Play all audios:
ABSTRACT Propylene production via propane dehydrogenation (PDH) requires high reaction temperatures to obtain sufficient propylene yields, which results to prominent catalyst deactivation
due to coke formation. Developing highly stable catalysts for PDH without deactivation even at high temperatures is of great interest and benefit for industry. Here, we report that
single-atom Pt included in thermally stable intermetallic PtGa works as an ultrastable and selective catalyst for PDH at high temperatures. Intermetallic PtGa displays three-hold-Pt
ensembles and single Pt atoms isolated by catalytically inert Ga at the surface, the former of which can be selectively blocked and disabled by Pb deposition. The PtGa-Pb/SiO2 catalyst
exhibits 30% conversion with 99.6% propylene selectivity at 600 °C for 96 h without lowering the performance. The single-atom Pt well catalyzes the first and second C–H activation, while
effectively inhibits the third one, which minimizes the side reactions to coke and drastically improves the selectivity and stability. SIMILAR CONTENT BEING VIEWED BY OTHERS SUPPRESSING COX
IN OXIDATIVE DEHYDROGENATION OF PROPANE WITH DUAL-ATOM CATALYSTS Article Open access 19 May 2025 DUAL-ATOM PT HETEROGENEOUS CATALYST WITH EXCELLENT CATALYTIC PERFORMANCES FOR THE SELECTIVE
HYDROGENATION AND EPOXIDATION Article Open access 26 May 2021 HIGH-ENTROPY INTERMETALLICS ON CERIA AS EFFICIENT CATALYSTS FOR THE OXIDATIVE DEHYDROGENATION OF PROPANE USING CO2 Article Open
access 29 August 2022 INTRODUCTION Propylene is one of the most important building blocks for the production of a wide range of chemicals, such as polymers, resins, surfactants, dyes, and
pharmaceuticals1. The supply of propylene has been reduced because of the recent shift in feedstock for steam crackers from oil-based naphtha to shale-based ethane. Catalytic propane
dehydrogenation (PDH) using Pt- or Cr2O3-based materials is a promising on-purpose technique to satisfy the increasing global demand of propylene production1,2,3. Owing to the
endothermicity, high reaction temperatures (preferably ≥600 °C) are required to obtain sufficient propylene yields. However, severe catalyst deactivation due to coke deposition and/or
sintering is inevitable under such harsh conditions; therefore, the catalysts in practical use must be regenerated continuously or in short cycles. Although a number of literatures on
catalytic PDH have been reported to this day, no catalyst that exhibits high catalytic activity, selectivity, and day-long stability at high temperatures (≥600 °C) has been developed to the
best of our knowledge1,2,3,4,5. Developing a catalyst to meet this demanding task is of a great challenge in pure and applied chemistry. Generally, selectivity and stability in PDH are
determined by the balance between whether the product propylene desorbs or undergoes undesired side reactions, such as further C–H(C) scissions and the subsequent coke formation6,7,8,9,10.
For Pt-based catalysts, Pt–Pt ensembles are known to be active for over-dehydrogenation of propylene and its hydrogenolysis1. The isolation of Pt atoms is a promising strategy to inhibit
these undesired side reactions in PDH11. For instance, alloying of active main metal (mostly Pt) with a certain inactive metal (mostly typical elements such as Sn) has been a conventional
approach to dilute Pt–Pt ensembles and enhance propylene selectivity and stability1. However, it is difficult to completely isolate Pt atoms by the conventional alloying approach.
Single-atom12,13,14,15 and single-atom alloy11,16 catalysts are also effective tools to use isolated Pt, where active metals are atomically dispersed on an oxide support and isolated by
excess amount of 11 group metal like Cu, respectively. However, it is difficult to apply them to high-temperature reactions such as PDH due to its insufficient thermal stability: Pt atoms17
or alloy nanoparticles11,16 without spatial separation13 are easily aggregated to form larger nanoparticles at very high temperatures. A possible candidate to solve this challenge is
single-atom-like isolated Pt included in thermally stable intermetallic compounds. For instance, the 1:1 compound of Pt and Ga with cubic _P_213 space group has thermal stability (Δ_H_f =
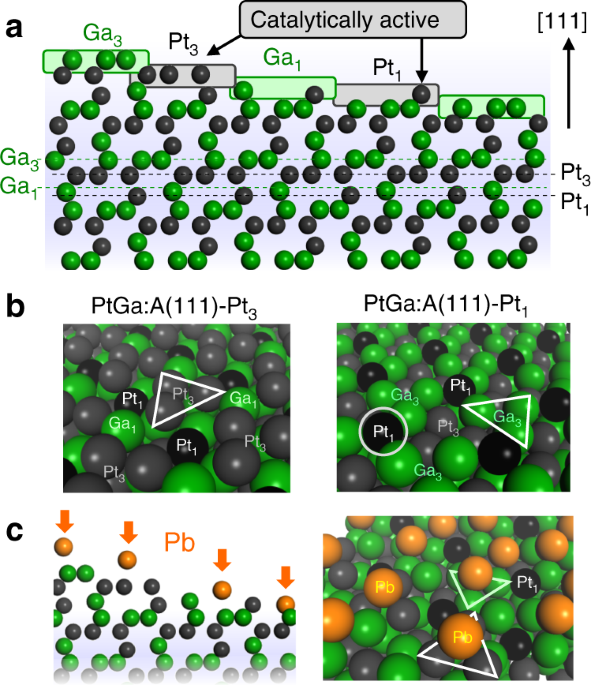
–55.6 kJ/mol−1) much greater than typical random alloys (–10 < Δ_H_f ≤ 0 kJ mol−1) and a unique structure for this purpose as shown in Fig. 1 (refs. 18,19). The stable (111) surface of
PtGa has four different terminations displaying isolated and threefold Pt and Ga sites (hereafter signed Pt1, Ga1, Pt3, and Ga3). Here, the Ga3 moiety can be regarded as a matrix to support
the isolated Pt1 atom; therefore, it may be possible to describe the Pt1 site as “single-atom Pt”. Note that there are two enantiomeric forms of PtGa unit cell (PtGa:A and PtGa:B, the former
is shown in Fig. 1), because the space group _P_213 is chiral. In an analogous system of PdGa (space group _P_213), such surface termination (Pd3 and Pd1, which were described as trimer and
single atom, respectively) has actually been observed by surface science techniques20,21. For the PdGa system, Pd3 is known to catalyze semihydrogenation of acetylene more selectively than
Pd1 (ref. 20). For PtGa in PDH, however, the Pt3 site is expected to be more active for further C–H(C) scissions. Therefore, some modification that makes only Pt3 sites disabled while Pt1
