have limited therapeutic options. In recent years, extensive
epidemiological, laboratory, and clinical investigations have been undertaken to tackle this life-threatening problem. One notable progression is the ground-breaking discovery of high-risk
human papillomavirus (HPV) as a major aetiological factor for cervical cancer.2 The subsequent prophylactic HPV vaccination and effective screening of precancerous lesions followed by
preventive treatment have yielded a dramatic reduction in the late-stage disease incidence.3,4 Although we envision that cervical cancer will be eventually eliminated with these efforts,
until it can be optimistically accomplished after decades,5 basic scientific advances still need to be made and novel lifesaving medicines are imminently desired to overcome this global
threat of public health.6,7 It has been well established that tumour microenvironment, especially the immune milieu, plays a crucial role in modulating disease progression and response to
anticancer therapies.8,9,10 As expected, cellular and molecular indicators of positive immune activities are typically associated with long-term patient survival, and vice versa.11,12
Indeed, numerous studies have identified certain immune contexture or immunity-related gene signatures as prognostic biomarkers in a wide spectrum of human malignancies.13,14,15,16 Along
similar lines, accumulating data suggest that the baseline immunologic state within tumour lesions determines the clinical outcome following pharmacological interventions, ranging from
conventional chemotherapeutics to targeted compounds.17,18,19 These anticancer agents may in turn trigger immunogenic cell death and alter the composition and phenotype of intratumoural
immune infiltrates.20,21,22 By exerting cytotoxic effects, many anti-neoplastic drugs often have the tendency to stimulate the innate and acquired immune system, thereby facilitating tumour
eradication.23,24,25 In fact, the ultimate therapeutic efficacy as a result of administered regimens sometimes hinges on their capacity to engage functional immune circuitries and restore
immunosurveillance.26,27,28,29 Therefore, malignant cells commonly co-opt multiple evasion mechanisms to avoid immune attack,30,31,32,33 and a rational approach unleashing the
immunoreactivity holds considerable promise for potentially curative remedies, as exemplified by the recent emergence of cancer immunotherapies to reinstate the immunological control of
diverse neoplasms.34,35,36 Hallmarked by HPV-driven carcinogenesis, cervical cancer is presumed to possess immunogenicity by nature.37,38,39 Meanwhile, persistent viral infection could also
induce host immune tolerance, thus leading to a more complicated scenario.40,41 Surprisingly, in-depth characterisation of its immunologic landscape has been rather scanty with only a few
reports focussing on specific cell subsets.42,43,44,45 We reasoned that a more thorough understanding of the immune components and their interrelation with empirical treatments would provide
enormous opportunities for improving patient management and optimising therapeutic protocols. In this study, using immunohistochemical (IHC) staining and RNA sequencing (RNA-seq), we
systematically surveyed various immune cell populations present in different stages of cervical cancer at baseline or upon neoadjuvant chemotherapy (NACT). These integrated analyses allowed
for a critical evaluation of tumour-infiltrating immune profiles and might contribute to the ongoing development of immunomodulatory therapies in cervical cancer. METHODS PATIENT COHORT The
study was conducted in accordance with ethical guidelines of the U.S. Common Rule and was approved by the Ethics Committee of Ren Ji Hospital. Appropriate written informed consent was
obtained from each patient. All patients were treated at the Department of Obstetrics and Gynecology, Ren Ji Hospital, and their clinical records and tissue specimens were retrospectively
retrieved. Formalin-fixed and paraffin-embedded (FFPE) sections were obtained in pathologic examination. For RNA-seq analysis, fresh-frozen tumour tissues were collected during diagnostic
biopsy (pre-chemotherapy) and debulking surgery (post-chemotherapy). A total of 14 patients (28 paired samples) were assayed. Magnetic resonance imaging (MRI) data were provided by the
Department of Radiology, Ren Ji Hospital. Detailed clinical characteristics of the patient cohort are described in Supplementary Table 1. CHEMOTHERAPY RESPONSE EVALUATION The clinical
response to NACT was assessed according to the Response Evaluation Criteria in Solid Tumours. The evaluation was performed by an experienced radiologist (J.C.) on the basis of MRI images
following 1–2 cycles of chemotherapy treatment. A complete response (CR) was defined as the disappearance of the initial lesions. A partial response (PR) was defined as the detection of at
least a 30% reduction in the sum of the longest dimensions of the primary tumours. Progressive disease (PD) was defined as a >20% increase in the sum of the longest dimensions of the
target lesions or the development of new lesions. Stable disease (SD) implied that none of the above applied. Patients with CR or PR were defined as good responders, and patients with SD or
PD were defined as poor responders. IMMUNOHISTOCHEMISTRY IHC was performed on 5-μm-thick FFPE tissue sections. Slides were baked, deparaffinised in xylene, passed through graded alcohols,
and antigen retrieved with 10 mM citrate buffer, pH 6.0 in a steam pressure cooker. Pre-processed tissues were treated with peroxidase block (Dako) to quench endogenous peroxidase activity,
blocked using protein block (Dako), and incubated with primary antibodies (Supplementary Table 2). Slides were then washed in 50 mM Tris-HCl, pH 7.4 and incubated with horseradish
peroxidase-conjugated secondary antibodies. Immunoperoxidase staining was developed using the DAB system according to the manufacturer’s instructions (Dako). Slides were counterstained with
haematoxylin, dehydrated in graded alcohol and xylene, and cover-slipped using mounting solution. IHC STAINING QUANTIFICATION Areas of necrosis or artefacts were ignored. Microscopically,
the cell membrane in the slices was stained. The slides were examined using a bright field microscope and were scored using a four-point scale. First, for progression-free survival (PFS)
survival analysis, the immune cellular staining of each antibody was semi-quantitatively scored as ‘−’ (no or <5% positive cells), ‘+’ (5–25% positive cells), ‘++’ (26–50% positive
cells), and ‘+++’ (>50% positive cells). Both tumour and immune cell staining of programmed death-ligand 1 (PD-L1) were scored. The IHC signals were enumerated in ten random ×20 fields,
and cell counts were normalised to the area of tumour tissues. The samples with staining scores of ‘−’ were considered as the negative group, whereas those with staining scores of ‘+’, ‘++’,
and ‘+++’ were combined into the positive group. Second, in order to perform correlation analysis and quantitative comparison before and after NACT accurately, the slides were also scanned
with an Aperio ScanScope system (Leica Biosystems) and quantified using the Aperio ImageScope software v12.1 with Positive Pixel Count v9 (PPCv9) algorithm for statistical analysis. RNA-SEQ
AND ANALYSIS We performed RNA-seq analysis on 14 patients (28 samples) with matched pre- and post-chemotherapy fresh-frozen tissues. Total RNA was extracted from shavings of fresh-frozen
specimens using the RNeasy Plus Kit (Qiagen) according to the manufacturer’s protocol. RNA purity and integrity were assessed by the NanoPhotometer spectrophotometer (Implen) and RNA Nano
6000 Assay Kit on Bioanalyzer 2100 system (Agilent Technologies), respectively. Total RNAs with RNA Integrity Number of >8 were subjected to next-generation sequencing. Total amount of 3
µg RNA for each sample was used as input materials for library preparation with the NEBNext Ultra Directional RNA Library Prep Kit (NEB). The index-coded libraries were clustered on a cBot
Cluster Generation System using the TreSeq PE Cluster Kit v3-cBot-HS (Illumina) and sequenced on an Illumina Hiseq X Ten platform to generate 125 bp paired-end reads (Novogene). Clean data
were obtained from FastQ raw data by removing adapter, poly-N sequences, and low-quality reads. All the downstream analyses were based on the clean data with high quality. Index of the
reference genome was built using Bowtie v2.0.6 and paired-end clean reads were aligned to the reference genome (Ensembl hg38 human genome) using TopHat v2.0.9.46 The mapped reads of each
sample were assembled by Cufflinks (v2.1.1) in a reference-based approach.47 Differential expression analysis was performed using Cuffdiff (v2.1.1). P-values were adjusted using the
Benjamini–Hochberg procedure for controlling the false discovery rate. Genes with an adjusted _P_ value of <0.05 were considered differentially expressed. The sequencing data have been
deposited in the NCBI BioProject database (http://www.ncbi.nlm.nih.gov/bioproject/) under the accession number SRP173984. STATISTICAL ANALYSIS Statistical analyses were performed with the R
language and Graphpad Prism 6. Unsupervised hierarchical clustering was conducted to define the immune subtypes based on the evaluated markers. Pearson correlation analysis was used to test
the associations between different immune measurements. Cumulative survival rate was calculated by the Kaplan–Meier method and analysed by log-rank test. Cox proportional models were used to
determine the hazard ratio that represents the relative risk of events among patients in the different groups. Gene ontology and pathway analyses were performed using Metascape
(http://metascape.org).48 Single sample gene set enrichment analysis implemented in the Bioconductor ‘GSVA’ package was applied to generate compound scores for the indicated gene
signatures.49 CIBERSORT was employed to estimate the relative abundance of diverse immune cell infiltrates from gene expression profiles.50 Antigen receptor repertoire present in bulk
RNA-seq data was inferred by MiXCR.51 Comparisons between two conditions were based on two-sided Student’s _t_ test. _P_ values of <0.05 were judged to be statistically significant.
RESULTS PATIENT CHARACTERISTICS The study cohort contained 109 cases of cervical cancer with high-quality FFPE tissues and clinicopathological information available (Supplementary Table 1).
The median age of the patients was 52 years (range, 25–83 years). The histological diagnosis was mainly squamous cell carcinoma (89.9%) and adenocarcinoma (9.2%) of different International
Federation of Gynecology and Obstetrics stages (IA–IVA). Forty (36.7%) and sixty-nine (63.3%) subjects received upfront radical hysterectomy and NACT followed by surgery or
chemoradiotherapy, respectively (Supplementary Fig. 1a). We were able to obtain 92 treatment-naive samples and 60 chemo-exposed specimens from the diagnostic biopsies or surgical procedures,
among which 43 pairs were matched pre- and post-NACT tissues. In total, 152 (92 treatment-naive and 60 chemo-exposed) FFPE blocks underwent IHC examination, and 28 fresh-frozen tumours (14
pre-NACT and 14 post-NACT) from the NACT group were subjected to RNA-seq analysis (RJCC1–14, all squamous cell carcinomas). PATTERNS OF BASELINE IMMUNE INFILTRATES IN CERVICAL CANCER To
systematically analyse the immune makeup of cervical cancer, CIBERSORT,50 a computational method for inferring the relative abundance of diverse cell infiltrates from bulk tumour
transcriptomes, was initially conducted on the gene expression data (RJCC1–14, 28 data sets) profiled by RNA-seq. This framework pinpointed that the major representative immune cell types
were B cells, T cells, natural killer (NK) cells, and macrophages (Supplementary Fig. 1b). Based on these findings, we assembled a panel of monoclonal antibodies to probe each
subset-specific marker, as well as immune checkpoint molecules including programmed death-1 (PD-1) and PD-L1 (Supplementary Table 2). These eight immunologic parameters displayed divergent
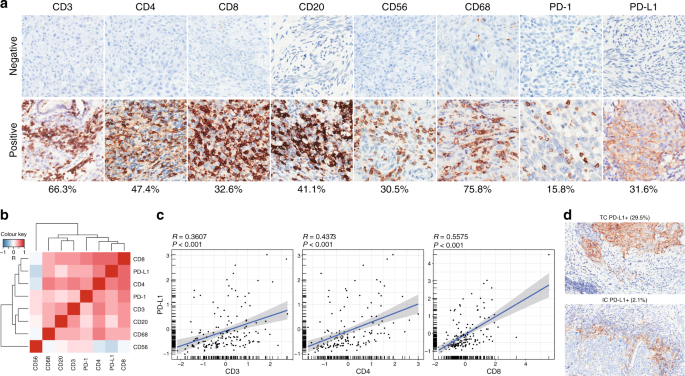
positive staining ratios in the 92 untreated samples. We observed CD3+ pan T cells (66.3%), CD4+ helper T cells (47.4%), CD20+ B cells (41.1%), and CD68+ macrophages (75.8%) in a prevalent
population of cervical tumours, whereas CD8+ cytotoxic T cells (32.6%), CD56+ NK cells (30.5%), PD-1 (15.8%), and PD-L1 (31.6%) signals were restricted to a smaller fraction of cancer
patients (Fig. 1a). While most immune markers were comparable between cervical adenocarcinoma and squamous cell carcinoma, histotype-specific CD20 and CD56 positivity was noted
(Supplementary Fig. 2). In order to better understand the complex immune characteristics in cervical cancer, we quantified the immune stains and assessed their interrelationships by
analysing pairwise correlation between the evaluated variables. Unsupervised hierarchical clustering of Pearson correlation coefficients (_R_) was visualised in a heatmap, which identified a
dominant array of co-modulated markers, including CD3, CD4, CD8, CD20, CD68, PD-1, and PD-L1 (Fig. 1b). There was a statistically significant positive correlation between PD-L1 intensity
and CD3+, CD4+, or CD8+ tumour-infiltrating lymphocytes (TILs) (Fig. 1c), consistent with the known role of T cell-derived cytotoxicity as a driver of PD-L1 expression.52 Among the 29 cases
(31.6%) showing PD-L1 staining, PD-L1 was mostly expressed on tumour cell surface (29.5%) and only sporadically detected in immune cells (2.1%; Fig. 1d). Taken together, these results
indicated that cervical cancer at baseline contained both innate and adaptive immune cells, as well as immune checkpoint expression within the tumour microenvironment. ASSOCIATION OF IMMUNE
INFILTRATES WITH PATIENT PROGNOSIS We explored the prognostic impact of baseline immune markers in cervical cancer and found CD8+ T cell infiltration as the most promising candidate to be
associated with beneficial clinical outcome regardless of neoadjuvant treatment (Supplementary Fig. 3a). We further considered the combination of immunologic features and performed
unsupervised hierarchical clustering of eight attributes. Four subgroups were revealed and arbitrarily designated as cluster 1 (16.8%), cluster 2 (37.9%), cluster 3 (26.3%), and cluster 4
(18.9%) (Fig. 2a). Cluster 1 (termed ‘immuno-active’) exhibited marked positivity for nearly all IHC markers other than CD56 and PD-1, hence resembling typical immunoreactive tumours (Fig.
2b). Cluster 2 (termed ‘immuno-medial’) showed moderate levels of immune contents (Fig. 2c). Cluster 3 (termed ‘immuno-deficient’) represented the immunologically inert prototype with low
immune cell densities (Fig. 2d). Cluster 4 (termed ‘immuno-NK’) was uniquely defined by prominent CD56+ NK cells (Fig. 2e). We found that the ‘immuno-deficient’ group (cluster 3) had
relatively shorter PFS than the ‘immuno-active’ group (cluster 1), and the ‘immuno-medial’ group (cluster 2) displayed intermediate risk of relapse (Fig. 2f). Interestingly, patients
categorised as ‘immuno-NK’ (cluster 4) demonstrated a PFS advantage compared to those in the ‘immuno-deficient’ and ‘immuno-medial’ classes (Fig. 2f). Of note, we discovered tertiary
lymphoid structures (TLSs) characterised by ectopic intratumoural aggregates of B and T lymphocytes (Fig. 2g and Supplementary Fig. 1b), which preferentially existed in the ‘immuno-active’
tumours (45.8%) and tended to correlate with improved PFS (Fig. 2h). Therefore, the magnitude and composition of baseline immune infiltrates aided the stratification of cervical cancer
patients into distinct molecular subtypes with prognostic relevance. IMMUNE AUGMENTATION UPON NACT Sixty-nine patients with locally advanced disease were first dosed with primary
chemotherapeutic regimens and subsequently evaluated to further receive surgical resection (62 patients) or chemoradiotherapy (7 patients). This neoadjuvant setting, although controversial,6
offered an unprecedented opportunity to investigate the potential impact of conventional systemic intervention on tumour microenvironment for rational combination with immunotherapeutics
and to explore the predictive determinants of chemosensitivity for patient-tailored medicine. To this end, we collected 60 specimens from debulking surgery after platinum-based doublets
(mostly cisplatin) and carried out IHC assessment using the same aforementioned antibody panel. Compared to the baseline (92 samples), cervical cancer following chemotherapy (60 samples)
experienced a significant reduction in Ki67 and PD-L1 positivity (Fig. 3a), in line with drug-invoked tumour cell death. By contrast, the densities of multiple immune markers, including CD4,
CD8, CD20, CD56, and PD-1, were evidently increased in chemo-treated samples (Fig. 3a). Treatment-conferred enrichment of CD4+, CD8+, CD20+, and CD56+ TIL was verified by performing paired
analysis (Fig. 3b) and inspecting representative IHC images (Fig. 3c) in the 43 cases with matched pre- and post-NACT sections. Although immunomodulatory effects of NACT were considerably
variable among these 43 individuals, an expansion of each immune cell population was noted in >50% of the patients without exception (Supplementary Fig. 4a). Of particular relevance, we
also observed CD14+ myeloid cell depletion by NACT (Supplementary Fig. 4b), which was shown to foster robust T cell reactivity in HPV16-based vaccination.53,54 In addition, TLSs were
markedly induced and arose de novo in some cases (Supplementary Fig. 4c). These data suggested that NACT fostered pronounced immune augmentation in cervical cancer. PATTERNS OF IMMUNE
AUGMENTATION UPON NACT We sought to delineate the overall patterns of immunostimulation by NACT in more detail. Analogous to the earlier immune profiles, the NACT cohort with paired samples
(43 patients) could be hierarchically divided into ‘immuno-active’, ‘immuno-medial’, ‘immuno-deficient’, and ‘immuno-NK’ subtypes as well. The most striking TIL accumulation occurred in the
initially classified ‘immuno-deficient’ tumours (Fig. 4a). Chemotherapeutic-elicited immunogenic phenotype was also manifested by frequent gain of CD56+ NK cells across all four molecular
clusters (Fig. 4a). We further compared TIL levels with respect to the clinical outcome by segregating NACT-treated patients (65 out of 69 evaluable) into good responders (with CR or PR) and
poor responders (with SD or PD) (Supplementary Fig. 5). As expected, 44 good responders exhibited better PFS than 21 poor responders (Fig. 4b). Of interest, decreased Ki67 and PD-L1
signals, as well as intensified CD4, CD8, CD20, CD56, and PD-1 staining, were specifically observed in good responders (Fig. 4c) but not in poor responders (Fig. 4d). Although the limited
number of cases and events did not allow for definitive assessment on the predictive value of immune augmentation, elevated abundance of diverse lymphatic cell populations, similar to the
TIL-enriched status regardless of medication, tended to be positively associated with chemotherapy response (Supplementary Fig. 6). Collectively, the immunomodulatory action of NACT was
affected by both baseline immunity and therapeutic efficacy. EVALUATION OF IMMUNOLOGIC PROPERTIES WITH RNA-SEQ We leveraged the RNA-seq data of 14 fresh-frozen sample pairs (RJCC1–14) to
validate the relationship between antitumour immunity and neoadjuvant treatment. NACT caused discrepant changes of gene expression in each patient and, across the cohort, resulted in 45
upregulated and 4 downregulated transcripts (Fig. 5a and Supplementary Fig. 7). Gene ontology and pathway analyses of differentially expressed genes pinpointed multiple significantly altered
modules related to immune activation upon chemotherapy, i.e. ‘TNFA signalling via NFKB’ and ‘inflammatory response’ (Fig. 5b). We conducted gene set variation analysis (GSVA) with
predefined transcriptional signatures for a range of biological processes and found that cell proliferation was indeed impaired, whereas relative amounts of CD8+ T cells, NK cells, and mast
cells were increased following NACT (Fig. 5c). In addition, by specifically analysing various immune checkpoints (Supplementary Fig. 8), we identified a significant upregulation of the
immunosuppressive CD200 molecule (Fig. 5d), which might serve as a potential immunotherapeutic target in chemo-treated cervical cancer. CIBERSORT algorithm confirmed the enlarged fractions
of CD4+ and CD8+ T cell subsets in the majority of residual lesions (Fig. 5e); on the contrary, there were relatively fewer remaining macrophages and T regulatory cells, both considered
negative mediators of immune function. Finally, the deep transcriptome sequencing enabled computational inference of antigen receptor diversities reflected by T cell receptor and
immunoglobulin repertoires using the MiXCR pipeline.51 More complementarity determining region 3 clonotypes were extracted from chemo-exposed tumours in 10 out of 14 sequenced subjects (Fig.
5f), implying enhanced lymphocyte infiltration. We concluded that the RNA-seq experiment substantially verified our IHC findings of chemotherapy-coupled immunostimulation in cervical
cancer. DISCUSSION In this study, by integrating IHC and RNA-seq analysis, we presented a rational approach for detailed interrogation of immune microenvironment in a large series of
cervical cancer. Our data revealed divergent baseline immunologic states that stratified patients into distinctive prognostic subgroups. Brief exposure and clinical response to NACT
seemingly incited a favourable reshaping of antitumour immunity against cervical carcinoma. These findings not only hold promise to better understand the impact of tumour–immune interactions
on disease behaviour and management but also provide the foundation to investigate synergistic treatment options of combining conventional chemotherapy with immunotherapeutic agents. We
employed a robust in silico deconvolution framework to estimate the immune constituents from bulk gene expression profiles,50 which indicated the highest degree of infiltrating B cells, T
cells, NK cells, and macrophages in cervical tumours. It is noteworthy that the computational measurements were at best approximate, and a definitive cellular composition and abundance can
be conceivably resolved using single-cell RNA-seq technology in the future.55,56 Nevertheless, these prevalent TIL populations were independently validated by immunostainings and
collectively segregated samples into four molecular subtypes. As with numerous other cancer types,57 cervical malignancies were vastly heterogeneous in the breadth of immune cell
infiltration. Remarkably, we found that B cells and T cells sporadically formed into TLSs, which were reported to play a direct role in the priming of antitumour immunity.58,59,60,61,62 In
addition, a unique subset of patients was revealed to contain disproportionate intratumoural NK cells and has exceptionally inferior risk of disease progression compared to other molecular
subtypes. This observation accords with the notion that NK cells are key to cancer immunosurveillance as both cytolytic effectors of the innate immune system and emerging regulators of the
adaptive immune cascade.63,64 Taken together, our in-depth characterisation of the immune portraits reinforced the immunogenic nature of virally driven cervical cancer. Chemotherapy,
including the mainstay cisplatin in cervical cancer, has traditionally been considered largely immunosuppressive due to its direct haematologic toxicity. However, such view is challenged by
cumulative evidence showing that it can enhance certain facets of locoregional immune response in a variety of human cancers.21,22,23,29,65 Along this line, we discovered that preoperative
chemotherapy indeed converted cervical lesion into a site permissive for antitumour immunity, as exemplified by selective enrichment of CD4+, CD8+, CD20+, and CD56+ TIL. The molecular
mechanisms underlying the inflammatory effects of cytotoxic chemotherapeutics have been predominantly attributed to the drug-evoked immunogenic cell death, involving for instance surface
calreticulin exposure,66 HMGB1 secretion,67 autophagic ATP release,68 NLRP3 inflammasome activation,69 cytokine production,70 and instigation of antigen-presenting dendritic cells.71
Alternatively, recent work showed that standard chemotherapy was able to reduce immunosuppressive myeloid cells and enhance T cell responses to therapeutic HPV16 vaccine in cervical
cancer.53,54 Of note, myriad preclinical and clinical studies have also unveiled differential immunostimulatory capacities of different chemotherapeutic agents.72,73 Given the pleiotropic
functions of chemotherapy, additional work is deserved to fully elucidate the mechanistic determinants responsible for the augmenting immune activities. Although ongoing trials with immune
checkpoint inhibitors in cervical cancer have shown early promising outcome, clinical responses are generally modest and variable.74 A disappointing 3% objective response rate (ORR) was
observed in a Phase 1/2 trial of 42 women who received ipilimumab (anti-CTLA-4) as monotherapy.75 In KEYNOTE-028 with pembrolizumab (anti-PD-1), ORR was 17% and median duration of response
was merely 5.4 months.76 Most recently, the KEYNOTE-158 Phase 2 basket trial presented an interim ORR of 12.2%, leading to the accelerated approval of pembrolizumab in advanced
PD-L1-positive cervical cancer.77 Overall, the potency of immune-based regimens is limited in unselected patient populations and should be tailored according to clinicopathological or
molecular attributes. Illuminated by current study, we propose the following paradigm shift toward precision immunotherapy for cervical cancer. The ‘immuno-active’ tumours may experience
spontaneous immunogenicity considering pronounced basal lymphocyte infiltration and PD-L1 expression and are likely poised to benefit from immunomodulatory medicine regardless of
chemotherapeutic education. For the ‘immuno-medial’ or ‘immuno-active’ subtypes, combined or induction chemotherapy is a plausible option to provoke iatrogenic immunogenicity. The
‘immuno-NK’ cluster illustrates an opportunity for pharmacological inhibition of NK cell checkpoints.78 Therefore, an improved understanding of the immune status at baseline and upon
specific treatments could yield valuable insights into more optimised and efficacious therapeutic modalities of cervical cancer. Several limitations of this exploratory study have to be
acknowledged. First, our investigations were retrospective in nature with potential biases owing to missing clinical records and unpredictable tissue availability. Second, these preliminary
results stemmed from one patient cohort in a single institution without internal and external validation data sets. Third, we mainly relied on conventional protein markers to define immune
cell types and ideally the corroborative RNA-seq analyses should have included a larger number of subjects. Finally, the prognostic and predictive significance of chemo-induced TIL
remodelling was underpowered to determine and future efforts with adequate sample size are warranted in this respect. CONCLUSIONS In summary, we provided for the first time a comprehensive
snapshot of baseline immunologic features within cervical tumour microenvironment and further uncovered the association between NACT effects and an immunostimulatory phenotype. Expanded
studies in the prospective setting are required to verify these findings, which may have clinical implications for tailoring immune-based treatment in women with cervical cancer. REFERENCES
* Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A. & Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers
in 185 countries. _CA Cancer J. Clin._ 68, 394–424 (2018). PubMed Google Scholar * Waggoner, S. E. Cervical cancer. _Lancet_ 361, 2217–2225 (2003). Article PubMed Google Scholar *
Crosbie, E. J., Einstein, M. H., Franceschi, S. & Kitchener, H. C. Human papillomavirus and cervical cancer. _Lancet_ 382, 889–899 (2013). Article PubMed Google Scholar * Ronco, G.,
Dillner, J., Elfstrom, K. M., Tunesi, S., Snijders, P. J., Arbyn, M. et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised
controlled trials. _Lancet_ 383, 524–532 (2014). Article PubMed Google Scholar * Simms, K. T., Steinberg, J., Caruana, M., Smith, M. A., Lew, J. B., Soerjomataram, I. et al. Impact of
scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020-99: a modelling study. _Lancet Oncol._
20, 394–440 (2019). Article PubMed Google Scholar * Cohen, P. A., Jhingran, A., Oaknin, A. & Denny, L. Cervical cancer. _Lancet_ 393, 169–182 (2019). Article PubMed Google Scholar
* Denny, L. Control of cancer of the cervix in low- and middle-income countries. _Ann. Surg. Oncol._ 22, 728–733 (2015). Article PubMed Google Scholar * Fridman, W. H., Galon, J., Pages,
F., Tartour, E., Sautes-Fridman, C. et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. _Cancer Res._ 71, 5601–5605 (2011). Article CAS PubMed Google
Scholar * Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. _Nat. Rev. Cancer_ 12, 298–306 (2012). Article
CAS PubMed Google Scholar * Galon, J., Angell, H. K., Bedognetti, D. & Marincola, F. M. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures.
_Immunity_ 39, 11–26 (2013). Article CAS PubMed Google Scholar * Gajewski, T. F., Schreiber, H. & Fu, Y. X. Innate and adaptive immune cells in the tumor microenvironment. _Nat.
Immunol._ 14, 1014–1022 (2013). Article CAS PubMed PubMed Central Google Scholar * Galon, J. & Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination
immunotherapies. _Nat. Rev. Drug Discov._ 18, 197–218 (2019). Article CAS PubMed Google Scholar * Galon, J., Costes, A., Sanchez-Cabo, F., Kirilovsky, A., Mlecnik, B., Lagorce-Pages, C.
et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. _Science_ 313, 1960–1964 (2006). Article CAS PubMed Google Scholar * Zhang,
L., Conejo-Garcia, J. R., Katsaros, D., Gimotty, P. A., Massobrio, M., Regnani, G. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. _N. Engl. J. Med._ 348,
203–213 (2003). Article CAS PubMed Google Scholar * Schalper, K. A., Brown, J., Carvajal-Hausdorf, D., McLaughlin, J., Velcheti, V., Syrigos, K. N. et al. Objective measurement and
clinical significance of TILs in non-small cell lung cancer. _J. Natl Cancer Inst._ 107, dju435 (2015). Article PubMed PubMed Central CAS Google Scholar * Mahmoud, S. M., Paish, E. C.,
Powe, D. G., Macmillan, R. D., Grainge, M. J., Lee, A. H. et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. _J. Clin. Oncol._ 29, 1949–1955 (2011).
Article PubMed Google Scholar * Fridman, W. H., Zitvogel, L., Sautes-Fridman, C. & Kroemer, G. The immune contexture in cancer prognosis and treatment. _Nat. Rev. Clin. Oncol._ 14,
717–734 (2017). Article CAS PubMed Google Scholar * Iwamoto, T., Bianchini, G., Booser, D., Qi, Y., Coutant, C., Shiang, C. Y. et al. Gene pathways associated with prognosis and
chemotherapy sensitivity in molecular subtypes of breast cancer. _J. Natl Cancer Inst._ 103, 264–272 (2011). Article CAS PubMed Google Scholar * Zitvogel, L., Kepp, O. & Kroemer, G.
Immune parameters affecting the efficacy of chemotherapeutic regimens. _Nat. Rev. Clin. Oncol._ 8, 151–160 (2011). Article CAS PubMed Google Scholar * Kroemer, G., Galluzzi, L., Kepp, O.
& Zitvogel, L. Immunogenic cell death in cancer therapy. _Annu. Rev. Immunol._ 31, 51–72 (2013). Article CAS PubMed Google Scholar * Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L.
& Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. _Cancer Cell_ 28, 690–714 (2015). Article CAS PubMed Google Scholar * de Biasi, A.
R., Villena-Vargas, J. & Adusumilli, P. S. Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. _Clin. Cancer Res._ 20, 5384–5391 (2014). Article
PubMed PubMed Central CAS Google Scholar * Galluzzi, L., Senovilla, L., Zitvogel, L. & Kroemer, G. The secret ally: immunostimulation by anticancer drugs. _Nat. Rev. Drug Discov._
11, 215–233 (2012). Article CAS PubMed Google Scholar * Krysko, D. V., Garg, A. D., Kaczmarek, A., Krysko, O., Agostinis, P. & Vandenabeele, P. Immunogenic cell death and DAMPs in
cancer therapy. _Nat. Rev. Cancer_ 12, 860–875 (2012). Article CAS PubMed Google Scholar * Frederick, D. T., Piris, A., Cogdill, A. P., Cooper, Z. A., Lezcano, C., Ferrone, C. R. et al.
BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. _Clin. Cancer Res._ 19, 1225–1231
(2013). Article CAS PubMed PubMed Central Google Scholar * Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease.
_Nat. Rev. Immunol._ 17, 97–111 (2017). Article CAS PubMed Google Scholar * Zitvogel, L., Rusakiewicz, S., Routy, B., Ayyoub, M. & Kroemer, G. Immunological off-target effects of
imatinib. _Nat. Rev. Clin. Oncol._ 13, 431–446 (2016). Article CAS PubMed Google Scholar * Knight, D. A., Ngiow, S. F., Li, M., Parmenter, T., Mok, S., Cass, A. et al. Host immunity
contributes to the anti-melanoma activity of BRAF inhibitors. _J. Clin. Investig._ 123, 1371–1381 (2013). Article CAS PubMed PubMed Central Google Scholar * Zitvogel, L., Galluzzi, L.,
Smyth, M. J. & Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. _Immunity_ 39, 74–88 (2013). Article CAS PubMed
Google Scholar * Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. _Science_ 331, 1565–1570 (2011).
Article CAS PubMed Google Scholar * Nirschl, C. J. & Drake, C. G. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer
immunotherapy. _Clin. Cancer Res._ 19, 4917–4924 (2013). Article CAS PubMed PubMed Central Google Scholar * Finn, O. J. Cancer immunology. _N. Engl. J. Med._ 358, 2704–2715 (2008).
Article CAS PubMed Google Scholar * Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. _Science_ 348, 74–80 (2015). Article CAS PubMed
Google Scholar * Sanmamed, M. F. & Chen, L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. _Cell_ 175, 313–326 (2018). Article CAS PubMed PubMed
Central Google Scholar * Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. _Cancer Discov._ 8, 1069–1086 (2018). Article PubMed
Google Scholar * Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. _Cell_ 161, 205–214 (2015). Article
CAS PubMed PubMed Central Google Scholar * Heusinkveld, M., Welters, M. J., van Poelgeest, M. I., van der Hulst, J. M., Melief, C. J., Fleuren, G. J. et al. The detection of circulating
human papillomavirus-specific T cells is associated with improved survival of patients with deeply infiltrating tumors. _Int. J. Cancer_ 128, 379–389 (2011). Article CAS PubMed Google
Scholar * Evans, E. M., Man, S., Evans, A. S. & Borysiewicz, L. K. Infiltration of cervical cancer tissue with human papillomavirus-specific cytotoxic T-lymphocytes. _Cancer Res._ 57,
2943–2950 (1997). CAS PubMed Google Scholar * van der Burg, S. H., Piersma, S. J., de Jong, A., van der Hulst, J. M., Kwappenberg, K. M., van den Hende, M. et al. Association of cervical
cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. _Proc. Natl Acad. Sci. USA_ 104, 12087–12092 (2007). Article PubMed CAS PubMed Central
Google Scholar * Tindle, R. W. Immune evasion in human papillomavirus-associated cervical cancer. _Nat. Rev. Cancer_ 2, 59–64 (2002). Article CAS PubMed Google Scholar * Karim, R.,
Jordanova, E. S., Piersma, S. J., Kenter, G. G., Chen, L., Boer, J. M. et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical
carcinoma. _Clin. Cancer Res._ 15, 6341–6347 (2009). Article CAS PubMed Google Scholar * de Vos van Steenwijk, P. J., Ramwadhdoebe, T. H., Goedemans, R., Doorduijn, E. M., van Ham, J.
J., Gorter, A. et al. Tumor-infiltrating CD14-positive myeloid cells and CD8-positive T-cells prolong survival in patients with cervical carcinoma. _Int. J. Cancer_ 133, 2884–2894 (2013).
PubMed Google Scholar * Jordanova, E. S., Gorter, A., Ayachi, O., Prins, F., Durrant, L. G., Kenter, G. G. et al. Human leukocyte antigen class I, MHC class I chain-related molecule A, and
CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? _Clin. Cancer Res._ 14, 2028–2035 (2008). Article CAS PubMed Google Scholar * Enwere, E.
K., Kornaga, E. N., Dean, M., Koulis, T. A., Phan, T., Kalantarian, M. et al. Expression of PD-L1 and presence of CD8-positive T cells in pre-treatment specimens of locally advanced cervical
cancer. _Mod. Pathol._ 30, 577–586 (2017). Article CAS PubMed Google Scholar * Heeren, A. M., van Luijk, I. F., Lakeman, J., Pocorni, N., Kole, J., de Menezes, R. X. et al. Neoadjuvant
cisplatin and paclitaxel modulate tumor-infiltrating T cells in patients with cervical cancer. _Cancer Immunol. Immunother._ 68, 1759–1767 (2019). Article CAS PubMed PubMed Central
Google Scholar * Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). Article CAS PubMed PubMed Central Google Scholar *
Trapnell, C., Roberts, A., Goff, L., Pertea, G., Kim, D., Kelley, D. R. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. _Nat.
Protoc._ 7, 562–578 (2012). Article CAS PubMed PubMed Central Google Scholar * Tripathi, S., Pohl, M. O., Zhou, Y., Rodriguez-Frandsen, A., Wang, G., Stein, D. A. et al. Meta- and
orthogonal integration of influenza “OMICs” data defines a role for UBR4 in virus budding. _Cell Host Microbe_ 18, 723–735 (2015). Article CAS PubMed PubMed Central Google Scholar *
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. _BMC Bioinformatics_ 14, 7 (2013). Article PubMed PubMed Central Google
Scholar * Gentles, A. J., Newman, A. M., Liu, C. L., Bratman, S. V., Feng, W., Kim, D. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. _Nat.
Med._ 21, 938–945 (2015). Article CAS PubMed PubMed Central Google Scholar * Bolotin, D. A., Poslavsky, S., Davydov, A. N., Frenkel, F. E., Fanchi, L., Zolotareva, O. I. et al. Antigen
receptor repertoire profiling from RNA-seq data. _Nat. Biotechnol._ 35, 908–911 (2017). Article CAS PubMed PubMed Central Google Scholar * Zou, W. & Chen, L. Inhibitory B7-family
molecules in the tumour microenvironment. _Nat. Rev. Immunol._ 8, 467–477 (2008). Article CAS PubMed Google Scholar * Welters, M. J., van der Sluis, T. C., van Meir, H., Loof, N. M., van
Ham, V. J., van Duikeren, S. et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. _Sci. Transl. Med._ 8, 334 (2016). Article CAS Google
Scholar * Melief, C. J. M., Welters, M. J. P., Vergote, I., Kroep, J. R., Kenter, G. G., Ottevanger, P. B. et al. Strong vaccine responses during chemotherapy are associated with prolonged
cancer survival. _Sci. Transl. Med._ 12, eaaz8235 (2020). Article CAS PubMed Google Scholar * Stubbington, M. J. T., Rozenblatt-Rosen, O., Regev, A. & Teichmann, S. A. Single-cell
transcriptomics to explore the immune system in health and disease. _Science_ 358, 58–63 (2017). Article CAS PubMed PubMed Central Google Scholar * Papalexi, E. & Satija, R.
Single-cell RNA sequencing to explore immune cell heterogeneity. _Nat. Rev. Immunol._ 18, 35–45 (2018). Article CAS PubMed Google Scholar * Binnewies, M., Roberts, E. W., Kersten, K.,
Chan, V., Fearon, D. F., Merad, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. _Nat. Med._ 24, 541–550 (2018). Article CAS PubMed PubMed Central
Google Scholar * Engelhard, V. H., Rodriguez, A. B., Mauldin, I. S., Woods, A. N., Peske, J. D. & Slingluff, C. L. Jr. Immune cell infiltration and tertiary lymphoid structures as
determinants of antitumor immunity. _J. Immunol._ 200, 432–442 (2018). Article CAS PubMed Google Scholar * Dieu-Nosjean, M. C., Goc, J., Giraldo, N. A., Sautes-Fridman, C. & Fridman,
W. H. Tertiary lymphoid structures in cancer and beyond. _Trends Immunol._ 35, 571–580 (2014). Article CAS PubMed Google Scholar * Dieu-Nosjean, M. C., Antoine, M., Danel, C., Heudes,
D., Wislez, M., Poulot, V. et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. _J. Clin. Oncol._ 26, 4410–4417 (2008). Article CAS
PubMed Google Scholar * Goc, J., Germain, C., Vo-Bourgais, T. K., Lupo, A., Klein, C., Knockaert, S. et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1
cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. _Cancer Res._ 74, 705–715 (2014). Article CAS PubMed Google Scholar * Kroeger, D. R.,
Milne, K. & Nelson, B. H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. _Clin.
Cancer Res._ 22, 3005–3015 (2016). Article CAS PubMed Google Scholar * Freud, A. G., Mundy-Bosse, B. L., Yu, J. & Caligiuri, M. A. The broad spectrum of human natural killer cell
diversity. _Immunity_ 47, 820–833 (2017). Article CAS PubMed PubMed Central Google Scholar * Poli, A., Michel, T., Patil, N. & Zimmer, J. Revisiting the functional impact of NK
cells. _Trends Immunol._ 39, 460–472 (2018). Article CAS PubMed Google Scholar * Khairallah, A. S., Genestie, C., Auguste, A. & Leary, A. Impact of neoadjuvant chemotherapy on the
immune microenvironment in advanced epithelial ovarian cancer: prognostic and therapeutic implications. _Int. J. Cancer_ 143, 8–15 (2018). Article CAS PubMed Google Scholar * Obeid, M.,
Tesniere, A., Ghiringhelli, F., Fimia, G. M., Apetoh, L., Perfettini, J. L. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. _Nat. Med._ 13, 54–61 (2007).
Article CAS PubMed Google Scholar * Apetoh, L., Ghiringhelli, F., Tesniere, A., Obeid, M., Ortiz, C., Criollo, A. et al. Toll-like receptor 4-dependent contribution of the immune system
to anticancer chemotherapy and radiotherapy. _Nat. Med._ 13, 1050–1059 (2007). Article CAS PubMed Google Scholar * Michaud, M., Martins, I., Sukkurwala, A. Q., Adjemian, S., Ma, Y.,
Pellegatti, P. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. _Science_ 334, 1573–1577 (2011). Article CAS PubMed Google Scholar *
Ghiringhelli, F., Apetoh, L., Tesniere, A., Aymeric, L., Ma, Y., Ortiz, C. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against
tumors. _Nat. Med._ 15, 1170–1178 (2009). Article CAS PubMed Google Scholar * Sistigu, A., Yamazaki, T., Vacchelli, E., Chaba, K., Enot, D. P., Adam, J. et al. Cancer cell-autonomous
contribution of type I interferon signaling to the efficacy of chemotherapy. _Nat. Med._ 20, 1301–1309 (2014). Article CAS PubMed Google Scholar * Shurin, G. V., Tourkova, I. L., Kaneno,
R. & Shurin, M. R. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. _J. Immunol._ 183, 137–144
(2009). Article CAS PubMed Google Scholar * Galluzzi, L., Humeau, J., Buque, A., Zitvogel, L., & Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint
inhibitors. _Nat. Rev. Clin. Oncol_. https://doi.org/10.1038/s41571-020-0413-z (2020). * Voorwerk, L., Slagter, M., Horlings, H. M., Sikorska, K., van de Vijver, K. K., de Maaker, M. et al.
Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. _Nat. Med._ 25, 920–928 (2019). Article CAS PubMed
Google Scholar * Dyer, B. A., Zamarin, D., Eskandar, R. N. & Mayadev, J. M. Role of immunotherapy in the management of locally advanced and recurrent/metastatic cervical cancer. _J.
Natl Compr. Cancer Netw._ 17, 91–97 (2019). Article CAS Google Scholar * Lheureux, S., Butler, M. O., Clarke, B., Cristea, M. C., Martin, L. P., Tonkin, K. et al. Association of
ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. _JAMA Oncol._ 4, e173776 (2018). Article PubMed Google
Scholar * Frenel, J. S., Le Tourneau, C., O’Neil, B., Ott, P. A., Piha-Paul, S. A., Gomez-Roca, C. et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand
1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. _J. Clin. Oncol._ 35, 4035–4041 (2017). Article CAS PubMed Google Scholar * Chung, H. C., Ros, W., Delord, J. P.,
Perets, R., Italiano, A., Shapira-Frommer, R. et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. _J.
Clin. Oncol_. https://doi.org/10.1200/JCO.18.01265 (2019). * Souza-Fonseca-Guimaraes, F., Cursons, J. & Huntington, N. D. The emergence of natural killer cells as a major target in
cancer immunotherapy. _Trends Immunol._ 40, 142–158 (2019). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors would like to thank Department of Pathology
and Biobank from Ren Ji Hospital for valuable assistance on coordinating IHC evaluation and preserving patient specimens. AUTHOR INFORMATION Author notes * These authors contributed
equally: Yi Zhang, Minhua Yu, Ying Jing AUTHORS AND AFFILIATIONS * State Key Laboratory of Oncogenes and Related Genes, Department of Obstetrics and Gynecology, Ren Ji Hospital, School of
Medicine, Shanghai Jiao Tong University, Shanghai, China Yi Zhang, Minhua Yu, Ying Jing, Lin Cheng, Haijiao Lu, Wenjing Wang, Weihua Lou, Lihua Qiu, Xia Yin, Guanglei Zhuang & Wen Di *
Shanghai Key Laboratory of Gynecologic Oncology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China Yi Zhang, Minhua Yu, Ying Jing, Lin Cheng, Haijiao Lu,
Wenjing Wang, Weihua Lou, Lihua Qiu, Xia Yin, Guanglei Zhuang & Wen Di * Department of Radiology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Jiejun Cheng * State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China Caiyan
Zhang & Mei-Chun Cai * Department of Pathology, The Affiliated Hospital of Qingdao University, Qingdao, China Jie Wu * Interdisciplinary Research Center on Biology and Chemistry,
Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, China Li Tan * Department of Gynecologic Oncology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University,
Guangzhou, China Huaiwu Lu Authors * Yi Zhang View author publications You can also search for this author inPubMed Google Scholar * Minhua Yu View author publications You can also search
for this author inPubMed Google Scholar * Ying Jing View author publications You can also search for this author inPubMed Google Scholar * Jiejun Cheng View author publications You can also
search for this author inPubMed Google Scholar * Caiyan Zhang View author publications You can also search for this author inPubMed Google Scholar * Lin Cheng View author publications You
can also search for this author inPubMed Google Scholar * Haijiao Lu View author publications You can also search for this author inPubMed Google Scholar * Mei-Chun Cai View author
publications You can also search for this author inPubMed Google Scholar * Jie Wu View author publications You can also search for this author inPubMed Google Scholar * Wenjing Wang View
author publications You can also search for this author inPubMed Google Scholar * Weihua Lou View author publications You can also search for this author inPubMed Google Scholar * Lihua Qiu
View author publications You can also search for this author inPubMed Google Scholar * Li Tan View author publications You can also search for this author inPubMed Google Scholar * Huaiwu Lu
View author publications You can also search for this author inPubMed Google Scholar * Xia Yin View author publications You can also search for this author inPubMed Google Scholar *
Guanglei Zhuang View author publications You can also search for this author inPubMed Google Scholar * Wen Di View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS Conception and design: X.Y., G.Z.; development of method: M.-C.C., L.T.; acquisition of data: Y.J., J.C., C.Z., L.C., H.L., M.-C.C., J.W., W.W.; analysis and
interpretation of data: Y.Z., M.Y., W.L., L.Q., H.L.; writing, review, and/or revision of manuscript: Y.Z., G.Z., W.D.; administrative, technical, or material support: W.L., L.Q., H.L.;
study supervision and approval of final version: W.D. All authors read and approved the final manuscript. CORRESPONDING AUTHORS Correspondence to Xia Yin, Guanglei Zhuang or Wen Di. ETHICS
DECLARATIONS ETHICS APPROVAL AND CONSENT TO PARTICIPATE Patient samples were obtained with informed consent at Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University. Studies
were performed under protocols approved by Ethics Committee of Ren Ji Hospital. Studies were performed in accordance with the Declaration of Helsinki. DATA AVAILABILITY All reagents used in
this study were commercially available. The RNA sequencing data have been deposited in NCBI BioProject database (http://www.ncbi.nlm.nih.gov/bioproject/) under the accession number
SRP173984. COMPETING INTERESTS The authors declare no competing interests. FUNDING INFORMATION This work was supported by the National Natural Science Foundation of China (81672714 and
81922047 to G.Z., 81772770 to W.D.); National Key Research and Development Program (863) of China (2016YFC1302900 to W.D.); National Program on Key Basic Research Project of China
(2016YFA0501900 and 2016YFA0501904 to L.T.); the Shanghai Municipal Key Clinical Specialty and the Program of Shanghai Hospital Development Center (16CR2001A to W.D.); the grants from
Shanghai Jiao Tong University School of Medicine (DLY201505 to W.D., YG2016MS51 to X.Y.); Doctoral Innovation Fund of Shanghai Jiao Tong University School of Medicine (CBXJ201805 to Y.Z.);
Shanghai Pujiang Program (17PJ1410700 to L.T.); Shanghai Municipal Commission of Health and Family Planning (2017ZZ02016 and ZY(2018-2020)-FWTX-3006 to W.D., 20174Y0043 to M.-C.C.,
20174Y0189 to Y.J.); and the grants from Science and Technology Commission of Shanghai Municipality (18441904800 to W.D., 16140904401 to X.Y.). ADDITIONAL INFORMATION NOTE This work is
published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0
International (CC BY 4.0). PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY FILES RIGHTS AND PERMISSIONS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Zhang, Y., Yu, M., Jing, Y. _et al._ Baseline immunity and impact of chemotherapy on immune microenvironment in cervical cancer. _Br J Cancer_ 124, 414–424 (2021).
https://doi.org/10.1038/s41416-020-01123-w Download citation * Received: 04 April 2020 * Revised: 01 October 2020 * Accepted: 02 October 2020 * Published: 22 October 2020 * Issue Date: 19
January 2021 * DOI: https://doi.org/10.1038/s41416-020-01123-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative