Play all audios:
ABSTRACT A faithful reconstitution of the complete process of oogenesis in vitro is helpful for understanding the molecular mechanisms, genetics, and epigenetic changes related to
gametogenesis; it can also be useful for clinical drug screening, disease research, and regenerative medicine. To this end, given the consensus that murine female germ cells initiate meiosis
at E13.5, substantial works have reported the successful generation of fertile oocytes using E12.5 female gonads as starting materials. Nevertheless, our data demonstrated that murine germ
cells at E12.5 have heterogeneously initiated a meiotic transcriptional program based on a measurement of pre‐mRNAs (unspliced) and mature mRNAs (spliced) at a single-cell level. Therefore,
to establish a platform that faithfully recapitulates the entire process in vitro (from premeiotic murine germ cells to fully developed oocytes), we here report a novel three-dimensional
organoid culture (3-DOC) system, which successfully induced fully developed oocytes from E11.5 premeiotic female germ cells (oogonia). Compared with 2D culture and other 3D culture methods,
this new culture system is more cost-effective and can create high-quality oocytes similar to in vivo oocytes. In summary, our new culture platform provides an experimental model for future
research in regenerative medicine and reproductive biology. SIMILAR CONTENT BEING VIEWED BY OTHERS GENERATION OF FUNCTIONAL OOCYTES FROM MALE MICE IN VITRO Article 15 March 2023 IPSCS
DERIVED FROM INFERTILE MEN CARRYING COMPLEX GENETIC ABNORMALITIES CAN GENERATE PRIMORDIAL GERM-LIKE CELLS Article Open access 22 August 2022 DERIVATION OF HUMAN PRIMORDIAL GERM CELL-LIKE
CELLS IN AN EMBRYONIC-LIKE CULTURE Article Open access 02 January 2024 INTRODUCTION In vitro culture systems are important research tools for investigating tissue development and clinical
diseases. However, traditional two-dimensional (2D) culture methods may hamper cell communication and cannot display the spatial distribution of tissue structures. Over the past few decades,
there has been tremendous development in the research of three-dimensional (3D) culture [1,2,3]. Compared with 2D culture, 3D culture can contribute to the formation of an organotypic
structure called organoid, which can be used to demonstrate the structure and function of organs. The construction of 3D culture models has become an important method of reconstructing
disease models in vitro, bringing great hope for regenerative medicine, drug research, and precision medicine [2,3,4]. Mammalian oogenesis is a complex and delicate process. To obtain
functional eggs, female germ cells must undergo a series of crucial events, including primordial germ cell (PGC) specification [5, 6], PGC migration and proliferation [7, 8], meiosis
initiation and arrest [9, 10], primordial follicle (PF) formation [11, 12], follicle activation and growth [13], and oocyte maturation and ovulation [14, 15]. The complexity of oogenesis
increases the difficulty of in vitro culture. Over the past decade, great progress has been made in the establishment of in vitro culture models of oogenesis. Since 2000, multiple studies
have created mature oocytes following heterotopic transplantation of newborn mouse ovaries and in vitro maturation [16,17,18]. The discovery of induced pluripotent stem cells (iPSCs) in 2006
provided a new source of germ cells [19], and then in 2011 and 2012, primordial germ cell-like cells (PGCLCs) with the capacity for normal spermatogenesis and oogenesis were obtained from
iPSCs, respectively, which provided a paradigm for the first step of complete in vitro gametogenesis [20, 21]. In the early protocols of in vitro culture, transplantation was necessary for
oogenesis, and it was not until 2016 that complete in vitro reconstruction of oogenesis and spermatogenesis was achieved [22,23,24]. However, all the above methods used germ cells at or
after E12.5 as the materials for in vitro culture. Furthermore, it was reported that some meiotic genes started to be expressed at E12.5 [25], which indicated that the model established from
E12.5 gonadal ridges (GRs) might not be as effective as a model from earlier GRs for studying the whole process of oogenesis. Mammalian embryonic ovaries contain a large number of PGCs, but
only a small fraction of them develop into mature oocytes due to apoptosis and follicular atresia [26]. The regulatory mechanisms of oogenesis are currently not well-known, and
reconstruction of oogenesis in vitro may help to elucidate these mechanisms. In this study, we focused on germ cells from E11.5 female fetal mice, and established a three-dimensional
organoid culture (3-DOC) system that enabled the successful generation of fully developed oocytes under complete in vitro conditions. Through mimicking the in vivo environment and
reconstructing the process of oogenesis in vitro, we established a platform to further reveal the mechanism of molecular regulation in early meiosis, oogenesis, and follicle formation, and
provided an experimental model for research in reproductive biology and reproductive medicine. RESULTS MEIOSIS WAS INITIATED IN FEMALE GERM CELLS BEFORE THE EXPRESSION OF THE MEIOTIC
GATEKEEPER _STRA8_ To unveil the transcriptome difference between germ cells at different stages, we firstly performed an integrated analysis of our recently published single-cell RNA seq
datasets (E11.5, E12.5, and E13.5) using uniform manifold approximation and projection (UMAP) [27, 28]. After stringent quality control standards to remove low-quality cells and potential