sites available for the reaction is needed for achieving highly selective and stable PDH. In this study, we design Pb-modified PtGa where the threefold Pt is selectively blocked by Pb
deposition while the single-atom Pt remains intact (Fig. 1c). As demonstrated later, the convex Pt1 site is unfavorable geometrically and energetically for Pb deposition. We prepare
SiO2-supported PtGa and PtGa–Pb (Pt/Pb = 2) catalysts by an impregnation method (reduced by H2 at 700 °C) and test in PDH at high temperatures (600 or 650 °C; note that it is lower than the
preparation temperature). Here, we show a different type of single-atom Pt and its outstandingly high catalytic performance in PDH at high temperature. RESULTS CHARACTERIZATION OF CATALYSTS
Figure 2a shows the high-resolution high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image of PtGa–Pb/SiO2 with a single nanoparticle. A crystal structure
with interplanar distances of 2.07 and 2.25 Å and dihedral angle of 56° was observed, which agreed with those of (211) and (20\(\bar 1\)) planes of intermetallic PtGa viewed along with
[1\(\bar 4\)2] direction22 (Fig. 2b). The particle size distribution was narrow (mostly 1.5–3 nm, Supplementary Fig. 1) with an average of 2.8 ± 0.6 nm. The elemental map acquired by
energy-dispersive X-ray (EDX) analysis showed that Pt and Ga were homogeneously distributed in each nanoparticle with approximately 1:1 ratio (Fig. 2c). Similar results of the HAADF-STEM-EDX
analyses were also obtained for PtGa/SiO2 (Supplementary Fig. 2, Supplementary Note 1). On the contrary, the Pb distribution in PtGa–Pb/SiO2 was focused on the shell part of nanoparticles
(areas 1 and 2 in Fig. 2c). Considering that the Pb content in the whole nanoparticle (area 1) is lower than those fed in the catalyst (Pt/Pb = 2), a part of Pb may present on SiO2 support.
X-ray photoelectron spectroscopy (XPS) analysis with Ar+ sputtering revealed that the Pt4_f_7/2-binding energy of PtGa–Pb was lower than that of PtGa (due to ligand effect of Pb)23, but came
close immediately after several sputtering (Fig. 2d, see Supplementary Fig. 3 for the spectra). This result strongly supports that Pb is located at the surface region of PtGa nanoparticles.
We also performed X-ray adsorption fine structure (XAFS) analysis (see Supplementary Notes 2 and 3, Supplementary Figs. 4–7, and Supplementary Table 1 for XAFS analysis: Pt LIII-edge X-ray
adsorption near edge spectra, extended XAFS (EXAFS) raw oscillations, EXAFS curve fits, magnitude of Fourier transform of EXAFS, and details of the curve fit). Pt–Ga scattering with 2.50 ±
0.01 Å was observed for PtGa–Pb, which is consistent finely with the interatomic distance of the nearest Pt and Ga in PtGa (2.499 Å)22. This result suggests that Pb atoms are not substituted
into the bulk of PtGa to increase the lattice constant. Pt–Pb scattering was also observed with a small coordination number of 1.0, which indicates that the surface Pt sites are partly
blocked by Pb deposition. CO pulse chemisorption experiment supported the partial coverage of surface Pt, where Pt dispersion decreased from 9.9% to 5.9% upon the Pb modification to
PtGa/SiO2 (Supplementary Table 2). To obtain further information about the surface of PtGa–Pb/SiO2, we then performed Fourier-transform infrared (FT-IR) spectroscopy with CO adsorption at
−196 °C (Fig. 3). For PtGa/SiO2, two peaks appeared at 2078 and 1885 cm−1 at the initial stage, which are assigned to stretching vibration of CO adsorbed on Pt with on-top and threefold
modes, respectively21. Upon the increase in CO pressure (_P_CO), the threefold CO disappeared and the intensity of the on-top CO increased with an appearance of a small shoulder feature at
around 2050 cm−1. This change could be attributed to the migration of threefold CO to on-top CO on the Pt3 site due to the increase of CO coverage. The new shoulder at around 2050 cm−1 might
be assigned to on-top CO adsorbed on Pt1 site21. On the contrary, for PtGa–Pb/SiO2, only a single symmetric adsorption band appeared at 2040 cm−1 with lower intensity even at saturation
coverage, which implies that the Pt3 sites are blocked by Pb while the remaining Pt1 sites are open for CO adsorption. We then simulated the theoretical _ν_C=O for the suggested
conformations by density functional theory (DFT) calculations (see Supplementary Fig. 8 for the detailed structures and _ν_C=O values). The calculated _ν_C=O values were consistent finely
(on-top CO) or roughly (threefold CO) with the corresponding experimental values (Fig. 3, vertical lines), which strongly supports the assignment mentioned above. The observed trend agreed
also with a relevant system of CO adsorption on PdGa:B(111) monitored by surface science techniques21. Only a slight red-shift in _ν_C=O (2043 to 2037 cm−1) was suggested when Pb was added
near the Pt1 site, likely because of electron-enriched Pt by the ligand effect of Pb as observed in Fig. 2d. Thus, we successfully prepared an ideal catalyst for PDH with single-atom-like
isolated Pt without any Pt–Pt ensembles. CATALYTIC PERFORMANCE IN PDH Next, we tested the catalytic performances of the prepared catalysts in PDH at 600 °C (Fig. 4), in which the equilibrium
propylene yield in this reaction condition was approximately 60% (Supplementary Fig. 9). Although PtGa exhibited high conversion and selectivity at the initial stage (40% conv., 99.1% sel.
at 0.5 h), conversion gradually decreased below half of its initial value within 50 h. Conversely, PtGa–Pb retained high conversion and excellent selectivity (>30% conv., >99.6% sel.)
for 50 h even under the harsh condition. It should be noted that almost no deactivation was observed even at 96 h (Supplementary Fig. 10). Thus, the Pb modification to PtGa significantly
improved the stability and selectivity. We achieved the long-term, continuous, and highly selective propylene production in PDH at high temperatures without deactivation (>580 °C: see
Supplementary Tables 3 and 4 and Supplementary Fig. 11 for comparison with literatures; deactivation rate constant was defined in Supplementary Note 4). We also tested Pt3Sn catalyst, the
well-known catalyst selective for PDH1,7, which gave lower conversion, selectivity, and stability (higher deactivation rate, Supplementary Table 5) than PtGa, highlighting the outstandingly
high catalytic performance of PtGa–Pb. The spent catalysts were then analyzed by temperature-programed oxidation (TPO) and the HAADF-STEM-EDX analysis. PtGa and Pt3Sn showed coke combustion
peaks in their TPO profiles, while PtGa–Pb gave no peak (Supplementary Fig. 12). This is consistent with the stability trend in Fig. 4 and suggests that the coke formation process is
strictly inhibited. The HAADF-STEM-EDX analysis revealed that, despite the long-term operation (50 h) in the harsh condition, PtGa–Pb retained its small particles sizes (flesh: 2.8 ± 0.6 nm,
spent: 3.0 ± 0.6 nm), intermetallic structure, and elemental distribution (Supplementary Fig. 13, Supplementary Note 5), demonstrating the high thermal stability and resistance to
sintering. The stability test was also conducted at 650 °C, where PtGa–Pb retained high conversion (37–38%) for several hours and then gradually decreased to approximately 20% over 50 h
(Supplementary Note 6, Supplementary Fig. 14). The gradual deactivation can be attributed to the contribution of thermal (noncatalytic) cracking24. This was confirmed by a control experiment
using SiO2, in which small amount of C1 and C2 were formed at 650 °C, while that was negligible at 600 °C (Supplementary Fig. 15). Other bimetallic combinations that have been reported to
be effective for PDH (PtSn (ref. 25) and Pt3In (ref. 8); see Supplementary Note 7 and Supplementary Fig. 16 for details and their X-ray diffraction (XRD) patterns, respectively) were also
tested at 600 °C. However, they all showed deactivation trends similar to that of Pt3Sn (Supplementary Fig. 17, Supplementary Table 5). Considering that Pt–Ga (Pt/Ga = 3) gave higher
deactivation rate and lower selectivity than PtGa, using 1:1 PtGa phase is a significant factor to develop a highly efficient catalytic system for PDH (Supplementary Fig. 17, Supplementary
Table 5). When the modifier for PtGa was changed from Pb to other metals such as In or Sn, no positive effects on activity and selectivity were obtained (Supplementary Fig. 14). We also
tested the recyclability of PtGa–Pb catalyst (Supplementary Note 8, Supplementary Fig. 18). The spent PtGa–Pb catalyst could be regenerated by O2 treatment to recover the original catalytic
performance after some induction period, whereas some other bimetallic or trimetallic Sn-containing catalysts (Pt3Sn, PtSn, and PtGa–Sn) did not (Supplementary Fig. 19). Therefore, the
combination of intermetallic PtGa and the Pb modification is suitable for stabilizing single-atom-like isolated Pt at high temperature. This is probably because (1) PtGa itself is
thermodynamically stable (Δ_H_f = –55.6 kJ mol−1)18,19 and (2) the atomic radius26 of Pb (1.80 Å) is much larger than those of Pt (1.35 Å) and Ga (1.30 Å): the diffusion of Pb into the bulk
of PtGa is likely to be unfavorable even at 600 °C. Although several researchers have pointed that Ga works as a good promotor for Pt-based PDH as well as other typical element such as Sn or
In (refs. 27,28,29,30,31,32,33), our results indicate that the geometry and appropriate design of an active site is more significant rather than the individual chemical property of the
additive element, that is, Pt should be strictly isolated. We also surveyed various Pt/Pb ratios and metal oxides as catalyst supports, which confirmed that PtGa–Pb/SiO2 (Pt/Pb = 2) was the
best (Supplementary Notes 9 and 10, Supplementary Figs. 20 and 21). Ga itself has also been known to be active for PDH34. However, a control experiment using Ga/SiO2 at 650 °C (Supplementary
Fig. 22) showed very low conversion (<3%), indicating the negligible contribution of Ga itself to the catalysis of much more active Pt-based materials. DFT CALCULATIONS Finally, we
conducted DFT calculations for the step-wise C–H scissions of propane to clarify the detailed property of isolated Pt for selective PDH. Figure 5 summarizes the reaction scheme of PDH and
the calculated energy barrier of each step (_E__x_: _x_ = 1, 2, 3, and _d_; see Supplementary Figs. 23–25, Supplementary Fig. 26, and Supplementary Table 6 for the detailed structures,
energy diagram, and summarized activation energies, respectively). The adsorbed propylene (C3H6(a)) formed via the first and second C–H scissions undergoes desorption to gas phase (C3H6(g))
or further (third) C–H scission to trigger undesired side reactions6,7,8,9,10. Here, propylene selectivity depends on the difference in the two energy barriers (Δ_E_ = _E_3 − _E_d, shaded
part in Fig. 5): the larger Δ_E_ is, the higher the selectivity is. PtGa-Pt3 gave Δ_E_ of 35.9 kJ mol−1, which was slightly larger than that of Pt3Sn(111) (24.1 kJ mol−1). Interestingly,
PtGa-Pt1 having the isolated Pt showed much larger Δ_E_ of 64.9 kJ mol−1. This is due to the remarkably high _E_3 (173.2 kJ mol−1) even though _E_1 (typically the rate-determining step of
PDH) and _E_d did not differ significantly from those of PtGa-Pt3 and Pt3Sn-Pt3 sites. The specifically high _E_3 could be attributed to the molecular rotation from lying 1,2-π-C3H6 to
vertically standing 2-σ-C3H5 conformations occurring at the convex Pt1 site (Supplementary Fig. 25c). Because of the molecular rotation and long Pt–Pt distance between the Pt1 site and the
nearest neighboring Pt3 site (3.06 Å), the hydrogen atom involved in the third C–H scission has to migrate a long distance toward the final state. The energy required for such an unfavorable
path becomes significantly high. We also estimated the theoretical propylene selectivity based on the Arrhenius equation with Δ_E_ (see Supplementary Note 11 for details), which are listed
in Table 1 with the corresponding experimental values. The calculated values and their order were consistent with the experimental results, which demonstrates the validity of our calculation
model. Thus, our calculation successfully reproduced the experimental trends in selectivity. The high propylene selectivity of Pt1 sites minimizes the accumulation of coke, which leads to
the outstandingly high catalyst stability. Finally, we investigated the affinity of Pb deposition to several Pt and Ga sites. Pb atoms adsorbed stably on the Pt3, Ga3, and concave Pt1 (in
PtGa-Pt3 termination) sites with large adsorption energies (−462 to −337 kJ mol−1, Supplementary Fig. 27), while could not on the convex Pt1 site: the Pb atom placed on the top of the Pt1
site migrated downward during structure optimization. This result indicates that the convex Pt1 site is unfavorable for Pb deposition geometrically and energetically. DISCUSSION In summary,
we designed and prepared the PtGa–Pb/SiO2 catalyst for highly selective PDH, in which threefold hollow Pt3 ensembles were successfully blocked by Pb deposition, while the single-atom-like
isolated Pt1 sites remained. The isolated Pt1 is highly selective (99.6%) for propylene production and the catalyst is outstandingly stable for long-term operation at high temperature (96 h,
600 °C). The catalytic performance in PDH is much superior to those of the reported systems. The combination of (1) the specific crystal structure of intermetallic PtGa providing isolated
Pt, (2) its thermal stability, and (3) the large atomic size of Pb enables the remarkably high selectivity and stability even in harsh conditions. The results obtained in this study provide
not only a highly efficient catalytic system for alkane dehydrogenation but also significant insights for material design to isolate and stabilize active metals. METHODS MATERIALS SiO2
(CARiACT G–6, Fuji Silysia, _S_BET = 673 m2 g−1), Al2O3 (prepared by the calcination of boehmite [γ-AlOOH, supplied by SASOL chemicals] at 900 °C for 3 h, γ-phase), CeO2 (JRC-CEO-2, _S_BET =
123.1 m2 g−1), ZrO2 (JRC-ZRO-6, _S_BET = 279.3 m2 g−1), and TiO2 (P-25, anatase). MgAl2O4 support was prepared by a co-precipitation method using urea as a precipitating agent. The
precursors and urea were precisely weighted and dissolved together in deionized water so that urea/precursors atomic ratio was 20. Mixed aqueous solution of Mg(NO3)3·6H2O, Al(NO3)3·9H2O and
urea was stirred overnight at 90 °C. After the precipitation, the solution was washed with water five times and dried overnight in oven at 90 °C, followed by calcination at 800 °C in the dry
air for 5 h. CeZrO2 and CaZrO3 were prepared in a same co-precipitation method used to prepare MgAl2O4. Ce(NO3)3·6H2O, Zr(NO3)2O·2H2O, and Ca(NO3)2·4H2O were used as precursors. CATALYST
PREPARATION (1) Pt-based bimetallic catalysts were prepared by the pore-filling co-impregnation method using SiO2 as the support (Pt3M/SiO2, and PtM/SiO2, where M = Ga, In, Sn, and Pb; Pt: 3
wt%). Ga(NO3)3·_n_H2O (_n_ = 7–9), SnCl2, Pb(NO3)2 were used as second metal precursors. The ratio of precursors was fixed at the desired ratio. Mixed aqueous solution of Pt(NH3)2(NO2)2 and
second metal was added dropwise to ground dried SiO2 so that the solutions just filled the pores of the SiO2. The mixture was kept in a sealed round-bottom flask overnight at room
temperature, followed by quick freezing with liquid nitrogen, freeze-drying in vacuum at −5 °C. The resulting powder was further dried in an oven at 90 °C overnight, calcined in dry air at
400 °C for 1 h, and finally reduced by H2 (0.1 MPa, 50 mL min−1) at 700 °C for 1 h. The catalysts except Pt/SiO2 were further annealed at 400 °C for 2 h under flowing H2 (0.1 MPa, 50 mL
min−1) to enhance alloying without further sintering. Ga/SiO2 catalyst with 5wt% loading was prepared using a similar method (reduction was carried out at 900 °C for 1 h). (2) The
corresponding silica-supported trimetallic catalysts were also prepared using a similar method for PtGa/SiO2 [PtGa–Pb/SiO2, where Pt/Pb = 5, 2.5, 2, and 1.5; PtGa-M/SiO2, where M = In and
Sn, Pt/M = 2; Pt: 3 wt%]. In(NO3)3·8.8H2O (determined by ICP-AES), SnCl2, and Pb(NO3)2·6H2O were used as third metal precursors. (3) A series of Pt–Ga bimetallic catalysts supported on
various oxides (PtGa/X, where _X_ = γ-Al2O3, MgAl2O4, CeO2, CeZrO2, ZrO2, CaZrO3, and TiO2; Pt/Ga = 1; Pt: 3 wt%) was prepared by the conventional impregnation method. To prepare the
precursor solution, Pt(NH3)2(NO3)2 and Ga(NO3)3·_n_H2O (_n_ = 7–9) were dissolved in an excess amount of water (ca. 25 mL of ion exchanged water per g of support). The oxide support was
added to a vigorously stirred aqueous solution of the metal precursors and kept with stirring at 90 °C for 3 h. The mixture was dried using a rotary evaporator at 50 °C and further dried
overnight in an oven at 90 °C. The resulting powder was treated in a similar manner for the SiO2-supported alloy catalysts as mentioned above. CATALYTIC REACTION PDH was carried out in a
vertical, quartz fixed-bed reactor with 6 mm of internal diameter under an atmospheric pressure. Generally, 15 mg of catalysts diluted with quartz sand (total: 1.5 g) were charged in the
reactor. For the experiments in Fig. 4, the catalyst amount was adjusted so that the number of exposed Pt was identical (4.5 μmol): PtGa (9.0 mg), PtGa–Pb (15 mg), Pt3Sn (3.7 mg). Prior to
the catalytic test, the catalyst was prereduced under flowing H2 at 650 °C and held at 650 °C for 0.5 h. After the pretreatment, the temperature was kept at 650 °C or decreased to 600 °C,
followed by feeding reactant gas mixture; C3H8:H2:He = 3.9:5:40, a total of 48.9 mL min−1 (WHSV = 30.7 h−1). The resulting product gas was analyzed by online thermal conductivity detector
(TCD) gas chromatograph (Shimadzu GC-8A with a column of Unipak S, GL Science) equipped downstream. For all the catalysts, C3H8, C2H4, C2H6, and CH4 were detected as reaction products. The
C3H8 conversion, C3H6 selectivity, C3H8 yield, and material balance were defined by Eqs. (1)–(4), respectively. Material balance typically ranged between 95% and 105% for all the reactions.
$${\mathrm{C}}_3{\mathrm{H}}_8\;{\mathrm{conversion}}\;\left( \% \right) = \frac{{\left[ {{\mathrm{{C}}}_3{\mathrm{{H}}}_{8}} \right]_{\mathrm{{inlet}}} \,- \, \left[
{{\mathrm{{C}}}_3{\mathrm{{H}}}_{8}} \right]_{\mathrm{{outlet}}}}}{{\left[ {{\mathrm{{C}}}_3{\mathrm{{H}}}_{8}} \right]_{{\mathrm{{inlet}}}}}} \times 100$$ (1)
$${\mathrm{C}}_3{\mathrm{H}}_6\;{\mathrm{selectivity}}\;\left( \% \right) = \frac{{\left[ {\mathrm{{C}}}_3{\mathrm{{H}}}_{6} \right]}}{{\left[ {\mathrm{{C}}}_3{\mathrm{{H}}}_{6} \right] +
\frac{2}{3}\left[ {\mathrm{{C}}}_2{\mathrm{{H}}}_{6} \right] + \frac{2}{3}\left[ {\mathrm{{C}}}_3{\mathrm{{H}}}_{4} \right] + \frac{1}{3}\left[ {\mathrm{{CH}}}_{4} \right]}} \times 100$$ (2)
$${\mathrm{C}}_3{\mathrm{H}}_6\;{\mathrm{yield}}\;\left(\% \right) = \frac{{\left[ {\mathrm{{C}}}_3{\mathrm{{H}}}_{6} \right]_{\mathrm{{outlet}}}}}{{\left[
{\mathrm{{C}}}_3{\mathrm{{H}}}_{8} \right]_{\mathrm{{inlet}}}}} \times 100$$ (3) $${\mathrm{Material}}\,{\mathrm{balance}}\;\left( \% \right) = \frac{{\left[
{\mathrm{{C}}}_3{\mathrm{{H}}}_{8} \right]_{\mathrm{{outlet}}} + \left[ {\mathrm{{C}}}_3{\mathrm{{H}}}_{6} \right] + \frac{2}{3}\left[ {\mathrm{{C}}}_2{\mathrm{{H}}}_{6} \right] +
\frac{2}{3}\left[ {\mathrm{{C}}}_2{\mathrm{{H}}}_{4} \right] + \frac{1}{3}\left[ {\mathrm{{CH}}}_{4} \right]}}{{\left[ {\mathrm{{C}}}_3{\mathrm{{H}}}_{8} \right]_{\mathrm{{inlet}}}}} \times
100$$ (4) CHARACTERIZATION XRD patterns of the Pt-based catalysts were obtained using a MiniFlex 700+D/teX Ultra (X-ray source: Cu _K_α radiation). HAADF-STEM analysis was performed by an
FEI Titan G2 or a JEOL JEM-ARM200 M microscope with an EDX detector. The volume averaged particle size in a TEM image (_d_TEM) was obtained by the following equation: $$d_{\mathrm{{TEM}}} =
\frac{{\mathop {\sum }\nolimits_i n_id_i^4}}{{\mathop {\sum }\nolimits_i n_id_i^3}},$$ (5) where _n__i_ and _d__i_ indicate the number of particles (having the size of _d__i_) and the
particle diameter, respectively. Pt dispersion in the catalysts (percentage of exposed Pt to the total amount of Pt) was measured by chemisorption of CO at room temperature. Prior to
chemisorption, the catalyst (50 mg) was treated by 5% H2/Ar (40 mL min−1) at 300 °C for 0.5 h, followed by cooling to room temperature with an He purge (40 mL min−1) to remove chemisorbed
hydrogen. We introduced a pulse of 10% CO/He into the reactor and quantified the CO passed through the catalyst bed using a TCD detector. This pulse measurement was repeated until no more CO
was adsorbed. We estimated the amount of chemisorbed CO assuming a 1:1 stoichiometry for CO chemisorption on a surface Pt atom. XPS study was conducted using a JEOL JPS-9010MC spectrometer
(X-ray source: Mg-_K_α radiation). The catalysts were treated by flowing H2 at 650 °C for 0.5 h in a quartz reactor, followed by transferring into the spectrometer in air. The surface of the
catalyst was sputtered by Ar+ (voltage: 400 V, rate: 20%, time: 1 s, at each cycle) for the depth analysis. Calibration of the binding energy was performed with the Si 2_p_ emission of the
SiO2 support (103.9 eV). FT-IR spectra of adsorbed CO were obtained with a JASCO FTIR-4100 spectrometer with a TGS detector in the transmission mode (resolution 4 cm−1) under a dynamic
condition. Prior to CO chemisorption, 50 mg of the catalyst was pressed into a pellet (diameter of 20 mm) and placed in a quartz cell equipped with CaF2 windows and a Dewar vessel, followed
by reduction under a flowing H2 at 550 ˚C for 1 h. The reduced sample was then kept in vacuum at 550 ˚C for 1 h, then the cell was cooled to ca −196 °C by liquid nitrogen. The sample was
exposed to a pulse of low-pressure CO, and then evacuated in vacuum to remove the gaseous CO and concentrated CO on the catalyst. This CO exposure was repeated several times until the CO
saturation coverage. TPO experiment was performed to quantify the amount of coke deposited on the spent catalysts after 20 h of PDH at 600 °C (15 mg of the catalyst without quartz sand). The
spent catalyst (10 mg) placed in a quartz tube reactor was treated under flowing He (40 mL min−1) at 150 °C for 30 min, followed by cooling to room temperature. Then, the catalyst bed
temperature was increased (25–900 °C, ramping rate: 5 °C min−1) under flowing O2/He (50%, 40 mL min−1). The amount of CO2 in the outlet gas was quantified by an online mass spectrometer.
XAFS spectra of the prepared catalysts were collected at the BL01B1 beamline of SPring-8, Japan Synchrotron Radiation Research Institute (JASRI) using an Si(111) double-crystal as a
monochromator. Prior to the measurement, the catalyst was pelletized (ca. 150 mg with a diameter of 10 mm) and pretreated by H2 at 650 °C for 0.5 h in a quartz tube. After the pretreatment,
the quartz tube containing the reduced pellet was sealed and transferred into an Ar grove box (O2: <0.1 ppm) without exposing to air. The pellet was sealed in a plastic film bag (Barrier
Nylon) together with an oxygen absorber (ISO A500-HS: Fe powder). The Pt LIII- and Ga K-edges XAFS spectra were recorded in a transmission mode at room temperature. Athena and Artemis
software ver. 0.9.25 implemented in the Demeter package35 was used for the analysis of the obtained XAFS spectra. Fourier-transform of the Pt LIII-edge EXAFS oscillation was obtained in the
_k_ range of 3−16 Å−1. The back Fourier-transform obtained in the _R_ range of 1.5−3.5 Å was used for curve-fitting. FEFF8 was used for the calculation of the back-scattering amplitude and
phase shift functions36. We defined the _R_-factor (_R_2) for curve-fitting as follows: $$R^2 = \Sigma _i\left\{ {k^3\chi _i^{\exp }\left( k \right) - k^3\chi _i^{\mathrm{{fit}}}\left( k
\right)} \right\}^2{\mathrm{per}}\,\Sigma _i\left\{ {k^3\chi _i^{\exp }\left( k \right)} \right\}^2.$$ (6) COMPUTATIONAL DETAILS DFT calculations were performed by using the CASTEP code37.
We used Vanderbilt-type ultrasoft pseudopotentials38 and the revised version of Perdew−Burke−Ernzerhof exchange-correlation functional39,40 based on the generalized gradient approximation. A
cut-off energy of 370 eV was used for the plane-wave basis set. A _k_-point mesh with a spacing of 0.04 Å−1 generated by the Monkhorst−Pack scheme41 was used to sample the Brillouin zone.
In this study, the PtGa:A(111) and Pt3Sn(111) planes were considered as the standard active surfaces for PDH. The supercell structure was constructed using a (2 × 2) unit cell slab with six
atomic layers and a vacuum spacing of 15 Å. We performed geometry optimizations on the supercell structures using a Fermi smearing of 0.1 eV, the OBS method for dispersion correlations, and
the following convergence criteria: (1) self-consistent field tolerance: 1.0 × 10−6 eV per atom; (2) energy tolerance: 1.0 × 10−5 eV per atom; (3) maximum force tolerance of 0.05 eV Å−1, and
(4) maximum displacement tolerance: 1.0 × 10−3 Å. Transition state search was carried out based on the complete linear synchronous transit/quadratic synchronous transit method42,43 with the
tolerance for all root-mean-square forces on an atom of 0.10 eV Å−1. DATA AVAILABILITY The data that support the findings of this study are available from the corresponding author upon
reasonable request. REFERENCES * Sattler, J. J. H. B., Ruiz-Martinez, J., Santillan-Jimenez, E. & Weckhuysen, B. M. Catalytic dehydrogenation of light alkanes on metals and metal oxides.
_Chem. Rev._ 114, 10613–10653 (2014). Article CAS PubMed Google Scholar * James, O. O., Mandal, S., Alele, N., Chowdhury, B. & Maity, S. Lower alkanes dehydrogenation: strategies
and reaction routes to corresponding alkenes. _Fuel Process. Technol._ 149, 239–255 (2016). Article CAS Google Scholar * Hu, Z. P., Yang, D., Wang, Z. & Yuan, Z. Y. State-of-the-art
catalysts for direct dehydrogenation of propane to propylene. _Chin. J. Catal._ 40, 1233–1254 (2019). Article CAS Google Scholar * Bhasin, M. M., McCain, J. H., Vora, B. V., Imai, T.
& Pujadó, P. R. Dehydrogenation and oxydehydrogenation of paraffins to olefins. _Appl. Catal. A Gen._ 221, 397–419 (2001). Article CAS Google Scholar * Sanfilippo, D. & Miracca,
I. Dehydrogenation of paraffins: synergies between catalyst design and reactor engineering. _Catal. Today_ 111, 133–139 (2006). Article CAS Google Scholar * Hauser, A. W., Horn, P. R.,
Head-Gordon, M. & Bell, A. T. A systematic study on Pt based, subnanometer-sized alloy cluster catalysts for alkane dehydrogenation: effects of intermetallic interaction. _Phys. Chem.
Chem. Phys._ 18, 10906–10917 (2016). Article CAS PubMed Google Scholar * Yang, M. L., Zhu, Y. A., Zhou, X. G., Sui, Z. J. & Chen, D. First-principles calculations of propane
dehydrogenation over PtSn catalysts. _ACS Catal._ 2, 1247–1258 (2012). Article CAS Google Scholar * Zha, S. et al. Identification of Pt-based catalysts for propane dehydrogenation: via a
probability analysis. _Chem. Sci._ 9, 3925–3931 (2018). Article CAS PubMed PubMed Central Google Scholar * Zhao, Z. J., Chiu, C. C. & Gong, J. Molecular understandings on the
activation of light hydrocarbons over heterogeneous catalysts. _Chem. Sci._ 6, 4403–4425 (2015). Article CAS PubMed PubMed Central Google Scholar * Nykänen, L. & Honkala, K.
Selectivity in propene dehydrogenation on Pt and Pt3Sn surfaces from first principles. _ACS Catal._ 3, 3026–3030 (2013). Article CAS Google Scholar * Sun, G. et al. Breaking the scaling
relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. _Nat. Commun._ 9, 4454 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Yang, X.
F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. _Acc. Chem. Res._ 46, 1740–1748 (2013). Article CAS PubMed Google Scholar * Zhang, Z. et al. Thermally stable
single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. _Nat. Commun._ 8, 16100 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Jones, J. et al. Thermally
stable single-atom platinum-on-ceria catalysts via atom trapping. _Science_ 353, 150–154 (2016). Article ADS CAS PubMed Google Scholar * Lin, L. et al. A highly CO-tolerant atomically
dispersed Pt catalyst for chemoselective hydrogenation. _Nat. Nanotechnol._ 14, 354 (2019). Article ADS CAS PubMed Google Scholar * Marcinkowski, M. D. et al. Pt/Cu single-atom alloys
as coke-resistant catalysts for efficient C-H activation. _Nat. Chem._ 10, 325–332 (2018). Article CAS PubMed Google Scholar * Duan, S., Wang, R. & Liu, J. Stability investigation of
a high number density Pt1/Fe2O3 single-atom catalyst under different gas environments by HAADF-STEM. _Nanotechnology_ 29, 204002 (2018). Article ADS PubMed CAS Google Scholar * Liu, W.
E. & Mohney, S. E. Condensed phase equilibria in transition metal-Ga-Sb systems and predictions for thermally stable contacts to GaSb. _J. Electron. Mater._ 32, 1090–1099 (2003).
Article ADS CAS Google Scholar * Furukawa, S. & Komatsu, T. Intermetallic compounds: promising inorganic materials for well-structured and electronically modified reaction
environments for efficient catalysis. _ACS Catal._ 7, 735–765 (2017). Article CAS Google Scholar * Prinz, J. et al. Adsorption of small hydrocarbons on the three-fold PdGa surfaces: the
road to selective hydrogenation. _J. Am. Chem. Soc._ 136, 11792–11798 (2014). Article CAS PubMed Google Scholar * Prinz, J. et al. Ensemble effect evidenced by CO adsorption on the
3-fold PdGa surfaces. _J. Phys. Chem. C_ 118, 12260–12265 (2014). Article CAS Google Scholar * Bhargava, M. K., Gadalla, A. A. & Schubert, K. Koexistente phasen vom FeSi-Typ in den
mischungen Ni-Pd-Ga und Ni-Pt-Ga. _J. Less Common Met._ 42, 69–76 (1975). Article CAS Google Scholar * Iihama, S., Furukawa, S. & Komatsu, T. Efficient catalytic system for
chemoselective hydrogenation of halonitrobenzene to haloaniline using PtZn intermetallic compound. _ACS Catal._ 6, 742–746 (2016). Article CAS Google Scholar * Buekens, A. G. &
Froment, G. F. Thermal cracking of propane: kinetics and product distributions. _Ind. Eng. Chem. Process Des. Dev._ 7, 435–447 (1968). Article CAS Google Scholar * Nykänen, L. &
Honkala, K. Density functional theory study on propane and propene adsorption on Pt(111) and PtSn alloy surfaces. _J. Phys. Chem. C._ 115, 9578–9586 (2011). Article CAS Google Scholar *
Slater, J. C. Atomic radii in crystals. _J. Chem. Phys._ 41, 3199–3204 (1964). Article ADS CAS Google Scholar * Searles, K. et al. Highly productive propane dehydrogenation catalyst
using silica-supported Ga-Pt nanoparticles generated from single-sites. _J. Am. Chem. Soc._ 140, 11674–11679 (2018). Article CAS PubMed Google Scholar * Redekop, E. A. et al. Delivering
a modifying element to metal nanoparticles via support: Pt-Ga alloying during the reduction of Pt/Mg(Al,Ga)Ox catalysts and its effects on propane dehydrogenation. _ACS Catal._ 4, 1812–1824
(2014). Article CAS Google Scholar * Wang, T. et al. Pyrolysis of heavy oil in the presence of supercritical water: the reaction kinetics in different phases. _AIChE J._ 61, 857–866
(2015). Article CAS Google Scholar * Siddiqi, G., Sun, P., Galvita, V. & Bell, A. T. Catalyst performance of novel Pt/Mg(Ga)(Al)O catalysts for alkane dehydrogenation. _J. Catal._
274, 200–206 (2010). Article CAS Google Scholar * Im, J. & Choi, M. Physicochemical stabilization of Pt against sintering for a dehydrogenation catalyst with high activity,
selectivity, and durability. _ACS Catal._ 6, 2819–2826 (2016). Article CAS Google Scholar * Jablonski, E. L., Castro, A. A., Scelza, O. A. & De Miguel, S. R. Effect of Ga addition to
Pt/Al2O3 on the activity, selectivity and deactivation in the propane dehydrogenation. _Appl. Catal. A Gen._ 183, 189–198 (1999). Article CAS Google Scholar * Xu, Y. et al.
Sintering-resistant Pt on Ga2O3 rods for propane dehydrogenation: the morphology matters. _Ind. Eng. Chem. Res._ 57, 13087–13093 (2018). Article CAS Google Scholar * Searles, K., Siddiqi,
G., Safonova, O. V. & Copéret, C. Silica-supported isolated gallium sites as highly active, selective and stable propane dehydrogenation catalysts. _Chem. Sci._ 8, 2661–2666 (2017).
Article CAS PubMed PubMed Central Google Scholar * Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. _J.
Synchrotron Radiat._ 12, 537–541 (2005). Article CAS PubMed Google Scholar * Ankudinov, A. & Ravel, B. Real-space multiple-scattering calculation and interpretation of
x-ray-absorption near-edge structure. _Phys. Rev. B_ 58, 7565–7576 (1998). Article ADS CAS Google Scholar * Segall, M. D. et al. First-principles simulation: Ideas, illustrations and the
CASTEP code. _J. Phys. Condens. Matter_ 14, 2717–2744 (2002). Article ADS CAS Google Scholar * Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue
formalism. _Phys. Rev. B_ 41, 7892–7895 (1990). Article ADS CAS Google Scholar * Zhang, Y. & Yang, W. Comment on “generalized gradient approximation made simple”. _Phys. Rev. Lett._
80, 890 (1998). Article ADS CAS Google Scholar * Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised
Perdew-Burke-Ernzerhof functionals. _Phys. Rev. B_ 59, 7413–7421 (1999). Article ADS Google Scholar * Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations.
_Phys. Rev. B_ 13, 5188–5192 (1976). Article ADS MathSciNet Google Scholar * Halgren, T. A. & Lipscomb, W. N. The synchronous-transit method for determining reaction pathways and
locating molecular transition states. _Chem. Phys. Lett._ 49, 225–232 (1977). Article ADS CAS Google Scholar * Govind, N., Petersen, M., Fitzgerald, G., King-Smith, D. & Andzelm, J.
A generalized synchronous transit method for transition state location. _Comput. Mater. Sci._ 28, 250–258 (2003). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work
was supported by JSPS KAKENHI (Grant Numbers 17H01341, 17H04965, and 20H02517), MEXT project Element Strategy Initiative (JPMXP0112101003), JST CREST (JPMJCR17J3), and JST PRESTO
(JPMJPR19T7). The XAFS analysis was performed with the approval of JASRI (No. 2019B1620 and 2019B1469). We appreciate the technical staffs of the faculty of engineering, Hokkaido University
and of Research Institute for Electronic Science, Hokkaido University for help with HAADF-STEM observation. Computation time was provided by the supercomputer systems in Institute for
Chemical Research, Kyoto University. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute for Catalysis, Hokkaido University, N21, W10, Sapporo, 001-0021, Japan Yuki Nakaya, Ken-ichi
Shimizu & Shinya Furukawa * Department of Chemistry, Graduate School of Science, Tokyo Metropolitan University, Hachioji-shi, Tokyo, 192-0397, Japan Jun Hirayama & Seiji Yamazoe *
Elements Strategy Initiative for Catalysts and Batteries, Kyoto University, Katsura, Kyoto, 615-8520, Japan Jun Hirayama, Seiji Yamazoe, Ken-ichi Shimizu & Shinya Furukawa * Japan
Science and Technology Agency, PRESTO, Chiyodaku, Tokyo, 102-0076, Japan Seiji Yamazoe & Shinya Furukawa Authors * Yuki Nakaya View author publications You can also search for this
author inPubMed Google Scholar * Jun Hirayama View author publications You can also search for this author inPubMed Google Scholar * Seiji Yamazoe View author publications You can also
search for this author inPubMed Google Scholar * Ken-ichi Shimizu View author publications You can also search for this author inPubMed Google Scholar * Shinya Furukawa View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.F. and Y.N. design the research and co-wrote the manuscript in discussion. Y.N. performed most of the
experimetal works. J.H. and S.Y. carried out the XAFS analysis. S.F. conducted the computational studies. S.F., Y.N., J.H., S.Y., and K.S. discussed the data and commented on the
manuscript. CORRESPONDING AUTHOR Correspondence to Shinya Furukawa. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW
INFORMATION _Nature Communications_ thanks Matthew Darby and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Nakaya, Y., Hirayama, J., Yamazoe, S. _et al._ Single-atom Pt in intermetallics as an ultrastable and selective catalyst for propane dehydrogenation. _Nat Commun_ 11, 2838
(2020). https://doi.org/10.1038/s41467-020-16693-9 Download citation * Received: 11 March 2020 * Accepted: 15 May 2020 * Published: 05 June 2020 * DOI:
https://doi.org/10.1038/s41467-020-16693-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative