Play all audios:
A long duration of treatment and emerging drug resistance pose significant challenges for global tuberculosis (TB) eradication efforts. Therefore, there is an urgent need to develop novel
strategies to shorten TB treatment regimens and to treat drug-resistant TB. Using an albumin-fusion strategy, we created a novel albumin-fused granulocyte-macrophage colony-stimulating
factor (albGM-CSF) molecule that harnesses albumin’s long half-life and targeting abilities to enhance the biostability of GM-CSF and direct it to the lymph nodes, where the effects of
GM-CSF can increase dendritic cell populations crucial for eliciting a potent immune response. In this study, we demonstrate that albGM-CSF serves as a novel immunotherapy for chronic
Mycobacterium tuberculosis (Mtb) infections by enhancing GM-CSF biostability in serum. Specifically, albumin is very safe, stable, and has a long half-life, thereby enhancing the
biostability of GM-CSF. In the lungs and draining lymph nodes, albGM-CSF is able to increase the numbers of dendritic cells, which are crucial for the activation of naive T cells and for
eliciting potent immune responses. Subcutaneous administration of albGM-CSF alone reduced the mean lung bacillary burden in mice with chronic tuberculosis infection. While GM-CSF
administration was associated with IL-1β release from Mtb-infected dendritic cells and macrophages, higher IL-1β levels were observed in albGM-CSF-treated mice with chronic tuberculosis
infection than in mice receiving GM-CSF. Albumin fusion with GM-CSF represents a promising strategy for the control of chronic lung tuberculosis infections and serves as a novel therapeutic
vaccination platform for other infectious diseases and malignancies.
Tuberculosis (TB) is currently the most common cause of death by a single infectious agent worldwide.1 Efforts have been made to implement a 6-month “short-course” combination regimen for
the treatment of drug-susceptible TB. Although this regimen has been shown to be efficacious, it requires proper provision and direct supervision, which can be taxing for health care
systems, especially in TB-endemic regions. Inadequate treatment of TB leads to excess morbidity and mortality, continued transmission, and emergence of drug resistance.2 Therefore, novel
strategies are needed to shorten the duration of curative TB treatment. In addition to novel antimicrobial agents, host-directed therapies represent attractive strategies to combat disease
due to drug-susceptible and drug-resistant Mycobacterium tuberculosis (Mtb).3 In particular, host-directed therapies may reverse TB-related lung inflammation and/or augment innate and
adaptive immune responses to accelerate mycobacterial clearance during anti-TB treatment.4
Effective immunity against TB depends on antigen presentation by MHC class I or class II molecules, which occurs in the draining lymph nodes (dLNs) at the site of infection.5 Dendritic cells
(DCs) are key antigen-presenting cells (APCs) that activate naive T cells by upregulating chemokine receptors and costimulatory molecules.5,6 Mature DCs are characterized by higher
expression of surface MHC class II molecules and the integrin-αX chain, as well as CD11c and other costimulatory molecules.7 Adoptive transfer of antigen-pulsed DCs has been shown to
significantly improve vaccination efficacy relative to control treatment, indicating that adequate antigen presentation in the lungs is one of the key factors for controlling Mtb infection.8
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a hematopoietic growth factor critical for DC generation, proliferation, and maturation.9,10,11 Other myeloid lineage cells,
including monocytes, macrophages, neutrophils, and eosinophils, are also activated by GM-CSF.9 Coadministration of GM-CSF during vaccination increases antigen-specific IFNγ-secreting T cells
and enhances protection against various infectious agents.12,13,14,15,16,17,18 Conversely, deficiency of GM-CSF is associated with reduced T-cell responses after vaccination.19 Mice
vaccinated with a bacillus Calmette–Guérin vaccine including cells expressing murine GM-CSF were found to have enhanced DC maturation in dLNs and increased protection against disseminated
TB.16 In addition to its promotion of the maturation of DCs, GM-CSF is one of the key cytokines that promotes the differentiation of M1 macrophages, which are key effectors in controlling
intracellular pathogens through the release of proinflammatory cytokines.20,21,22 GM-CSF enhances the ability of human macrophages to inhibit Mtb growth ex vivo.23 GM-CSF−/− mice are highly
susceptible to Mtb infection, and anti-GM-CSF autoantibodies increase the risk of cryptococcal meningitis and pulmonary TB in patients.10,24,25,26 GM-CSF secreted by T cells has been shown
to offer protection against Mtb infection in murine models.25 A greater proportion of GM-CSF+ multifunctional CD4+ T cells are present in latently infected individuals than in those with
active TB.27 Furthermore, GM-CSF secretion was significantly reduced when CD3+ T cells were cocultured with myeloid-derived suppressor cells in patients with active TB.28 These observations
indicate that GM-CSF plays an important role in innate immunity and initiating adaptive immunity, indicating the potential utility of this cytokine in anti-TB immunotherapy. Indeed, GM-CSF
enhances the bactericidal activity of anti-TB drugs in both mouse models and in humans.29,30,31 However, the observed synergy of GM-CSF is relatively limited due to its side effects, short
half-life of ~7 h, and reduced penetration into the lungs.32 Therefore, an alternative strategy is required to improve the bioavailability of GM-CSF in the lungs, which are the primary site
of TB.
A fusion strategy using the fragment crystallizable region (Fc region) has been used to improve the biostability and half-life of proteins, as well as for mucosal targeting.33,34,35 However,
one limitation of this approach is the potential development of autoantibodies directed against the Fc region.33,34 Similar to immunoglobulin, albumin has an extended serum half-life of 3
weeks due to its size and its ability to undergo neonatal Fc receptor (FcRn)-mediated recycling, thus preventing intracellular degradation.33 Since albumin is very safe and stable and has a
very long half-life, it has been frequently used for drug delivery. Currently, there are six albumin-based drugs that are commercially available, with many more being tested in clinical
trials.36 Labeled human albumin has been used for LN identification and imaging, suggesting that albumin can traffic to LNs.37 We reasoned that these favorable properties of albumin may be
exploited by fusing this protein to GM-CSF to enhance the serum levels of GM-CSF and augment its effect in the lungs and LNs, thus achieving organ-targeting vaccination. Specifically, we
determined whether murine albumin conjugation was able to increase the effects of GM-CSF in mice. In our proof-of-concept studies, we show that this albumin-fusion strategy (albGM-CSF)
enhances the serum levels of GM-CSF, leading to increased DC populations, cytokine secretion, and CD4+ T-cell activation, thus improving the control of chronic TB in mice.
To generate albumin-fused GM-CSF (albGM-CSF), mouse albumin was first amplified with PCR using the cDNA template of mouse albumin (AAH49971, transOMIC Technologies, Huntsville, AL, USA) and
a set of primers, 5′-AAATCTAGAGCCACCATGAAGTGGGTAACCTTT-3′ and 5′-TTTGAATTCGGCTAAGGCGTCTTTGCATC-3′. The amplified product was then cloned into the XbaI/EcoRI sites of a pcDNA3 vector
(Invitrogen Corp., Carlsbad, CA, USA). Next, for the generation of pcDNA3-AlbGM-CSF, mouse GM-CSF was first amplified via PCR with a cDNA template of the mouse GM-CSF (NM_009969.4) gene
synthesized from Genscript (Piscataway, NJ, USA) and the following primers: 5′-TTTGAATTCGCACCCACCCGCTCACCCAT-3′ and 5′-AAACTTAAGTCATTTTTGGACTGGTTTTTTG-3′. The amplified product was then
cloned into the EcoRI/Afl II sites of pcDNA3-Alb. For the generation of pcDNA3-albGLuc, Gaussia luciferase (GLuc) was first amplified via PCR with a cDNA template of phGLuc (gifted from Dr
John Schiller, NIH) and the primers 5′-AAAGAATTCATGGGAGTCAAAGTTCTGTTTG-3′ and 5′-TTTAAGCTTTTAGTCACCACCGGCCCCCTTG-3′. The amplified product was then cloned into the EcoRI/HindIII sites of
pcDNA3-Alb. For the generation of pET28a-GLuc, GLuc was first amplified via PCR with a cDNA template of phGLuc and the following primers: 5′-AAAGAATTCGAGGCCAAGCCCACCGAGAAC-3′ and
5′-TTTCTCGAGGTCACCACCGGCCCCCTTGA-3′. The amplified product was then cloned into the EcoRI/XhoI sites of the PET28a vector (Novagen Inc., Madison, WI, USA). All plasmid constructs were
confirmed by DNA sequencing. The AlbGM-CSF and albumin-GLuc (albGLuc) proteins were expressed using the Expi293F Expression System Kit (Thermo Fisher Scientific, Waltham, MA, USA) according
to the manufacturer’s instructions. Expi293F cells were transfected with albGM-CSF and alb-GLuc, and the transfection efficiency was determined by the expression levels of the target
protein. Proteins were purified by a HiTrap albumin column (GE Healthcare Life Sciences, Marlborough, MA, USA). GLuc was expressed in E. coli BL21 (Rosetta cells; Novagen) and purified by
Ni+ affinity chromatography (Ni-NTA agarose, Qiagen Sciences, Germantown, MD, USA) according to the manufacturer’s protocol). Mouse GM-CSF was purchased from Genscript.
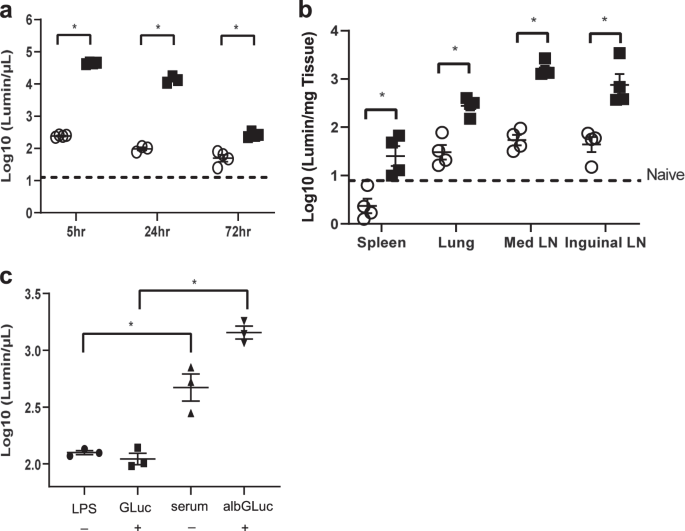
Eight-to-ten-week-old female C57BL/6J (n = 3–4) (NCI, Frederick, MD, USA) or eight-to-ten-week-old FcRn-knock-out (KO) mice (n = 3–4) (B6.129X1-Fcgrttm1Dcr/DcrJ, Jackson Laboratory, Bar
Harbor, ME, USA) received retro-orbital injections with either Gaussia luciferase (GLuc) (20 μg) or albGLuc (2.8 μg) in 20 μL of phosphate-buffered saline (PBS) following anesthesia by
ketamine/xylazine intraperitoneal injection. Seventy-two hours after injection, mice were euthanized, and the serum, inguinal LNs, and lungs were removed and disrupted by bead-beating. GLuc
activity was measured with coelenterazine-H (Regis) by a GloMax Luminometer (Promega, Madison, WI, USA). The total luminescence was normalized to tissue weight.
The mice were housed in the Oncology Center Animal Facility at the Johns Hopkins Medical Institutes (Baltimore, MD, USA). All animal procedures were performed according to the approved
protocols and in accordance with the recommendations for the proper use and care of laboratory animals. To ensure that animal discomfort, distress, pain, and injury were kept to a minimum, a
maximum of five mice were housed in the same cage. All animals were maintained and all experiments were performed according to the protocols approved by the Institutional Animal Care and
Use Committee at the Johns Hopkins University School of Medicine.
Female C57BL/6J mice (6–8 weeks old; n = 3–4) received 20 μg of lipopolysaccharide (LPS) from E. coli O26:B6 (Sigma-Aldrich, St. Louis, MO, USA) via the intranasal route. The control group
received PBS. One day later, both groups of mice received either GLuc (20 μg) or albGLuc (2.8 µg) by intranasal injection. To measure luciferase activity, sera from each group were collected
the following day. Luciferase expression was taken to indicate transcytosis activity in the airway, as previously described.38
Wild-type Mtb H37Rv was grown in Middlebrook 7H9 broth (Difco, Sparks, MD, USA) supplemented with 10% oleic acid-albumin-dextrose-catalase (Difco), 0.1% glycerol, and 0.05% Tween-80 at 37 °C
in a roller bottle.39
Bone marrow was harvested from C57BL/6J mice, as previously described.40 To compare the efficacy of GM-CSF and albGM-CSF, bone marrow cells were incubated with either GM-CSF (0.625 µM) or
albGM-CSF (0.625 µM) at 37 °C in 5% CO2 and RPMI media with 10% fetal bovine serum (FBS) for 7 days (Sigma-Aldrich). Antigen-containing supplemented media were replenished on day 3. The
cells were collected to analyze the percentage of CD11c+ cells and CD11c+MHCII+ cells. For Mtb infection studies, bone marrow cells were incubated with GM-CSF (10 ng/mL, Genscript) to obtain
BMDCs or with macrophage colony-stimulating factor (10 ng/mL, Genscript) to obtain BMDMs. A total of 2 × 105 BMDMs or 5 × 105 BMDCs were plated in a 24-well plate 1 day prior to infection.
H37Rv was used to infect BMDCs at an MOI of 1:2.5 (1.25 × 106 bacteria) for 1 day or BMDMs (5 × 105 bacteria) for 2 days in 1 mL of complete RPMI medium (Gibco Laboratories, Gaithersburg,
MD, USA) with 10% FBS (Sigma-Aldrich) and 0.625 µM GM-CSF or albGM-CSF.
The levels of GM-CSF in sera and IL-1β in cell culture media were determined by ELISAs with mouse GM-CSF or IL-1β DuoSet ELISA kits from R&D Systems (Minneapolis, MN, USA). The IL-1β levels
of lung lysates were normalized to the lysate protein concentration using a Qubit protein assay kit (Thermo Fisher Scientific).
At predetermined time points, the mice were euthanized, and cells from LNs and spleens were collected, as previously described.41,42 To determine ESAT6 antigen-specific CD4+ or TB10
antigen-specific CD8+ T-cell responses, splenocytes were incubated with ESAT6 ((MTEQQW NFAGIEAAA) or TB10 (IMYNYPAM) peptides (Genscript) and GolgiPlug (BD Biosciences, San Jose, CA, USA)
overnight.43 After incubation, the cells were washed once with FACScan buffer and then stained with a PE-conjugated monoclonal rat anti-mouse CD4 antibody (BD Biosciences) and/or an
APC-conjugated monoclonal rat anti-mouse CD8 antibody (eBioscience, Inc., San Diego, CA, USA). Cells were permeabilized using the Cytofix/Cytoperm kit (BD Biosciences). Intracellular IFN-γ
was stained using a FITC-conjugated rat anti-mouse IFN-γ antibody. Flow cytometry was performed on a FACSCalibur instrument, and the results were analyzed with FlowJo software (Supplementary
Fig. 1). To collect pneumocytes, the lungs were perfused with 1 mL of normal saline by direct injection into the right ventricle of the heart at necropsy. A section of the lung was used for
cytometry analysis, and the tissue samples were incubated at 37 °C for 1 h with intermittent agitation in RPMI medium (Gibco Laboratories) containing collagenase D (1 mg/mL, Sigma-Aldrich),
DNAase (0.25 mg/mL, Sigma-Aldrich), and hyaluronidase type V (1 mg/mL, Sigma-Aldrich). The cells were then filtered through a 70-μm nylon filter mesh to remove undigested tissue fragments
and washed with complete RPMI medium. To identify surface markers of DCs from lungs, spleens, or LNs, PE-conjugated anti-mouse MHCII, FITC-conjugated anti-mouse CD11c, APC-conjugated
anti-mouse CD103, APC-conjugated anti-mouse CD11b, and APC-conjugated anti-mouse DEC205 (eBioscience, Inc.) antibodies were used to stain cells from each of these tissues (Supplementary
Figs. 2 and 3). After washing with FACScan buffer, the cells were counted with a FACSCalibur and analyzed using FlowJo software (Supplementary Figs. 2 and 3).
Female 6–8-week-old C57BL/6J mice were aerosol-infected with ~100 bacilli of wild-type Mtb H37Rv. After 1 month of infection, groups of mice received human-equivalent doses of isoniazid (10
mg/kg) by esophageal gavage once daily (5 days/week). In the experimental groups, mice received 0.375 μM GM-CSF or albGM-CSF in 100 µL of PBS by retro-orbital or subcutaneous injection at
predetermined time points. Mice were euthanized on days 30, 60, and 90 after aerosol challenge, lungs were homogenized, and the cells were plated for colony-forming unit assays to evaluate
the potential synergistic effect of each therapeutic vaccine.44
Mean differences between groups were compared using one-way analysis of variance with Tukey–Kramer post hoc analyses (MedCalc software, Ostend, Belgium). If the data did not pass through the
normality test by the D’Agostino–Pearson test, the differences of groups were compared by the Kruskal–Wallis test with post hoc analyses. Data from at least three biological replicates were
used to calculate means and standard errors of the mean (SEMs) for graphing purposes. To compare differences between experimental and control groups, statistical analyses employed the
Mann–Whitney test for sample sizes less than 4 or an unpaired Student’s t test for sample sizes >4, and a p value of