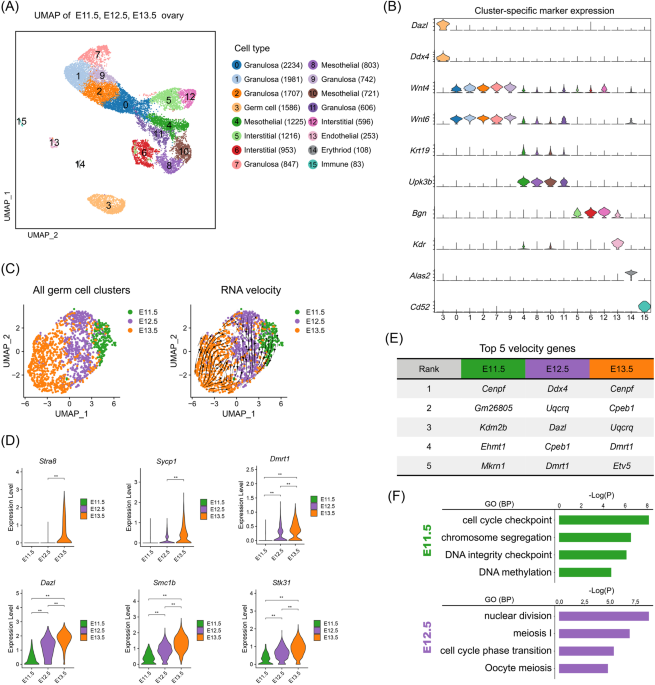
doublets (Fig. S1A, see materials and methods for details), we identified 15 cell clusters across three developmental stages (Figs. 1A and S1B), and seven cell types were characterized
according to their expression of canonical cell markers (Fig. 1B), including germ cells expressing _Dazl_ and _Ddx4_ [29], granulosa cells expressing _Wnt4_ and _Wnt6_ [30], mesothelial
cells expressing _Upk3b_ and _Krt19_ [30], interstitial cells expressing _Bgn_ [31], endothelial cells expressing _Kdr_ [32], erythroid cells expressing _Alas2_ [33], and immune cells
expressing _Cd52_ [34]. To conduct a focused analysis of germ cells, we extracted the scRNA-seq data of germ cells after the identification of gonadal cells. After UMAP projection, we
observed the highest transcriptional heterogeneity in germ cells at E13.5, while E11.5 germ cells showed the lowest transcriptional heterogeneity. Projection of RNA velocity vectors
confirmed the developmental trajectories from the top right to the bottom left (Fig. 1C), which was also consistent with the developmental stages (from E11.5 to E13.5). Noteworthy, E11.5
germ cells and E12.5 germ cells were located differently in the UMAP plot, thus suggesting that these germ cells were heterogeneous at the transcription level. To evaluate the meiotic
potential of these germ cells at different time points, we then explored the expression of canonical meiotic initiation-related gene expression across the three stages. The results showed
that a small number of cells began to express _Stra8_ and _Sycp1_ as early as E12.5, while other meiotic regulatory genes such as _Dazl_, _Smc1b_, _Stk31,_ and _Dmrt1_, showed significantly
higher expression in E12.5 germ cells when compared with E11.5 germ cells (Fig. 1D). To clarify that germ cells at E12.5 had initiated meiotic programs, we further performed RNA velocity
analysis to infer cellular fate by leveraging splicing kinetics [35, 36]. By comparing the stage-specific top velocity genes, we found that mitosis-related velocity driving genes were mainly
enriched in E11.5 germ cells, while at E12.5, meiotic-related genes were identified in velocity driving genes, such as _Dazl_ and _Cpeb1_ (Fig. 1E, and Table S6) [37, 38]. Furthermore, we
performed GO enrichment analysis on germ cells from different time points. It was found that “meiosis I” and “oocyte meiosis” could be enriched at E12.5 (Fig. 1F), while the E11.5 top
enriched RNA velocity genes were related to the GO terms of “cell cycle checkpoint” and “chromosome segregation”, thus representing a mitotic state. In summary, germ cells at E12.5 had
initiated a meiotic program even though the meiotic gatekeeper _Stra8_ was barely expressed. FEMALE GERM CELLS FROM E12.5 GRS SHOW HIGHER MEIOTIC POTENTIAL COMPARED WITH E11.5 GERM CELLS
UNDER IN VITRO CONDITIONS Based on our previously established adherent culture methods [39, 40], we next explored the developmental potential of E11.5 and E12.5 female germ cells (Fig. S2A).
We firstly collected E11.5 female GRs using _Sry_ genotyping because GRs are sexually indistinguishable at this development time point. Morphologically, E11.5 GRs showed an obvious
elongated length when compared with E12.5 and E13.5 GRs, and were characterized by a lower number of germ cells (Fig. 2A). We next cultured these GRs under adherent conditions, and
representative follicle structures were observed at around Day 11 of in vitro culture (Fig. 2B). However, E11.5 GRs generated lower numbers of follicles when compared with E12.5 GRs after in
vitro culture (11 d and 14 d for E11.5 GRs, 10 d and 13 d for E12.5 GRs). Besides, germ cells from E11.5 formed oocytes with significantly smaller diameters after 14 days of in vitro
culture (Fig. 2C). We further compared the ratio of oocyte-generating GRs, and the results also confirmed that E12.5 showed a significantly greater ability to produce follicle structures
(Figs. 2D and S2B). As the onset and arrest of meiosis is a crucial event during oogenesis, we then focused on early meiosis and performed chromatin spread analysis on both in vitro and in
vivo oocytes at the same stage. The protein synaptonemal complex protein 3 (SYCP3), one component of the synaptonemal complex, was stained for oocyte stage classification [41, 42]. Then
oocytes were divided into leptotene, zygotene, pachytene, and diplotene according to the morphology of the synaptonemal complex, and the percentages of oocytes at the four stages were
calculated respectively (Fig. 2E). As Fig. 2E indicates, oocytes could enter meiosis, undergo normal homologous synapsis, and develop to the pachytene and diplotene stages after in vitro 2D
culture. Nevertheless, most of the oocytes from E11.5 GRs were still in the leptotene stage, which indicated a later meiotic process than that in E12.5 GRs. Taken together, these data
verified our scRNA-seq analysis and demonstrated that the onset of meiosis had taken place in some female germ cells at E12.5, which indicated that E11.5 female germ cells were superior for
an in vitro culture model considering that they remained mitotic. THE ESTABLISHMENT OF 3-DOC ENABLES THE PROPER PROGRESSION OF MEIOSIS FOR E11.5 FEMALE GERM CELLS To generate fully developed
oocytes from premeiotic female germ cells under complete in vitro conditions, we created a new 3D culture method (Fig. 3A). The female GRs of E11.5 in our 3D culture method were cultured on
agarose blocks and Matrigel. Noteworthy, our 3-DOC here did not require the application of hanging cell inserts (commonly used consumables for 3D culture), which makes it more
cost-effective when compared with other 3D culture methods [43, 44]. After an 11-day 3-DOC culture, E11.5 female germ cells efficiently formed ovarian follicle structures, and of particular
note, these ovarian follicle structures showed distinct boundaries, with multiple layers of granulosa cells around the central oocyte at Day 17 of in vitro culture (Fig. 3B). The comparison
of the ovarian follicle formation efficiency and ovarian follicle diameter demonstrated that E11.5 female GRs cultured under the current 3-DOC conditions were much more effective in
promoting ovarian follicle formation (Fig. S3A). After 4 days of in vitro culture, we next prepared meiotic chromosome spreads from E11.5 female GRs and the spreads were co-immunostained for
the synaptonemal complex marker SYCP3 and homologous recombination marker RAD51. Analyzing RAD51 foci at pachytene verified that under 3-DOC conditions, E11.5 female germ cells showed a
comparable number of RAD51 foci to their in vivo counterparts, thus indicating that these E11.5 derived germ cells faithfully recapitulated meiotic recombination (Figs. 3C and S3B). We
further compared the progression of meiotic prophase I of E11.5 female germ cells after 2, 3, 4, and 5 days of in vitro 3-DOC culture, and the results showed that those germ cells cultured
in the 3-DOC conditions gradually entered meiosis prophase I with prolonged culture; of particular notice, the percentage of germ cells at different meiotic stages resembled their in vivo
counterparts (Fig. 3D). As most of oocytes have entered meiosis in E16.5 [45], we further evaluated the homologous chromosome synapsis of the in vitro cultured E11.5 female germ cells by
co-staining SYCP3 and SYCP1, and the results showed that approximately 30% of germ cells cultured in vitro showed asynapsis, which was higher when compared with E16.5 female germ cells in
vivo (Fig. 3E–G). In general, the majority of germ cells (approximately 70%) underwent normal synapsis under in vitro conditions. Together, these results demonstrated that our established
3-DOC efficiently enabled the progression of meiosis and recapitulated key meiotic events under complete in vitro conditions. PROLONGED CULTURE OF E11.5 FEMALE GERM CELLS UNDER 3-DOC
CONDITIONS RECAPITULATED KEY EVENTS DURING PF ASSEMBLY During the process of meiosis, germ cell cysts were formed and then underwent programmed cyst breakdown to form PFs (Fig. 4A). To
investigate the process of PF assembly of E11.5 female germ cells under 3-DOC conditions, we then performed immunofluorescence on the E11.5 female GRs after being cultured in vitro, and on
GRs from postnatal mice at the corresponding time points (Fig. 4B). It was observed that female germ cells under 3-DOC successfully formed germ cell cyst structures after 7 days of in vitro
culture. After prolonged culture, these germ cell cyst structures underwent programmed breakdown and finally formed PFs in the center of GRs. To evaluate the progression of PF assembly, we
then analyzed the ratio of germ cells within cysts and follicles after 7, 10, and 13 days of 3-DOC culture (Fig. 4C). According to the results of immunofluorescence, a single oocyte
surrounded by a single layer of granulosa cells was classified as the oocyte in the follicle, while oocytes in clusters were identified as oocytes in cysts. An accurate estimation method
described by Myers, M. et al. was used to count the numbers of oocytes in follicles and cysts [46]. The results showed that female germ cells derived from E11.5 GRs underwent PF assembly
resembling their in vivo counterparts (no significance). To further validate the process of PF assembly in vitro, we performed an ultrastructure analysis of 3-DOC cultured E11.5 GRs (Fig.
4D). The cyst structure can be found in both E11.5 female GRs cultured for 7 days in vitro and ovaries from 0 day post-partum (0 dpp) mice. Meanwhile, the complete structure of follicles
that included an oocyte enclosed in a single layer of flattened granulosa cells could be observed in E11.5 female GRs cultured for 10 days in vitro and ovaries from 3 dpp mice, indicating
these germ cells formed representative PF structures at this stage. Moreover, in E11.5 female GRs cultured for 13 days in vitro and ovaries from 5 dpp mice, we discovered that some oocytes
were surrounded by multiple layers of granulosa cells, indicating they were in the secondary follicle stage (Fig. 4D). Taken together, the results of immunofluorescence and Transmission
electron microscopy (TEM) demonstrated that, after 3D culture, the E11.5 female GRs were capable of cyst breakdown and PF assembly. FULL DEVELOPMENT OF 3-DOC DERIVED PREMEIOTIC FEMALE GERM
CELLS INTO MII STAGE OOCYTES UNDER IN VITRO CONDITIONS After 16 days of 3-DOC culture, E11.5 derived premeiotic female germ cells successfully generated primordial and secondary follicles;
we next investigated whether these oocytes could develop beyond the growth stage. To this end, we adopted the two-step culture system established in 1996 by Eppig [47], and isolated the
primordial follicles and secondary follicles from ovary organoids after 16–18 days of 3-DOC culture. The follicles had a single layer or multiple layers of granulosa cells, which was similar
to their in vivo counterparts (Figs. 5A and S4). We next isolated these single follicles for in vitro single follicle culture using a PVP supplemented medium as described by Morohaku et
al., and the results showed that granulosa cells proliferated rapidly during single follicle culture and follicle cavities gradually formed after 7 days of single follicle culture [43] (Fig.
5B). With prolonged culture, the diameter of the follicle cavity increased, and granulosa cells expanded to form a follicular structure. Oocytes featuring well-defined germinal vesicles
could also be observed in our in vitro cultured follicles. On day 11 of the in vitro single follicle culture, we observed representative cumulus-oocyte-complexes (COCs), and these oocytes
showed comparable diameters to their in vivo counterparts (Fig. 5C). Moreover, to examine whether oocytes were sufficient for maturation after 3D culture, we then cultured oocytes with
follicle-stimulating hormone (FSH) and human chorionic gonadotropin (hCG) for 13–17 h [48]. After in vitro maturation (IVM), the oocytes resumed meiosis and the first polar body was
extruded, which indicated that oocytes were induced into the MII stage (Fig. 5D). The success of IVM demonstrated the feasibility of our 3-DOC system, which might become a cost-effective
alternative method for in vitro oogenesis (Table S7). DISCUSSION Normal oogenesis produces functional oocytes, while abnormal oogenesis can result in multiple diseases related to
infertility, such as premature ovarian failure, oocyte maturation defects, and polycystic ovary syndrome [49]. According to the World Health Organization (WHO), it is reported that
approximately 48.5 million couples globally are affected by infertility, and assisted reproductive technology is one of the most useful interventions [50, 51]. However, problems remain for
women with severe infertility, as the source of high-quality oocytes may be limited and cause ethical issues. Reconstruction of oogenesis in vitro is an alternative approach to investigating
female infertility, and great progress has been made in this area [52]. However, there is still a long way to go before these achievements can be used in clinical reproductive medicine.
Nevertheless, any improvement to in vitro experimental models could be of great significance for future clinical applications. To investigate the feasibility of inducing premeiotic female
germ cells into mature oocytes in vitro, we selected female germ cells from E11.5 GRs as materials. Oocytes from E11.5 female GRs using the method previously established by our lab [53]
showed a delayed process of meiosis compared with those from the E12.5 female GRs and in vivo. Furthermore, the quantity and quality of oocytes from E11.5 female GRs were significantly lower
than those from E12.5 GRs at the same stage after culture. These results indicated that the distinct status between E11.5 female GRs and E12.5 female GRs results in different
characteristics during in vitro culture. Recently, with the development of various RNA sequencing technologies, research on embryonic development has entered the "omics era"
[54,55,56]. Using analysis at the single-cell level, the dynamic changes of the entire developmental period can be obtained. The application of new technologies has led to new perspectives
and insights into the timing of meiotic initiation [57]. The initiation of meiosis is one of the keys to successful gamete development. It was previously thought that mouse female germ cells
start to enter meiosis between E13.5 and E14.5. In our analysis results, some genes related to meiosis initiation such as _Dazl_ and _Dpeb1_ had already started to be expressed at E12.5,
while E11.5 germ cells were still undergoing mitosis. The different expression patterns between E11.5 female germ cells and E12.5 female germ cells might be the reason for the low efficiency
of the traditional 2D culture method. Therefore, a new culture system was needed for the culture of E11.5 female GRs. To improve culture efficiency, we established a novel 3-DOC platform
for the in vitro culture of E11.5 female germ cells. As Fig. 3A indicates, the GRs were placed on agarose blocks which were flooded to their upper surface with medium. We also used Matrigel
to support cell development during 3-DOC. In recent years, natural extracellular matrices or synthetic hydrogels have been commonly used in cell culture [58, 59]. Matrigel, which is secreted
by mouse tumor cells, is a mixture of proteins including collagen IV, laminin, and enactin [60, 61]. Matrigel can mimic the extracellular environment, provide cells with structural support,
and promote cell differentiation and development [62]. In our 3-DOC model, we added Matrigel to mimic the in vivo microenvironment and improve cell culture conditions. Unlike oocytes from
the 2D culture method, female germ cells from E11.5 GRs showed a similar meiotic process to oocytes in vivo. In addition, oocytes can also undergo PF formation after 3D culture and the
structure of follicles was similar to those from in vivo ovaries. In female mice, the diameter of oocytes during meiotic arrest is 15–20 μm. When the diameter reaches 75 μm or more, the
oocytes are considered to be in a state that can be matured in vitro [63]. In our experiments, the oocytes after single follicle culture were greater than 75 μm in diameter. To further
verify the quality of our oocytes from the 3-DOC system, we performed IVM after single follicle culture. It was discovered that MII oocytes could be obtained after our 3D culture, and the
first polar body could be extruded. Through the scRNA-seq analysis, we demonstrated the differences between E11.5 and E12.5 germ cells on the meiosis initiation. Then we successfully
established a 3-DOC model in this paper and completed the in vitro maturation of oocytes from E11.5 female GRs. Compared with other methods, our method is more cost-effective and can
generate high-quality oocytes that closely resemble their in vivo counterparts. Overall, our findings offer a new experimental method for future research in reproductive biology and
reproductive medicine. MATERIALS AND METHODS ANIMALS The healthy six-week-old CD1 mice used in our experiments were purchased from the Qingdao Daren Fortune Animal Technology Co., Ltd
(Qingdao, China). The mice were provided with free access to food and water, and housed under a cycle of 12 h light and 12 h dark. For mating, mice were randomly selected by the random
number table method. One male mouse was mated with one or two female mice at 17:00. The presence of a vaginal plug was checked the next morning and the female mice with vaginal plugs were
considered as 0.5 day post coitum (dpc) at noon. All the experiments were permitted by the Ethics Committee of Qingdao Agriculture University. ISOLATION OF GRS AND GENDER IDENTIFICATION The
11.5 dpc or 12.5 dpc pregnant mice were sacrificed, and embryos were collected from the uteri and washed with normal saline. Female GRs of E11.5 or E12.5 were carefully isolated from the
embryos under a stereoscope (Nikon SMZ-1000, Japan) and the mesonephroi were cut off using a 1 mL syringe needle. For E11.5 GRs, the gender cannot be distinguished through morphology. So we
performed PCR on the E11.5 embryos and selected the female GRs based on the band of sex-determining gene _Sry_. The primer sequences of _Sry_ and the internal reference gene _Gapdh_ are
listed in Table S1. The collected GRs were washed three times with normal saline for further experiments. 2D CULTURE OF GRS The medium was prepared according to Table S2 and balanced at 37°C
for 10 min before culture. Subsequently, the collected GRs were washed in medium, transferred to the 24-well plate, and cultured as a whole at 37°C and 5% CO2 for three days. Subsequently,
half of the medium in the 24-well plate was replaced by the new balanced medium every other day. The entire culture took 17 days in total. 3-DOC SYSTEM OF GRS To construct the 3-DOC system,
2% agarose gel was prepared and sterilized in advance. Before culture, the agarose gel was cut into cubes and placed in the culture medium to replace the water in the gel. We applied
Matrigel to our culture system, which was placed on the agarose blocks and balanced in the medium for 30 min. The GRs were then put on the agarose blocks, and the blocks were transferred
into the 24-well plate. The medium was the same as that of the 2D culture system, and an appropriate amount of medium was added to ensure that the liquid level reached the upper surface of
the agarose blocks. The GRs were cultured at 37°C and 5% CO2 for six days, and the medium was replaced every three days. On the sixth day, we replaced the normal medium with medium
containing 5 μM ICI182780 (R&D Systems 1047, USA), which is an inhibitor of estrogen receptors. Half of the medium containing ICI182780 was changed every other day. On the 12th day, we
replaced the medium containing ICI182780 with the normal GR culture medium again and the culture was continued until the 17th day. ISOLATION AND IN VITRO CULTURE OF FOLLICLES The isolation
of follicles was performed when most of follicles reached the stage of secondary follicles with multiple layers of granulosa cells surrounding them. To conduct follicle isolation, the
ovary-like tissue was first separated from the surface of agarose blocks and transferred into the isolation medium. The follicle isolation medium was prepared as shown in Table S3. A
tungsten needle was then used to isolate follicles carefully. We isolated follicles mechanically and washed them in follicle isolation medium and follicle growth medium in turn. The follicle
growth medium was prepared according to Table S4. Before the isolation of follicles, we placed several droplets of follicle growth medium on the dishes, covered them with mineral oil, and
equilibrated them for 5 h in the incubator. After follicles were isolated and washed, each follicle was placed in one droplet. The follicles were cultured at 37°C and 5% CO2, and half of the
medium was changed every two days. Follicle growth took place over a period of 12–14 days. INDUCTION OF FOLLICLE MATURATION The maturation medium was prepared as indicated in Table S5. Four
droplets of the maturation medium were pipetted on the dishes, covered by mineral oil, and equilibrated in the incubator for 12 h. Follicles with multiple layers of granulosa cells were
selected and transferred to the droplets for maturation. Follicle maturation was identified by the extrusion of the first polar body. THE ANALYSIS OF SCRNA-SEQ The scRNA-seq of E11.5–E13.5
gonadal cells used in this paper were previously used by Novogene (Beijing, China) [64] and the data are deposited in the Gene Expression Omnibus (GEO) database under accession number
GSE128553. The software CellRanger v6.1.2 was used for quality control of the data and genome alignment according to the CellRanger pipeline. After obtaining the expression matrix, R v.3.6.3
was used for downstream analysis of scRNA-seq data and doublets were removed by the “DoubletFinder” [65]. The “Seurat” Package v.4.0.0 was used for the aggregation of the data from three
groups of GRs based on the official vignette (https://satijalab.org/seurat/index.html) [66]. Uniform Manifold Approximation and Projection was applied for dimension reduction and the
function “FindAllMarkers” were used to identify the marker genes of each cluster. We also performed RNA velocity with the “scVelo” [67] package and the RNA velocity streamlines were embedded
in the UMAP plot from the “Seurat” package. GO enrichment analysis was performed on Metascape (https://metascape.org/gp/index.html) [68]. TRANSMISSION ELECTRON MICROSCOPY To prepare TEM
samples, the ovary-like tissue was initially fixed in 2.5% glutaraldehyde and Osmium tetroxide. Samples were subsequently dehydrated in an alcohol and acetone solution, and finally embedded
in resin. After resin solidification under different temperatures, the samples were sectioned at approximately 70 nm. Then the slides were treated with uranyl acetate for 15 min and lead
citrate for 8 min, in turn. After staining, the slides were observed under an electron microscope (Hitachi HT7700, Japan). IMMUNOFLUORESCENCE The GR samples were first collected, fixed in 4%
paraformaldehyde at 4°C overnight and then washed with water for 4 h. After dehydration in an alcohol solution, the samples were finally embedded in paraffin and sliced at a thickness of 5
μm. Subsequently, the slides were placed in the 60°C incubator for 2 h and deparaffinized with xylene, alcohol, and PBS solution. For antigen retrieval, slides were treated with 0.01 M
sodium citrate solution for 10 min at 96°C. After cooling to room temperature, slides were treated with TBS and TBST for 5 minutes, respectively. Blocking buffer (0.05 M TBS supplemented
with 3% bovine serum albumin and 10% goat serum) was added to block the slides. After blocking for 30 min, the slides were incubated with the anti-DDX4 antibody (Abcam ab27591, UK) at 4°C
overnight. We then washed the slides three times with TBST. After washing, the slides were incubated with the secondary antibody at 37°C for 45 min. The Alexa Fluor® 488 goat anti-Rabbit
antibody (Abcam ab150077, UK) was used as the secondary antibody. Slides were washed with TBST after incubation and 60 μL propidium iodide (PI) was added to each slide for nuclei staining.
Finally, slides were washed with PBS and an antifade mounting medium (Boster, AR1109, Wuhan, China) was added before mounting. Photos were taken under a fluorescence microscope (Olympus
BX51, Japan) or LSCM (Leica TCS SP5 II, Germany). CHROMOSOME SPREAD ANALYSIS To perform chromosome spread analysis, the GRs were isolated, washed with normal saline, and finally placed in a
hypo-extraction buffer, which contained 30 mM Tris, 50 mM sucrose, 17 mM citric acid, 5 mM EDTA, 2.5 mM DTT, and 1 mM PMSF. After treatment with the hypo-extraction buffer for 1 h at 4°C,
the GRs were placed in a droplet of 0.1 M sucrose on a slide and torn using forceps. Then 500 μL PFA was added to fix the cells at room temperature overnight. The slides were air-dried the
next day and washed with 0.04% Photo-Flo for 4 min. Subsequently, the slides were washed with TBS and blocked with blocking buffer (4 mL TBS supplemented with 12 mg bovine serum albumin, 40
μL goat serum, and 0.2 μL Triton-X-100) for 1 h at 37°C. After blocking, the slides were incubated with primary antibodies at 37°C for 8 h. The primary antibodies used in the experiment
included anti-SYCP3 (Abcam ab97672, UK), anti-SYCP1 (Abcam 15090, UK), and anti-RAD51 (Abcam ab133534, UK). Then the slides were washed with TBS and incubated with the secondary antibodies
at 37°C for 2 h. After incubation and TBS washing, Hoechst 33342 was used to stain nuclei for 8 min. The slides were washed with PBS after nucleus staining and mounted using an antifade
mounting medium. Images were taken under a fluorescence microscope (Olympus BX51, Japan) or LSCM (Leica TCS SP5 II, Germany). STATISTICAL ANALYSIS The software ImageJ 1.53c was used for
image analysis. At least three replicates were performed in each experiment. Two-sided _t_ test and _F_ test were conducted using Graphpad Prism 5. The data in this paper are presented as
mean ± SD. * and ** represent _p_ < 0.05 and _p_ < 0.01, respectively. DATA AVAILABILITY The scRNA-seq data analyzed during the current study are available in the Gene Expression
Omnibus (GEO) database under accession number GSE128553. Other data are included in this published article and supplementary information files. CODE AVAILABILITY Main scripts used for
scRNA-seq data processing and analyses are available at this link: https://github.com/Flyingcattle/mouse-ovary-organoids. REFERENCES * Lancaster MA, Knoblich JA. Organogenesis in a dish:
modeling development and disease using organoid technologies. Science. 2014;345:1247125. Article PubMed Google Scholar * Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid
research. Nat Rev Genet. 2018;19:671–87. Article CAS PubMed Google Scholar * Suarez-Martinez E, Suazo-Sanchez I, Celis-Romero M, Carnero A. 3D and organoid culture in research:
physiology, hereditary genetic diseases and cancer. Cell Biosci. 2022;12:39. Article CAS PubMed PubMed Central Google Scholar * Clevers H. Modeling development and disease with
organoids. Cell. 2016;165:1586–97. Article CAS PubMed Google Scholar * Hansen CL, Pelegri F. Primordial germ cell specification in vertebrate embryos: phylogenetic distribution and
conserved molecular features of preformation and induction. Front Cell Dev Biol. 2021;9:730332. Article PubMed PubMed Central Google Scholar * Hancock GV, Wamaitha SE, Peretz L &
Clark AT. Mammalian primordial germ cell specification. Development 2021;148:dev189217. * Grimaldi C, Raz E. Germ cell migration-Evolutionary issues and current understanding. Semin Cell Dev
Biol. 2020;100:152–9. Article CAS PubMed Google Scholar * Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol
Cell Biol. 2010;11:37–49. Article CAS PubMed PubMed Central Google Scholar * Sou IF, Pryce RM, Tee WW, McClurg UL. Meiosis initiation: a story of two sexes in all creatures great and
small. Biochem J. 2021;478:3791–805. Article CAS PubMed Google Scholar * Jaffe LA, Egbert JR. Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian
follicle. Annu Rev Physiol. 2017;79:237–60. Article CAS PubMed Google Scholar * Wang C, Zhou B, Xia G. Mechanisms controlling germline cyst breakdown and primordial follicle formation.
Cell Mol Life Sci. 2017;74:2547–66. Article CAS PubMed Google Scholar * Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–71. Article
PubMed Google Scholar * McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–14. CAS PubMed Google Scholar * Robker RL, Hennebold JD,
Russell DL. Coordination of ovulation and oocyte maturation: a good egg at the right time. Endocrinology. 2018;159:3209–18. Article CAS PubMed PubMed Central Google Scholar * Duffy DM,
Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: parallels with inflammatory processes. Endocr Rev. 2019;40:369–416. Article PubMed Google Scholar * Liu J, Van Der Elst J, Van Den Broecke
R, Dumortier F, Dhont M. Maturation of mouse primordial follicles by combination of grafting and in vitro culture. Biol Reprod. 2000;62:1218–23. Article CAS PubMed Google Scholar * Shen
W, Li L, Zhang D, Pan Q, Ding M, Deng H. Mouse oocytes derived from fetal germ cells are competent to support the development of embryos by in vitro fertilization. Mol Reprod Dev.
2006;73:1312–7. Article CAS PubMed Google Scholar * Matoba S, Ogura A. Generation of functional oocytes and spermatids from fetal primordial germ cells after ectopic transplantation in
adult mice. Biol Reprod. 2011;84:631–8. Article CAS PubMed Google Scholar * Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 2006;126:663–76. Article CAS PubMed Google Scholar * Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell
specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–32. Article CAS PubMed Google Scholar * Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M.
Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338:971–5. Article CAS PubMed Google Scholar * Hikabe O, Hamazaki N, Nagamatsu G,
Obata Y, Hirao Y, Hamada N, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539:299–303. Article PubMed Google Scholar * Morohaku K,
Tanimoto R, Sasaki K, Kawahara-Miki R, Kono T, Hayashi K, et al. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc Natl Acad Sci USA. 2016;113:9021–6.
Article CAS PubMed PubMed Central Google Scholar * Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, et al. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem
Cell. 2016;18:330–40. Article CAS PubMed Google Scholar * Zhao ZH, Ma JY, Meng TG, Wang ZB, Yue W, Zhou Q, et al. Single-cell RNA sequencing reveals the landscape of early female germ
cell development. FASEB J. 2020;34:12634–45. Article CAS PubMed Google Scholar * Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005;11:162–77. Article
PubMed Google Scholar * McInnes L, Healy J & Melville JJapa. Umap: Uniform manifold approximation and projection for dimension reduction. https://arxiv.org/abs/1802.03426 2018. * Becht
E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol 2018;34:38–44. * Saiti D, Lacham-Kaplan O.
Mouse germ cell development in-vivo and in-vitro. Biomarker insights. 2007;2:241–52. Article PubMed PubMed Central Google Scholar * Niu W, Spradling AC. Two distinct pathways of
pregranulosa cell differentiation support follicle formation in the mouse ovary. Proc Natl Acad Sci USA. 2020;117:20015–26. Article CAS PubMed PubMed Central Google Scholar * Piprek RP,
Kolasa M, Podkowa D, Kloc M, Kubiak JZ. Transcriptional profiling validates involvement of extracellular matrix and proteinases genes in mouse gonad development. Mech Dev. 2018;149:9–19.
Article CAS PubMed Google Scholar * Schoen K, Hirschberg RM, Plendl J, Kaessmeyer S. Identification of CD133-, CD34- and KDR-positive cells in the bovine ovary: a new site of vascular
wall resident endothelial progenitor cells. Clin Hemorheol Microcirc. 2012;52:67–84. Article PubMed Google Scholar * Tchaikovskii V, Desnick RJ, Bishop DF. Molecular expression,
characterization and mechanism of ALAS2 gain-of-function mutants. Mol Med. 2019;25:4. Article PubMed PubMed Central Google Scholar * Zhao Y, Su H, Shen X, Du J, Zhang X, Zhao Y. The
immunological function of CD52 and its targeting in organ transplantation. Inflamm Res. 2017;66:571–8. Article CAS PubMed Google Scholar * Svensson V, Pachter L. RNA velocity: molecular
kinetics from single-cell RNA-seq. Mol Cell. 2018;72:7–9. Article CAS PubMed Google Scholar * La Manno G, Soldatov R, Zeisel A, Braun E, Hochgerner H, Petukhov V, et al. RNA velocity of
single cells. Nature. 2018;560:494–8. Article PubMed PubMed Central Google Scholar * Smorag L, Xu X, Engel W, Pantakani DV. The roles of DAZL in RNA biology and development. Wiley
Interdiscip Rev RNA. 2014;5:527–35. Article CAS PubMed Google Scholar * Igea A, Mendez R. Meiosis requires a translational positive loop where CPEB1 ensues its replacement by CPEB4. EMBO
J. 2010;29:2182–93. Article CAS PubMed PubMed Central Google Scholar * Liang GJ, Zhang XF, Wang JJ, Sun YC, Sun XF, Cheng SF, et al. Activin A accelerates the progression of fetal
oocytes throughout meiosis and early oogenesis in the mouse. Stem Cells Dev. 2015;24:2455–65. Article CAS PubMed Google Scholar * Wang JJ, Zhai QY, Zhang RQ, Ge W, Liu JC, Li L, et al.
Effects of activin A on the transcriptome of mouse oogenesis in vitro. J Cell Physiol. 2019;234:14339–50. Article CAS PubMed Google Scholar * Syrjanen JL, Pellegrini L, Davies OR. A
molecular model for the role of SYCP3 in meiotic chromosome organisation. Elife. 2014;3:e02963. Article PubMed PubMed Central Google Scholar * Syrjanen JL, Heller I, Candelli A, Davies
OR, Peterman EJ, Wuite GJ et al. Single-molecule observation of DNA compaction by meiotic protein SYCP3. Elife 2017;6:e22582. * Morohaku K, Hirao Y, Obata Y. Development of fertile mouse
oocytes from mitotic germ cells in vitro. Nat Protoc. 2017;12:1817–29. Article CAS PubMed Google Scholar * Hayashi K, Hikabe O, Obata Y, Hirao Y. Reconstitution of mouse oogenesis in a
dish from pluripotent stem cells. Nat Protoc. 2017;12:1733–44. Article CAS PubMed Google Scholar * Hwang GH, Hopkins JL & Jordan PW. Chromatin spread preparations for the analysis of
mouse oocyte progression from prophase to metaphase II. J Vis Exp 2018;26:56736. * Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the
mouse ovary. Reproduction. 2004;127:569–80. Article CAS PubMed Google Scholar * Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod.
1996;54:197–207. Article CAS PubMed Google Scholar * Lamb JD, Shen S, McCulloch C, Jalalian L, Cedars MI, Rosen MP. Follicle-stimulating hormone administered at the time of human
chorionic gonadotropin trigger improves oocyte developmental competence in in vitro fertilization cycles: a randomized, double-blind, placebo-controlled trial. Fertil Steril.
2011;95:1655–60. Article CAS PubMed Google Scholar * Guerri G, Maniscalchi T, Barati S, Gerli S, Di Renzo GC, Della, et al. Non-syndromic monogenic female infertility. Acta Biomed.
2019;90:68–74. CAS PubMed PubMed Central Google Scholar * Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence
since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. Article PubMed PubMed Central Google Scholar * Inhorn MC, Patrizio P. Infertility around the globe:
new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21:411–26. Article PubMed Google Scholar * Saitou M, Hayashi K.
Mammalian in vitro gametogenesis. Science. 2021;374:eaaz6830. Article CAS PubMed Google Scholar * Zhang ZP, Liang GJ, Zhang XF, Zhang GL, Chao HH, Li L, et al. Growth of mouse oocytes to
maturity from premeiotic germ cells in vitro. PLoS One. 2012;7:e41771. Article CAS PubMed PubMed Central Google Scholar * Stark R, Grzelak M, Hadfield J. RNA sequencing: the teenage
years. Nat Rev Genet. 2019;20:631–56. Article CAS PubMed Google Scholar * Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, et al. mRNA-Seq whole-transcriptome analysis of a single
cell. Nat Methods. 2009;6:377–82. Article CAS PubMed Google Scholar * Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive
epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–8. Article CAS PubMed PubMed Central Google Scholar * Ge W, Wang JJ,
Zhang RQ, Tan SJ, Zhang FL, Liu WX, et al. Dissecting the initiation of female meiosis in the mouse at single-cell resolution. Cell Mol Life Sci. 2021;78:695–713. Article CAS PubMed
Google Scholar * Aisenbrey EA, Murphy WL. Synthetic alternatives to matrigel. Nat Rev Mater. 2020;5:539–51. Article CAS PubMed PubMed Central Google Scholar * Xing H, Lee H, Luo L,
Kyriakides TR. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv. 2020;42:107421. Article CAS PubMed Google Scholar * Hughes CS, Postovit LM, Lajoie
GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–90. Article CAS PubMed Google Scholar * Benton G, Arnaoutova I, George J,
Kleinman HK, Koblinski J. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev. 2014;79-80:3–18. Article CAS PubMed Google Scholar *
Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. Article CAS PubMed Google Scholar * Mehlmann LM. Stops and starts
in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–9. Article CAS PubMed Google Scholar * Ge W,
Wang JJ, Zhang RQ, Tan SJ, Zhang FL, Liu WX et al. Dissecting the initiation of female meiosis in the mouse at single-cell resolution. Cell Mol Life Sci 2020;78:695–713. * McGinnis CS,
Murrow LM, Gartner ZJ. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 2019;8:329–37. Article CAS PubMed PubMed Central
Google Scholar * Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol.
2018;36:411–20. Article CAS PubMed PubMed Central Google Scholar * Bergen V, Lange M, Peidli S, Wolf FA, Theis FJ. Generalizing RNA velocity to transient cell states through dynamical
modeling. Nat Biotechnol. 2020;38:1408–14. Article CAS PubMed Google Scholar * Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a
biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This
work was supported by the National Nature Science Foundation of China (32100683 and 32072734), the Natural Science Foundation of Shandong Province, China (ZR2021QC003), the National Key
Research and Development Program of China (2018YFC1003400), the Taishan Scholar Construction Foundation of Shandong Province of China (ts20190946), and the Start-up Fund for High-level
Talents of Qingdao Agricultural University (6651121003). AUTHOR INFORMATION Author notes * These authors contributed equally: Lu Wang, Zi-Hui Yan. AUTHORS AND AFFILIATIONS * College of Life
Sciences, Key Laboratory of Animal Reproduction and Biotechnology in Universities of Shandong, Qingdao Agricultural University, Qingdao, 266109, China Lu Wang, Zi-Hui Yan, Tao-Ran He,
Hai-Xia Liu, Yu-Kang Li, Yi-Lin Niu, Jun-Jie Wang, Wei Ge & Wei Shen * Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, 00133, Italy Massimo De Felici
Authors * Lu Wang View author publications You can also search for this author inPubMed Google Scholar * Zi-Hui Yan View author publications You can also search for this author inPubMed
Google Scholar * Tao-Ran He View author publications You can also search for this author inPubMed Google Scholar * Hai-Xia Liu View author publications You can also search for this author
inPubMed Google Scholar * Yu-Kang Li View author publications You can also search for this author inPubMed Google Scholar * Yi-Lin Niu View author publications You can also search for this
author inPubMed Google Scholar * Jun-Jie Wang View author publications You can also search for this author inPubMed Google Scholar * Massimo De Felici View author publications You can also
search for this author inPubMed Google Scholar * Wei Ge View author publications You can also search for this author inPubMed Google Scholar * Wei Shen View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS LW and WG designed the research. LW, ZY, and YL performed the experiments. YN, TH, and HL analyzed data and prepared figures. ZY
drafted the manuscript. JW, MDF, WG, and WS revised the manuscript. All authors read and approved the final paper. CORRESPONDING AUTHORS Correspondence to Wei Ge or Wei Shen. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, L., Yan, ZH., He, TR. _et al._ In vitro oogenesis from murine premeiotic germ
cells using a new three-dimensional culture system. _Cell Death Discov._ 9, 276 (2023). https://doi.org/10.1038/s41420-023-01577-w Download citation * Received: 05 May 2023 * Revised: 18
July 2023 * Accepted: 20 July 2023 * Published: 31 July 2023 * DOI: https://doi.org/10.1038/s41420-023-01577-w SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative