Play all audios:
ABSTRACT Ductal carcinoma in situ (DCIS) is a non-obligate precursor of invasive carcinoma. Multiple studies have shown that DCIS lesions typically possess a driver mutation associated with
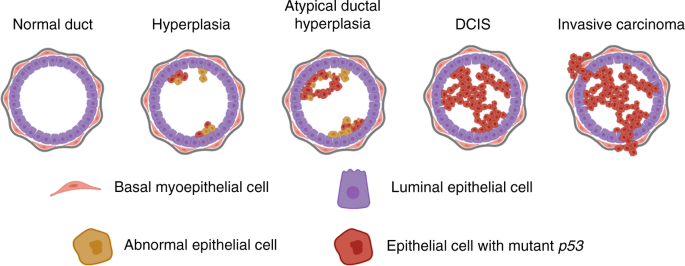
cancer development. Mutation in the _TP53_ tumour suppressor gene is present in 15–30% of pure DCIS lesions and in ~30% of invasive breast cancers. Mutations in _TP53_ are significantly
associated with high-grade DCIS, the most likely form of DCIS to progress to invasive carcinoma. In this review, we summarise published evidence on the prevalence of mutant _TP53_ in DCIS
(including all DCIS subtypes), discuss the availability of mouse models for the study of DCIS and highlight the need for functional studies of the role of _TP53_ in the development of DCIS
and progression from DCIS to invasive disease. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Subscribe to this journal Receive 24 print issues and online access $259.00 per year only $10.79 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MOLECULAR SIGNATURES OF IN SITU TO INVASIVE PROGRESSION FOR BASAL-LIKE BREAST CANCERS: AN
INTEGRATED MOUSE MODEL AND HUMAN DCIS STUDY Article Open access 18 July 2022 EPIGENETIC ACTIVATION OF SOX11 IS ASSOCIATED WITH RECURRENCE AND PROGRESSION OF DUCTAL CARCINOMA IN SITU TO
INVASIVE BREAST CANCER Article 17 May 2024 PROGRESSION FROM DUCTAL CARCINOMA IN SITU TO INVASIVE BREAST CANCER: MOLECULAR FEATURES AND CLINICAL SIGNIFICANCE Article Open access 03 April 2024
REFERENCES * Schnitt SJ. Local outcomes in ductal carcinoma in situ based on patient and tumor characteristics. JNCI Monogr. 2010;2010:158–61. https://doi.org/10.1093/jncimonographs/lgq031.
Article Google Scholar * Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. JNCI Monogr. 2010;2010:134–8.
https://doi.org/10.1093/jncimonographs/lgq035. Article Google Scholar * Alsheh Ali M, Czene K, Hall P, Humphreys K. Association of microcalcification clusters with short-term invasive
breast cancer risk and breast cancer risk factors. Sci Rep. 2019;9:14604 http://www.nature.com/articles/s41598-019-51186-w. Article PubMed PubMed Central Google Scholar * Bent CK,
Bassett LW, D’Orsi CJ, Sayre JW. The positive predictive value of BI-RADS microcalcification descriptors and final assessment categories. Am J Roentgenol. 2010;194:1378–83.
https://doi.org/10.2214/AJR.09.3423. Article Google Scholar * Burnside ES, Ochsner JE, Fowler KJ, Fine JP, Salkowski LR, Rubin DL, et al. Use of microcalcification descriptors in BI-RADS
4th edition to stratify risk of malignancy. Radiology. 2007;242:388–95. https://doi.org/10.1148/radiol.2422052130. Article PubMed Google Scholar * Uematsu T, Kasami M, Yuen S. Usefulness
and limitations of the Japan Mammography Guidelines for the categorization of microcalcifications. Breast Cancer. 2008;15:291–7. https://doi.org/10.1007/s12282-008-0033-4. Article PubMed
Google Scholar * van Seijen M, Lips EH, Thompson AM, Nik-Zainal S, Futreal A, Hwang ES, et al. Ductal carcinoma in situ: to treat or not to treat, that is the question. Br J Cancer.
2019;121:285–92. http://www.nature.com/articles/s41416-019-0478-6. Article PubMed PubMed Central Google Scholar * Goh CW, Wu J, Ding S, Lin C, Chen X, Huang O, et al. Invasive ductal
carcinoma with coexisting ductal carcinoma in situ (IDC/DCIS) versus pure invasive ductal carcinoma (IDC): a comparison of clinicopathological characteristics, molecular subtypes, and
clinical outcomes. J Cancer Res Clin Oncol. 2019;145:1877–86. https://doi.org/10.1007/s00432-019-02930-2. Article PubMed Google Scholar * Casasent AK, Edgerton M, Navin NE. Genome
evolution in ductal carcinoma in situ: invasion of the clones. J Pathol. 2017;241:208–18. https://doi.org/10.1002/path.4840. Article PubMed Google Scholar * Tozbikian G, Brogi E, Vallejo
CE, Giri D, Murray M, Catalano J, et al. Atypical ductal hyperplasia bordering on ductal carcinoma in situ. Int J Surg Pathol. 2017;25:100–7. https://doi.org/10.1177/1066896916662154.
Article PubMed Google Scholar * Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clin Med Insights Pathol. 2015;8:CPath.S31563.
https://doi.org/10.4137/CPath.S31563. * Norton K-A, Wininger M, Bhanot G, Ganesan S, Barnard N, Shinbrot T. A 2D mechanistic model of breast ductal carcinoma in situ (DCIS) morphology and
progression. J Theor Biol. 2010;263:393–406. https://linkinghub.elsevier.com/retrieve/pii/S0022519309005669. Article PubMed Google Scholar * Salvatorelli L, Puzzo L, Vecchio GM,
Caltabiano R, Virzì V, Magro G. Ductal carcinoma in situ of the breast: an update with emphasis on radiological and morphological features as predictive prognostic factors. Cancers (Basel).
2020;12:609 https://www.mdpi.com/2072-6694/12/3/609. Article CAS PubMed Central Google Scholar * Maxwell AJ, Clements K, Hilton B, Dodwell DJ, Evans A, Kearins O, et al. Risk factors for
the development of invasive cancer in unresected ductal carcinoma in situ. Eur J Surg Oncol. 2018;44:429–35. https://linkinghub.elsevier.com/retrieve/pii/S074879831830009X. Article PubMed
Google Scholar * Gorringe KL, Fox SB. Ductal carcinoma in situ biology, biomarkers, and diagnosis. Front Oncol. 2017. https://doi.org/10.3389/fonc.2017.00248/full. * Harrison BT, Hwang
ES, Partridge AH, Thompson AM, Schnitt SJ. Variability in diagnostic threshold for comedo necrosis among breast pathologists: implications for patient eligibility for active surveillance
trials of ductal carcinoma in situ. Mod Pathol. 2019;32:1257–62. http://www.ncbi.nlm.nih.gov/pubmed/30980039. Article PubMed Google Scholar * van Seijen M, Jóźwiak K, Pinder SE, Hall A,
Krishnamurthy S, Thomas JS, et al. Variability in grading of ductal carcinoma in situ among an international group of pathologists. J Pathol Clin Res. 2021;7:233–42.
http://www.ncbi.nlm.nih.gov/pubmed/33620141. Article PubMed PubMed Central Google Scholar * Thompson AM, Clements K, Cheung S, Pinder SE, Lawrence G, Sawyer E, et al. Management and
5-year outcomes in 9938 women with screen-detected ductal carcinoma in situ: the UK Sloane Project. Eur J Cancer. 2018;101:210–9.
https://linkinghub.elsevier.com/retrieve/pii/S0959804918309419. Article PubMed Google Scholar * Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms
of breast cancer screening: an independent review. Br J Cancer. 2013;108:2205–40. http://www.nature.com/articles/bjc2013177. Article CAS PubMed PubMed Central Google Scholar * Ryser MD,
Weaver DL, Zhao F, Worni M, Grimm LJ, Gulati R, et al. Cancer outcomes in DCIS patients without locoregional treatment. JNCI J Natl Cancer Inst. 2019;111:952–60.
https://academic.oup.com/jnci/article/111/9/952/5318677. Article PubMed Google Scholar * Kanbayashi C, Thompson AM, Hwang E-SS, Partridge AH, Rea DW, Wesseling J, et al. The international
collaboration of active surveillance trials for low-risk DCIS (LORIS, LORD, COMET, LORETTA). J Clin Oncol. 2019;37:TPS603–TPS603. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS603.
Article Google Scholar * Hwang ES, Hyslop T, Lynch T, Frank E, Pinto D, Basila D, et al. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III
randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open. 2019;9:e026797 http://www.ncbi.nlm.nih.gov/pubmed/30862637. Article PubMed PubMed Central
Google Scholar * Elshof LE, Tryfonidis K, Slaets L, van Leeuwen-Stok AE, Skinner VP, Dif N, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase
III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ – The LORD study. Eur J Cancer. 2015;51:1497–510.
https://linkinghub.elsevier.com/retrieve/pii/S0959804915003949. Article PubMed Google Scholar * Francis A, Thomas J, Fallowfield L, Wallis M, Bartlett JMS, Brookes C, et al. Addressing
overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer. 2015;51:2296–303. https://linkinghub.elsevier.com/retrieve/pii/S0959804915006978. Article PubMed Google Scholar * Van
Bockstal MR, Berlière M, Duhoux FP, Galant C. Interobserver variability in ductal carcinoma in situ of the breast. Am J Clin Pathol. 2020;154:596–609.
https://academic.oup.com/ajcp/article/154/5/596/5860662. Article PubMed Google Scholar * Kanbayashi C, Iwata H. Current approach and future perspective for ductal carcinoma in situ of the
breast. Jpn J Clin Oncol. 2017;47:671–7. https://academic.oup.com/jjco/article/47/8/671/3807293*. Article PubMed PubMed Central Google Scholar * Pang J-MB, Savas P, Fellowes AP, Mir
Arnau G, Kader T, Vedururu R, et al. Breast ductal carcinoma in situ carry mutational driver events representative of invasive breast cancer. Mod Pathol. 2017;30:952–63.
http://www.nature.com/articles/modpathol201721. Article CAS PubMed Google Scholar * Bergholtz H, Kumar S, Wärnberg F, Lüders T, Kristensen V, Sørlie T. Comparable cancer‐relevant
mutation profiles in synchronous ductal carcinoma in situ and invasive breast cancer. Cancer Rep. 2020. https://doi.org/10.1002/cnr2.1248. * Zhu C, Hu H, Li J, Wang J, Wang K, Sun J.
Identification of key differentially expressed genes and gene mutations in breast ductal carcinoma in situ using RNA-seq analysis. World J Surg Oncol. 2020;18:52
https://doi.org/10.1186/s12957-020-01820-z. Article PubMed PubMed Central Google Scholar * Kaur H, Mao S, Li Q, Sameni M, Krawetz SA, Sloane BF, et al. RNA-seq of human breast ductal
carcinoma in situ models reveals aldehyde dehydrogenase isoform 5A1 as a novel potential target. PLoS ONE. 2012;7:e50249. https://doi.org/10.1371/journal.pone.0050249. * van der Groep P, van
Diest PJ, Menko FH, Bart J, de Vries EGE, van der Wall E. Molecular profile of ductal carcinoma in situ of the breast in BRCA1 and BRCA2 germline mutation carriers. J Clin Pathol.
2009;62:926–30. https://doi.org/10.1136/jcp.2009.065524. Article PubMed Google Scholar * Petridis C, Arora I, Shah V, Megalios A, Moss C, Mera A, et al. Frequency of pathogenic germline
variants in BRCA1, BRCA2, PALB2, CHEK2 and TP53 in ductal carcinoma in situ diagnosed in women under the age of 50 years. Breast Cancer Res. 2019;21:58
https://doi.org/10.1186/s13058-019-1143-y. Article PubMed PubMed Central Google Scholar * Kuba MG, Lester SC, Bowman T, Stokes SM, Taneja KL, Garber JE, et al. Histopathologic features
of breast cancer in Li–Fraumeni syndrome. Mod Pathol. 2021;34:542–8. http://www.nature.com/articles/s41379-020-0610-4. Article CAS PubMed Google Scholar * Casasent AK, Schalck A, Gao R,
Sei E, Long A, Pangburn W, et al. Multiclonal invasion in breast tumors identified by topographic single cell sequencing. Cell. 2018;172:205–.e12.
http://www.ncbi.nlm.nih.gov/pubmed/29307488. Article CAS PubMed PubMed Central Google Scholar * Lane DP. p53, guardian of the genome. Nature. 1992;358:15–6.
http://www.nature.com/articles/358015a0. Article CAS PubMed Google Scholar * Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol.
2009;1:a001883–a001883. https://doi.org/10.1101/cshperspect.a001883. Article CAS PubMed PubMed Central Google Scholar * Moyer SM, Wasylishen AR, Qi Y, Fowlkes N, Su X, Lozano G. p53
drives a transcriptional program that elicits a non-cell-autonomous response and alters cell state in vivo. Proc Natl Acad Sci USA. 2020;117:23663–73.
https://doi.org/10.1073/pnas.2008474117. Article PubMed PubMed Central Google Scholar * Liu Y, Gu W. p53 in ferroptosis regulation: the new weapon for the old guardian. Cell Death
Differ. 2022;29:895–910. https://www.nature.com/articles/s41418-022-00943-y. Article CAS PubMed Google Scholar * Silwal-Pandit L, Vollan HKM, Chin S-F, Rueda OM, McKinney S, Osako T, et
al. TP53 mutation spectrum in breast cancer is subtype specific and has distinct prognostic relevance. Clin Cancer Res. 2014;20:3569–80. https://doi.org/10.1158/1078-0432.CCR-13-2943.
Article CAS PubMed Google Scholar * Wasylishen AR, Lozano G. Attenuating the p53 pathway in human cancers: many means to the same end. Cold Spring Harb Perspect Med. 2016;6:a026211
https://doi.org/10.1101/cshperspect.a026211. Article CAS PubMed PubMed Central Google Scholar * Abubakar M, Guo C, Koka H, Sung H, Shao N, Guida J, et al. Clinicopathological and
epidemiological significance of breast cancer subtype reclassification based on p53 immunohistochemical expression. npj Breast Cancer. 2019;5:20
http://www.nature.com/articles/s41523-019-0117-7. Article PubMed PubMed Central Google Scholar * Gencel-Augusto J, Lozano G. p53 tetramerization: at the center of the dominant-negative
effect of mutant p53. Genes Dev. 2020;34:1128–46. https://doi.org/10.1101/gad.340976.120. Article CAS PubMed PubMed Central Google Scholar * Kim MP, Lozano G. Mutant p53 partners in
crime. Cell Death Differ. 2018;25:161–8. http://www.nature.com/articles/cdd2017185. Article CAS PubMed Google Scholar * Brosh R, Rotter V. When mutants gain new powers: news from the
mutant p53 field. Nat Rev Cancer. 2009;9:701–13. http://www.nature.com/articles/nrc2693. Article CAS PubMed Google Scholar * Lang GA, Iwakuma T, Suh Y-A, Liu G, Rao VA, Parant JM, et al.
Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–72. https://linkinghub.elsevier.com/retrieve/pii/S0092867404010487. Article CAS
PubMed Google Scholar * Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–60.
https://linkinghub.elsevier.com/retrieve/pii/S0092867404010463. Article CAS PubMed Google Scholar * O’Malley FP, Vnencak-Jones CL, Dupont WD, Parl F, Manning S, Page DL. p53 mutations
are confined to the comedo type ductal carcinoma in situ of the breast. Immunohistochemical and sequencing data. Lab Invest. 1994;71:67–72. http://www.ncbi.nlm.nih.gov/pubmed/8041120. PubMed
Google Scholar * Rajan PB, Scott DJ, Perry RH, Griffith CDM. p53 protein expression in ductal carcinoma in situ (DCIS) of the breast. Breast Cancer Res Treat. 1997;42:283–90.
https://doi.org/10.1023/A:1005741723479. Article CAS PubMed Google Scholar * Done SJ, Eskandarian S, Bull S, Redston M, Andrulis IL. p53 missense mutations in microdissected high-grade
ductal carcinoma in situ of the breast. JNCI J Natl Cancer Inst. 2001;93:700–4. https://doi.org/10.1093/jnci/93.9.700. Article CAS PubMed Google Scholar * Visser LL, Elshof LE, Van de
Vijver K, Groen EJ, Almekinders MM, Sanders J, et al. Discordant marker expression between invasive breast carcinoma and corresponding synchronous and preceding DCIS. Am J Surg Pathol.
2019;43:1574–82. http://journals.lww.com/00000478-201911000-00015. Article PubMed Google Scholar * Radford DM, Fair K, Thompson AM, Ritter JH, Holt M, Steinbrueck T, et al. Allelic loss
on a chromosome 17 in ductal carcinoma in situ of the breast. Cancer Res. 1993;53:2947–9. http://www.ncbi.nlm.nih.gov/pubmed/8391383. CAS PubMed Google Scholar * Bouchalova P, Nenutil R,
Muller P, Hrstka R, Appleyard MV, Murray K, et al. Mutant p53 accumulation in human breast cancer is not an intrinsic property or dependent on structural or functional disruption but is
regulated by exogenous stress and receptor status. J Pathol. 2014;233:238–46. https://doi.org/10.1002/path.4356. Article CAS PubMed Google Scholar * Pareja F, Brown DN, Lee JY, Da Cruz
Paula A, Selenica P, Bi R, et al. Whole-exome sequencing analysis of the progression from non–low-grade ductal carcinoma in situ to invasive ductal carcinoma. Clin Cancer Res.
2020;26:3682–93. https://doi.org/10.1158/1078-0432.CCR-19-2563. Article CAS PubMed PubMed Central Google Scholar * Zhou W, Muggerud AA, Vu P, Due EU, Sørlie T, Børresen-Dale A-L, et al.
Full sequencing of TP53 identifies identical mutations within in situ and invasive components in breast cancer suggesting clonal evolution. Mol Oncol. 2009;3:214–9.
https://doi.org/10.1016/j.molonc.2009.03.001. Article CAS PubMed PubMed Central Google Scholar * Abba MC, Gong T, Lu Y, Lee J, Zhong Y, Lacunza E, et al. A molecular portrait of
high-grade ductal carcinoma In situ. Cancer Res. 2015;75:3980–90. https://doi.org/10.1158/0008-5472.CAN-15-0506. Article CAS PubMed PubMed Central Google Scholar * Pereira B, Chin S-F,
Rueda OM, Vollan H-KM, Provenzano E, Bardwell HA, et al. The somatic mutation profiles of 2,433 breast cancers refine their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479
http://www.nature.com/articles/ncomms11479. Article CAS PubMed PubMed Central Google Scholar * Curtis C, Shah SP, Chin S-F, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and
transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. http://www.nature.com/articles/nature10983. Article CAS PubMed PubMed Central Google
Scholar * Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data: Fig. 1.
Cancer Discov. 2012;2:401–4. https://doi.org/10.1158/2159-8290.CD-12-0095. Article PubMed Google Scholar * Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative
analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6. https://doi.org/10.1126/scisignal.2004088. * Lukas J, Niu N, Press MF. p53 mutations and
expression in breast carcinoma in situ. Am J Pathol. 2000;156:183–91. https://linkinghub.elsevier.com/retrieve/pii/S0002944010647189. Article CAS PubMed PubMed Central Google Scholar *
Done SJ, Arneson NC, Ozçelik H, Redston M, Andrulis IL. p53 mutations in mammary ductal carcinoma in situ but not in epithelial hyperplasias. Cancer Res. 1998;58:785–9.
http://www.ncbi.nlm.nih.gov/pubmed/9485035. CAS PubMed Google Scholar * Lips EH, Kumar T, Megalios A, Visser LL, Sheinman M, Fortunato A, et al. Genomic analysis defines clonal
relationships of ductal carcinoma in situ and recurrent invasive breast cancer. Nat Genet. 2022. https://www.nature.com/articles/s41588-022-01082-3. * Guy CT, Cardiff RD, Muller WJ.
Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61.
http://www.ncbi.nlm.nih.gov/pubmed/1312220. CAS PubMed PubMed Central Google Scholar * Green JE, Shibata MA, Yoshidome K, Liu ML, Jorcyk C, Anver MR, et al. The C3(1)/SV40 T-antigen
transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene. 2000;19:1020–7. http://www.ncbi.nlm.nih.gov/pubmed/10713685.
Article CAS PubMed Google Scholar * Schulze-Garg C, Löhler J, Gocht A, Deppert W. A transgenic mouse model for the ductal carcinoma in situ (DCIS) of the mammary gland. Oncogene.
2000;19:1028–37. http://www.ncbi.nlm.nih.gov/pubmed/10713686. Article CAS PubMed Google Scholar * Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, et al. Transgenic
Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–305. http://www.ncbi.nlm.nih.gov/pubmed/11719463. CAS PubMed Google Scholar * Maglione JE, McGoldrick
ET, Young LJT, Namba R, Gregg JP, Liu L, et al. Polyomavirus middle T-induced mammary intraepithelial neoplasia outgrowths: single origin, divergent evolution, and multiple outcomes. Mol
Cancer Ther. 2004;3:941–53. http://www.ncbi.nlm.nih.gov/pubmed/15299077. Article CAS PubMed Google Scholar * Namba R, Maglione JE, Young LJT, Borowsky AD, Cardiff RD, MacLeod CL, et al.
Molecular characterization of the transition to malignancy in a genetically engineered mouse-based model of ductal carcinoma in situ. Mol Cancer Res. 2004;2:453–63.
http://www.ncbi.nlm.nih.gov/pubmed/15328372. Article CAS PubMed Google Scholar * Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, et al. Mice deficient for p53
are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. http://www.nature.com/articles/356215a0. Article CAS PubMed Google Scholar * Jacks T,
Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7.
https://linkinghub.elsevier.com/retrieve/pii/S0960982200000026. Article CAS PubMed Google Scholar * Koch JG, Gu X, Han Y, El-Naggar AK, Olson MV, Medina D, et al. Mammary tumor modifiers
in BALB/cJ mice heterozygous for p53. Mamm Genome. 2007;18:300–9. http://link.springer.com/10.1007/s00335-007-9028-2. Article CAS PubMed Google Scholar * Kuperwasser C, Hurlbut GD,
Kittrell FS, Dickinson ES, Laucirica R, Medina D, et al. Development of spontaneous mammary tumors in BALB/c p53 heterozygous mice. Am J Pathol. 2000;157:2151–9.
https://linkinghub.elsevier.com/retrieve/pii/S0002944010648535. Article CAS PubMed PubMed Central Google Scholar * Medina D, Kittrell FS, Shepard A, Stephens LC, Jiang C, Lu J, et al.
Biological and genetic properties of the p53 null preneoplastic mammary epithelium. FASEB J. 2002;16:881–3. https://doi.org/10.1096/fj.01-0885fje. Article CAS PubMed Google Scholar *
Ross SR. Mouse mammary tumor virus molecular biology and oncogenesis. Viruses. 2010;2:2000–12. http://www.mdpi.com/1999-4915/2/9/2000. Article CAS PubMed PubMed Central Google Scholar *
Lu X, Liu DP, Xu Y. The gain of function of p53 cancer mutant in promoting mammary tumorigenesis. Oncogene. 2013;32:2900–6. http://www.ncbi.nlm.nih.gov/pubmed/22824795. Article CAS PubMed
Google Scholar * Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:1–9.
https://www.hindawi.com/journals/mbi/2014/852748/. Article Google Scholar * Yallowitz AR, Li D, Lobko A, Mott D, Nemajerova A, Marchenko N. Mutant p53 amplifies epidermal growth factor
receptor family signaling to promote mammary tumorigenesis. Mol Cancer Res. 2015;13:743–54. http://www.ncbi.nlm.nih.gov/pubmed/25573952. Article CAS PubMed PubMed Central Google Scholar
* Alexandrova EM, Yallowitz AR, Li D, Xu S, Schulz R, Proia DA, et al. Improving survival by exploiting tumour dependence on stabilized mutant p53 for treatment. Nature. 2015;523:352–6.
http://www.nature.com/articles/nature14430. Article CAS PubMed PubMed Central Google Scholar * Alexandrova EM, Mirza SA, Xu S, Schulz-Heddergott R, Marchenko ND, Moll UM. p53
loss-of-heterozygosity is a necessary prerequisite for mutant p53 stabilization and gain-of-function in vivo. Cell Death Dis. 2017;8:e2661 http://www.ncbi.nlm.nih.gov/pubmed/28277540.
Article CAS PubMed PubMed Central Google Scholar * Wijnhoven SWP, Zwart E, Speksnijder EN, Beems RB, Olive KP, Tuveson DA, et al. Mice expressing a mammary gland–specific R270H mutation
in the p53 tumor suppressor gene mimic human breast cancer development. Cancer Res. 2005;65:8166–73. https://doi.org/10.1158/0008-5472.CAN-05-1650. Article CAS PubMed Google Scholar *
Triplett AA, Sakamoto K, Matulka LA, Shen L, Smith GH, Wagner K-U. Expression of the whey acidic protein (Wap) is necessary for adequate nourishment of the offspring but not functional
differentiation of mammary epithelial cells. Genes. 2005;43:1–11. http://www.ncbi.nlm.nih.gov/pubmed/16106354. Article CAS Google Scholar * Heinlein C, Krepulat F, Löhler J, Speidel D,
Deppert W, Tolstonog GV. Mutant p53R270H gain of function phenotype in a mouse model for oncogene-induced mammary carcinogenesis. Int J Cancer. 2007;122:1701–9.
https://doi.org/10.1002/ijc.23317. Article CAS Google Scholar * Behbod F, Kittrell FS, LaMarca H, Edwards D, Kerbawy S, Heestand JC, et al. An intraductal human-in-mouse transplantation
model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res. 2009;11:R66 http://www.ncbi.nlm.nih.gov/pubmed/19735549. Article PubMed PubMed Central Google Scholar * Kittrell
F, Valdez K, Elsarraj H, Hong Y, Medina D, Behbod F. Mouse mammary intraductal (MIND) method for transplantation of patient derived primary DCIS cells and cell lines. Bio Protoc. 2016.
http://www.ncbi.nlm.nih.gov/pubmed/27446983. * Valdez KE, Fan F, Smith W, Allred DC, Medina D, Behbod F. Human primary ductal carcinoma in situ (DCIS) subtype-specific pathology is preserved
in a mouse intraductal (MIND) xenograft model. J Pathol. 2011;225:565–73. http://www.ncbi.nlm.nih.gov/pubmed/22025213. Article CAS PubMed PubMed Central Google Scholar * Hong Y,
Limback D, Elsarraj HS, Harper H, Haines H, Hansford H, et al. Mouse‐INtraDuctal (MIND): an in vivo model for studying the underlying mechanisms of <scp>DCIS</scp> malignancy. J
Pathol. 2022;256:186–201. https://doi.org/10.1002/path.5820. Article CAS PubMed Google Scholar * Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in
epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96:8551–6. https://doi.org/10.1073/pnas.96.15.8551. Article CAS PubMed PubMed Central
Google Scholar * Bado I, Nikolos F, Rajapaksa G, Wu W, Castaneda J, Krishnamurthy S, et al. Somatic loss of estrogen receptor beta and p53 synergize to induce breast tumorigenesis. Breast
Cancer Res. 2017;19:79 http://breast-cancer-research.biomedcentral.com/articles/10.1186/s13058-017-0872-z. Article PubMed PubMed Central Google Scholar * Bowman-Colin C, Xia B, Bunting
S, Klijn C, Drost R, Bouwman P, et al. Palb2 synergizes with Trp53 to suppress mammary tumor formation in a model of inherited breast cancer. Proc Natl Acad Sci USA. 2013;110:8632–7.
https://doi.org/10.1073/pnas.1305362110. Article PubMed PubMed Central Google Scholar * Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the
maintenance of genomic stability. Oncogene. 2006;25:5864–74. http://www.nature.com/articles/1209874. Article CAS PubMed Google Scholar * Winter C, Nilsson MP, Olsson E, George AM, Chen
Y, Kvist A, et al. Targeted sequencing of BRCA1 and BRCA2 across a large unselected breast cancer cohort suggests that one-third of mutations are somatic. Ann Oncol. 2016;27:1532–8.
https://linkinghub.elsevier.com/retrieve/pii/S0923753419347404. Article CAS PubMed PubMed Central Google Scholar * Hollern DP, Contreras CM, Dance-Barnes S, Silva GO, Pfefferle AD,
Xiong J, et al. A mouse model featuring tissue-specific deletion of p53 and Brca1 gives rise to mammary tumors with genomic and transcriptomic similarities to human basal-like breast cancer.
Breast Cancer Res Treat. 2019;174:143–55. http://link.springer.com/10.1007/s10549-018-5061-y. Article CAS PubMed Google Scholar * Liu H, Murphy CJ, Karreth FA, Emdal KB, White FM,
Elemento O, et al. Identifying and targeting sporadic oncogenic genetic aberrations in mouse models of triple-negative breast cancer. Cancer Discov. 2018;8:354–69.
https://doi.org/10.1158/2159-8290.CD-17-0679 . Article CAS PubMed Google Scholar * Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor
suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–25. http://www.nature.com/articles/ng747z. * Macedo GS, Alemar B, Ashton-Prolla P.
Reviewing the characteristics of BRCA and PALB2-related cancers in the precision medicine era. Genet Mol Biol. 2019;42(1 suppl 1):215–31.
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1415-47572019000200215&tlng=en. Article CAS PubMed PubMed Central Google Scholar * Gusterson BA, Ross DT, Heath VJ, Stein
T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005;7:143
http://breast-cancer-research.biomedcentral.com/articles/10.1186/bcr1041. Article CAS PubMed PubMed Central Google Scholar * Rutkowski MR, Allegrezza MJ, Svoronos N, Tesone AJ, Stephen
TL, Perales-Puchalt A, et al. Initiation of metastatic breast carcinoma by targeting of the ductal epithelium with adenovirus-cre: a novel transgenic mouse model of breast cancer. J Vis Exp.
2014. http://www.jove.com/video/51171/initiation-metastatic-breast-carcinoma-targeting-ductal-epithelium. * Tao L, Xiang D, Xie Y, Bronson RT, Li Z. Induced p53 loss in mouse luminal cells
causes clonal expansion and development of mammary tumours. Nat Commun. 2017;8:14431 http://www.nature.com/articles/ncomms14431. Article CAS PubMed PubMed Central Google Scholar * Zhang
Y, Xiong S, Liu B, Pant V, Celii F, Chau G, et al. Somatic Trp53 mutations differentially drive breast cancer and evolution of metastases. Nat Commun. 2018;9:3953
http://www.nature.com/articles/s41467-018-06146-9. Article PubMed PubMed Central Google Scholar * Brock EJ, Ji K, Shah S, Mattingly RR, Sloane BF. In vitro models for studying invasive
transitions of ductal carcinoma in situ. J Mammary Gland Biol Neoplasia. 2019;24:1–15. http://link.springer.com/10.1007/s10911-018-9405-3. Article PubMed Google Scholar * Bischel LL,
Beebe DJ, Sung KE. Microfluidic model of ductal carcinoma in situ with 3D, organotypic structure. BMC Cancer. 2015;15:12
https://bmccancer.biomedcentral.com/articles/10.1186/s12885-015-1007-5. Article PubMed PubMed Central Google Scholar * Kang J, Kim S, Noh D-Y, Choe K, Lee E, Kang H-S. The timing and
characterization of p53 mutations in progression from atypical ductal hyperplasia to invasive lesions in the breast cancer. J Mol Med. 2001;79:648–55.
http://link.springer.com/10.1007/s001090100269. Article CAS PubMed Google Scholar Download references FUNDING This work was supported by the NIH/NCI under award number F31CA246917, the
Cancer Prevention Research Institute of Texas under award number RP180313 and Cancer Research UK and KWF Kankerbestrijding (ref. C38317/A24043). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Genetics and Epigenetics Program at The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, Houston, TX, USA Rhiannon L. Morrissey &
Guillermina Lozano * Department of Genetics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA Rhiannon L. Morrissey & Guillermina Lozano * Division of Surgical
Oncology, Department of Surgery, Baylor College of Medicine, Houston, TX, USA Alastair M. Thompson Authors * Rhiannon L. Morrissey View author publications You can also search for this
author inPubMed Google Scholar * Alastair M. Thompson View author publications You can also search for this author inPubMed Google Scholar * Guillermina Lozano View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS RLM, AMT and GL wrote and revised the manuscript. CORRESPONDING AUTHOR Correspondence to Guillermina Lozano. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Morrissey, R.L., Thompson, A.M. & Lozano, G. Is loss
of p53 a driver of ductal carcinoma in situ progression?. _Br J Cancer_ 127, 1744–1754 (2022). https://doi.org/10.1038/s41416-022-01885-5 Download citation * Received: 10 February 2022 *
Revised: 17 May 2022 * Accepted: 01 June 2022 * Published: 28 June 2022 * Issue Date: 09 November 2022 * DOI: https://doi.org/10.1038/s41416-022-01885-5 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative