Play all audios:
ABSTRACT Cellular homeostasis depends on the supply of metabolic energy in the form of ATP and electrochemical ion gradients. The construction of synthetic cells requires a constant supply
of energy to drive membrane transport and metabolism. Here, we provide synthetic cells with long-lasting metabolic energy in the form of an electrochemical proton gradient. Leveraging the
L-malate decarboxylation pathway we generate a stable proton gradient and electrical potential in lipid vesicles by electrogenic L-malate/L-lactate exchange coupled to L-malate
decarboxylation. By co-reconstitution with the transporters GltP and LacY, the synthetic cells maintain accumulation of L-glutamate and lactose over periods of hours, mimicking nutrient
feeding in living cells. We couple the accumulation of lactose to a metabolic network for the generation of intermediates of the glycolytic and pentose phosphate pathways. This study
underscores the potential of harnessing a proton motive force via a simple metabolic network, paving the way for the development of more complex synthetic systems. SIMILAR CONTENT BEING
VIEWED BY OTHERS CREATING ENZYMES AND SELF-SUFFICIENT CELLS FOR BIOSYNTHESIS OF THE NON-NATURAL COFACTOR NICOTINAMIDE CYTOSINE DINUCLEOTIDE Article Open access 09 April 2021 SYNTHETIC
SYNTROPHY FOR ADENINE NUCLEOTIDE CROSS-FEEDING BETWEEN METABOLICALLY ACTIVE NANOREACTORS Article Open access 21 October 2024 METABOLOME AND PROTEOME ANALYSES REVEAL TRANSCRIPTIONAL
MISREGULATION IN GLYCOLYSIS OF ENGINEERED _E. COLI_ Article Open access 13 August 2021 INTRODUCTION Living cells require energy to fuel essential biosynthetic processes, to grow and divide,
and to maintain homeostasis and an out-of-equilibrium metabolic state. The two main metabolic energy currencies of a cell are ATP and H+ (or Na+) electrochemical gradients; the latter are
referred to as proton and sodium motive force (SMF), respectively. A proton motive force (PMF) can be generated by respiration, light-driven electron transfer reactions, or ATP
hydrolysis1,2. The PMF is composed of a H+ chemical gradient, ΔpH (typically alkaline inside), and an electrical potential, ΔΨ (typically negative inside): $${{{\rm{PMF}}}}=\Delta {\Psi}
-2.303\frac{{{{\mathrm{RT}}}}}{{{\mathrm{{F}}}}}\Delta {{{\rm{pH}}}}$$ (1) where _R_, _T_, and _F_ correspond to the gas constant, temperature, and Faraday constant, respectively, and ΔpH =
pHi–pHo. Fermentative bacteria are unable to form a PMF by respiration or photosynthetic reactions, and the PMF can be formed via ATP hydrolysis by F1F0-ATPase3,4. However, it is also
possible to generate a PMF without involvement of high-energy intermediates like ATP, using electrogenic uniport or electrogenic precursor-product exchange in combination with metabolic
breakdown of the substrate inside the cell3,5. An example is the internal decarboxylation of substrate (precursor), catalyzed by a soluble decarboxylase, coupled to the uptake of precursor
and extrusion of product, mediated by a specific transport protein6. Bacteria of the genera _Lactobacillus, Lactococcus, Leuconostoc,_ and _Pedicoccus_ possess an L-malate decarboxylation
pathway, also known as malolactic fermentation, which generates a PMF and counterbalances intracellular acidification7,8. In _Lactococcus lactis_, the cytosolic L-malate decarboxylase
(malolactic enzyme, MleS) catalyzes the decarboxylation of L-malate to L-lactate plus CO2, while a membrane-embedded secondary transporter, MleP, exchanges di-anionic L-malate for L-lactate
or mono-anionic L-malate for L-lactic acid. The decarboxylation reaction results in an inward gradient for L-malate and an outward gradient for L-lactate, establishing the driving forces for
the L-malate/L-lactate exchange5. The CO2 may leave the cell by passive diffusion without affecting pH7. Consumption of scalar protons during the decarboxylation reaction leads to an
intracellular alkalinization and, therefore, generates a ΔpH across the plasma membrane. The gradual decrease in external L-malate and increase in L-lactate can rise the external pH, because
the molecules have a different acidity (L-malate: pKa1 = 3.4, pKa2 = 5.1; and L-lactate: pKa = 3.8)7,9, but generally the impact of L-malate decarboxylation will be highest for the internal
pH. The exchange of di-anionic L-malate for L-lactate or mono-anionic L-malate for L-lactic acid is electrogenic and thus generates a membrane potential (ΔΨ, inside negative). Both
components of the PMF are generated in different but coupled steps, which is mechanistically very different from how the PMF is generated in respiration or photosynthesis or upon ATP
hydrolysis by F1F0-ATPase. We refer to the combined action of MleP and MleS as the L-malate decarboxylation pathway. The compartmentalization of the L-malate decarboxylation pathway makes it
possible to conserve the low amount of free energy from the decarboxylation reaction (−17 to −25 kJ mol−1)6, chemiosmotically into a PMF10. The free energy change of a carboxylation
reaction is too small for the synthesis of ATP from ADP plus Pi, but the formed PMF can be used to supply the cell with ATP and fuel other essential functions like the transport of
nutrients. The PMF can also facilitate processes like cell division11, (membrane) protein insertion/secretion12 and intercellular communication13,14. Various other PMF-generating
precursor-product exchange–decarboxylation pathways have been described (oxalate2−/formate−, citrate2−/L-lactate−, arginine+/agmatine2+, ornithine+/putrescine2+, glutamate−/γ-aminobutyrate,
histidine/histamine+, tyrosine/tyramine+, aspartate−/alanine)15,16,17,18,19,20,21,22. In this work, we explore the potential of the L-malate decarboxylation pathway for the generation of a
PMF in submicrometer-size lipid vesicles. We co-reconstituted the pathway with _Escherichia coli_ glutamate transporter GltP23,24 and lactose transporter LacY25, and we show long-lasting
transport and high steady-state levels of these solutes. We also demonstrate the utilization of L-malate-dependent lactose accumulation in downstream metabolic reactions. The sustainable
energy conversion by the L-malate decarboxylation pathway enables more complex cell-like metabolic functions and sets the foundations for further out-of-equilibrium networks in synthetic
cells. RESULTS The L-malate decarboxylation pathway generates a proton motive force by the action of two proteins: the integral membrane L-malate/L-lactate exchanger (MleP) and the soluble,
luminal, L-malate decarboxylase (MleS). To guide the reconstitution of this system in lipid vesicles we characterized both proteins. A summary of the data obtained and in literature is
presented in Table 1. MLEP MEDIATES ELECTROGENIC L-MALATE/L-LACTATE EXCHANGE AND L-MALATE UNIPORT MleP belongs to the 2-hydroxycarboxylate transporter family (2HCT), which function as
symporters or exchangers26. MleP has been described as a L-malate/L-lactate exchanger7 with a molecular weight of 47.9 kDa and 9–14 predicted TMS26,27. We overexpressed the 10× His-tagged
MleP in _L. lactis_, purified the protein via immobilized metal-affinity chromatography (IMAC) and incorporated the protein in lipid vesicles composed of dioleoyl-phospholipids
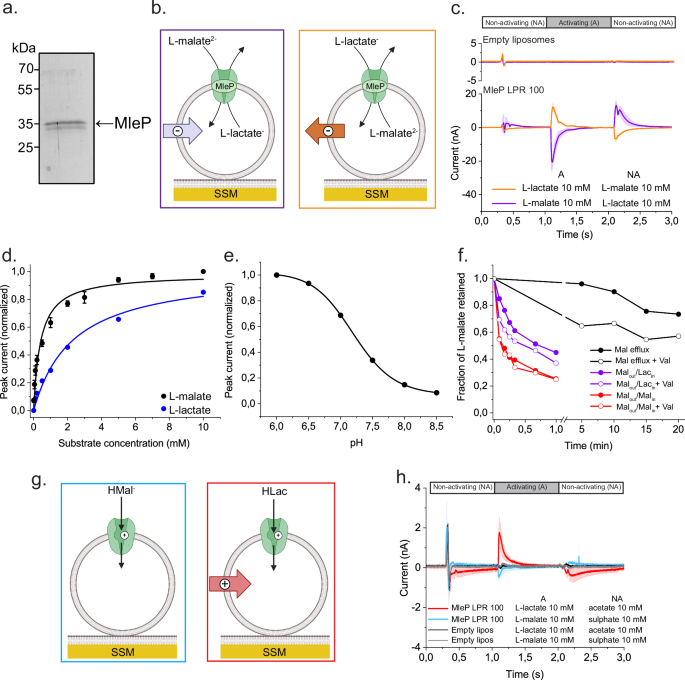
DOPE:DOPG:DOPC 1:1:2 (mol ratio) or _E. coli_ polar lipids: egg PC 3:1 (mol ratio). Figure 1a shows that MleP is reconstituted with an efficiency of 51 ± 9% (Supplementary Fig. 1); the
double band is assigned to different structural conformations and incomplete denaturation by SDS. We also observe some dimeric MleP, similar to what has been reported for other members of
the 2HCT family26. We determined the electrogenic nature of L-malate/L-lactate exchange by solid-supported membrane (SSM)-based electrophysiological measurements (Fig. 1). The net transfer
of charge by the exchange of divalent L-malate for monovalent L-lactate is detected as a transient current via the capacitive coupling between the supported membrane and the vesicles28. A
negative current is observed when external L-lactate (non-activating solution) is replaced with L-malate (activating solution) with L-lactate-loaded MleP-vesicles adsorbed to the supporting
membrane (Fig. 1b, c). A positive peak current is obtained when a L-lactate jump is triggered on L-malate-loaded MleP vesicles, because the charge transport is now in the opposite direction
(Fig. 1b, c). There is no substantial current when the same solution exchange is performed in liposomes without MleP (empty liposomes) (Fig. 1c). We reduced the possibility of obtaining
electrical artifacts from differences in ionic strength between the activating and non-activating solutions by replacing lactate and malate with acetate and sulfate, respectively, which
carry the same charge but are not recognized as substrates by MleP. These results confirm the electrogenic character of the L-malate/L-lactate exchange. We found that MleP reconstituted at a
lipid-to-protein (LPR) ratio of 100 was able to exchange L-malate for L-lactate in the chemically defined synthetic lipid mixture DOPE:DOPG:DOPC 1:1:2 (mol ratio), but the activity was 7
times higher in liposomes composed of _E. coli_ polar lipids: egg PC 3:1 (Supplementary Table 1, Supplementary Fig. 2). Therefore, we used the _E. coli_ polar lipid/egg PC mixture for the
majority of the reconstitutions at LPR 100 and further SSM measurements. The amplitude of the peak current (_I__p_) is proportional to the steady state L-malate/L-lactate exchange
activity29. The peak current amplitude increases in a hyperbolic manner with the increment in the outside concentration of L-malate or L-lactate, while keeping the internal concentration of
L-lactate or L-malate at 30 mM (Fig. 1d and Supplementary Fig. 3a–c). We find that the _K_mapp for L-lactate is 7-fold higher than for L-malate (Table 1 and Fig. 1d). Since the L-malate
decarboxylation leads to an internal as well as external pH change, (see Introduction and7), we performed L-malate jumps on L-lactate-loaded vesicles at pH values between 6 and 8.5
(Supplementary Fig. 3d). Here, we used MleP LPR 250 vesicles to have a similar number of transporters per vesicle as in the experiments with the L-malate decarboxylation pathway. The
L-malate/L-lactate activity is highest at pH 6 and decreased at more alkaline pHs (Fig. 1e). The pH dependence most likely reflects the activity of MleP and not the availability of
substrate, because the change in concentration of di-anionic L-malate is only 4% between pH 6 and pH 8.5. Besides L-malate/L-lactate exchange, MleP facilitates uniport of L-malate27, which
would also be electrogenic and enable L-malate decarboxylation because L-lactate can leave the vesicles in the protonated form (L-lactic acid) by passive diffusion. Indeed, transport assays
with vesicles loaded with radiolabelled L-malate (Fig. 1f) show efflux of L-malate but with a rate at least one-order of magnitude slower than L-malate/L-lactate exchange. The electrogenic
nature of both the uniport and exchange is shown by the increase of activity in the presence of the K+ ionophore valinomycin, which dissipates the membrane potential. Thus, two different
methodologies (SSM-based electrophysiology and efflux of radiolabelled substrate) confirm that MleP is an electrogenic secondary antiporter/uniporter. The slow kinetics of the uniport
reaction complicates the SSM measurements, but we recorded small peak currents when L-malate or L-lactate jumps were applied on MleP vesicles without counter-substrate (Fig. 1g, h). These
peak currents can be interpreted as pre-steady state currents that originate from a half turnover, _i.e_. L-malate or L-lactate influx, which is followed by a slow return of the empty
carrier. Interestingly, the peak current from the L-lactate jump is not only 5-fold larger in magnitude but also has a positive direction, indicative of movement of positive charge in or
negative charge out of the vesicles. L-MALATE DECARBOXYLATION CATALYZED BY MLES L-malate decarboxylase MleS catalyzes the decarboxylation of L-malate to L-lactate, releasing carbon dioxide
and consuming a proton (Fig. 2a). Protons are used to compensate for the free electron pair remaining in the organic intermediate after the heterolytic cleavage that releases CO2
(Supplementary Fig. 4)6. MleS is a homodimeric protein with a molecular weight of 60–65 kDa per subunit and has NAD+ and Mn2+ as bound cofactors30,31,32. The decarboxylation reaction
proceeds in three consecutive steps without detectable accumulation of intermediates: (i) L-malate oxidation to oxaloacetate; (ii) decarboxylation of oxaloacetate to pyruvate; and (iii)
pyruvate reduction to L-lactate (Supplementary Fig. 4)26. The NAD+ consumed in the first step is recycled in the third step. The proton consumption leads to alkalinization of the cytoplasm.
We overexpressed _L. lactis_ MleS and purified the protein by IMAC and size-exclusion chromatography (SEC). A single and symmetrical peak in the SEC and SDS-polyacrylamide gel confirms the
production of a monodisperse protein (Fig. 2b). The L-malate decarboxylation activity of MleS was determined by pH measurements in 2 mM of potassium phosphate and is presented as H+
consumption rate (µmol H+ consumed min−1 mg MleS−1) (Fig. 2c–f). The H+ consumption was calibrated by titration of the reaction buffer with NaOH (Supplementary Fig. 5). We noticed that on
longer timescales the amount of consumed H+ was lower than expected for a reaction with a _K_eq of 5.5 × 102, calculated by eQuilibrator33 (Supplementary Fig. 6d). To verify if the enzymatic
reaction was not running to completeness, we followed the production of L-lactate by HPLC after derivatization with 9-chloromethyl anthracene (Supplementary Fig. 6). Virtually complete
decarboxylation of 5 mM L-malate was confirmed by the production of approximately 5 mM of L-lactate (Supplementary Fig. 6 d). We explain the leveling off of the pH by the dissolution of CO2
and the formation of bicarbonate plus a proton, which opposes the alkalinization of the decarboxylation reaction. The pH recordings and L-lactate measurements show excellent correspondence
for the initial 30 s of the reaction (Supplementary Fig. 6d, inset), and therefore, the initial rates of H+ consumption were used to determine the kinetic parameters of MleS (Table 1 and
Fig. 2c–f). There is no deleterious effect of L-lactate up to 5 mM (Fig. 2c and Supplementary Fig. 7), but the initial rate of H+ consumption was reduced by 50% in 25 mM L-lactate (Fig. 2c).
The _K_mapp for L-malate is 1.4 ± 0.4 mM and _K_mapp for NAD+ is 42 µM (Supplementary Fig. 8) at pH 7. In summary, we show that MleS is a relatively fast enzyme with a _k_cat of 266 ± 14
s−1 around pH 7. L-MALATE DECARBOXYLATION PATHWAY GENERATES PMF IN VESICLES QUANTIFICATION OF THE H+ GRADIENT (ΔPH) We determined the coupled activities of MleP and MleS in vesicles in which
we first reconstituted MleP and then encapsulated MleS, along with NAD+ plus MnCl2 (Fig. 3a). We used MleS concentrations and MleP LPRs that would yield at least one dimer, even in the
smallest vesicles (~100 nm); the other components were encapsulated in a large excess (Table 2). We encapsulated the hydrophilic fluorescent probe pyranine (8-hydroxypyrene-1,3,6-trisulfonic
acid or HPTS) for ratiometric quantification of the intravesicular pH (Supplementary Fig. 10). Preliminary experiments with liposomes showed that a small fraction of pyranine is retained at
the outer surface of the vesicles, even after extensive washing (two cycles of ultracentrifugation and resuspension, and a final gel filtration step) (Supplementary Fig. 11). Therefore, we
included the collisional quencher DPX (p-xylene-bis-pyridinium bromide) in the external medium for every measurement34. Additionally, we inhibited any MleS, possibly adsorbed to the outer
surface of the vesicles, by using EDTA to chelate Mn2+ ions that are required for activity (Supplementary Fig. 12a). Due to the low rate of L-malate uniport by MleP (Fig. 1f), we included 2
mM of L-lactate inside the vesicles to enable rapid L-malate/L-lactate exchange. We kept the same concentration in the external medium, because L-lactic acid (in fast equilibrium with
L-lactate) rapidly permeates the membrane (Supplementary Fig. 18a). Indeed, when L-lactate is not initially present in the external medium, the alkalinization is slower because initially,
only L-malate uniport is possible, but the internal pH reaches a higher point than in the presence of external L-lactate (Supplementary Fig. 13). Upon addition of 10 mM L-malate to the
MleP-MleS containing vesicles, the internal pH increased from 7.0 to 7.50 ± 0.03 (Fig. 3c) and then over a period of 10 h gradually decreased to 7.34 ± 0.07 (Supplementary Fig. 14). The drop
in internal pH was not observed at pH 6.0 (Fig. 3d). No alkalinization was observed when either MleP or MleS were absent (Fig. 3c), indicating that the formation of a pH gradient (ΔpH)
requires the coupled activities of MleP and MleS. The rate of alkalinization increases with lower LPR (more MleP per vesicle) and higher amounts of encapsulated MleS (Supplementary Fig. 15).
Although the _k_cat of the enzymatic reaction is ≈10× higher than the estimated turnover number of MleP, the MleP/MleS ratio (in molecules per vesicle) was always higher than 1 (range
2–13), explaining the increase in activity with MleS concentration (Supplementary Fig. 15). However, the rate of acidification shows a stronger dependence on the MleP than MleS
concentration. A slight decrease in internal pH was observed upon addition of L-malate to vesicles lacking MleS (Fig. 3c), which may reflect uniport of L-malateH− and dissociation of the
proton in the vesicle lumen. To demonstrate that the internal alkalinization results in a H+ gradient across the membrane we used the ionophore nigericin, which exchanges K+ for H+. Indeed,
nigericin collapses the H+ gradient (Fig. 3e). The formation of a membrane potential by L-malate decarboxylation is evident from the accelerated alkalinization in the presence of the
K+-selective ionophore valinomycin, which dissipates the membrane potential ΔΨ (Fig. 3e). The ΔΨ (inside negative) slows down the L-malate/L-lactate exchange decreasing thereby the activity
of the L-malate decarboxylation pathway. Interestingly, when the L-malate decarboxylation pathway runs at pH 6, which is the optimal pH for MleS and MleP (Figs. 1e and 2f), the H+ gradient
is maintained constant for longer periods of time (Fig. 3d). Finally, CO2 can leave the vesicles by passive diffusion but it can also be converted into bicarbonate plus a proton and thus
contribute to acidification of the vesicle lumen. QUANTIFICATION OF MEMBRANE POTENTIAL (ΔΨ) Next, we monitored the formation of the ΔΨ, using the fluorescent probe DiSC3(5)
(3,3’-dipropylthiadicarbocyanine iodide)35,36. This carbocyanine distributes uniformly over the inner and outer leaflet when ΔΨ = 0 (Fig. 4a). ΔΨ <0 leads to accumulation of the probe in
the inner leaflet and quenching of fluorescence (Fig. 4a, b). After equilibration of DiSC3(5) in the membrane of L-lactate-loaded MleP vesicles, the addition of 10 mM L-malate results in a
fast quenching of fluorescence (Fig. 4b). This indicates the generation of ΔΨ <0 as a consequence of the L-malate/L-lactate exchange. The exchange rapidly reaches electrochemical
equilibrium, in which the L-malate gradient is opposed by the ΔΨ, after which the membrane potential slowly decreasing (Fig. 4b). When the same experiment is performed with MleP vesicles
containing MleS, a larger quenching is observed (Fig. 4b). The internal conversion of L-malate into L-lactate, catalyzed by MleS, maintains the inward gradient of L-malate and hence, the
membrane potential is larger and sustained for a longer period of time (Fig. 4b). Competition between L-malate and L-lactate leads to a gradual decrease in ΔΨ, because fewer molecules of
L-malate are transported per unit of time when the L-lactate concentration in the external medium increases. The dissipation of ΔΨ starts earlier than the dissipation of ΔpH, which reflects
the low capacitance of the lipid bilayer as the translocation of a few charges is sufficient to reduce ΔΨ substantially. The dequenching of DiSC3(5) fluorescence upon addition of valinomycin
confirms that the L-malate-induced quenching corresponds to the formation of a ΔΨ across the membrane. We calibrated the fluorescence quenching by comparison of the signal generated with K+
diffusion potentials of varying magnitudes (Supplementary Fig. 16). We find ΔΨ = −79 ± 9 mV three min after addition of L-malate when the decarboxylation reaction is done at pH 7 (Fig. 4b,
d). Similar as seen with the ΔpH, the build-up of ΔΨ is faster and subsequent dissipation occurs at a lower rate when the pathway is operated at pH 6 (Fig. 4c, e), which is in line with the
activity of MleP and MleS. The dynamics of the PMF generated by the L-malate decarboxylation at pH 7 and 6 follows from the corresponding ΔpH and ΔΨ curves, using Eq. 1 (Fig. 4d, e, orange
curves). The PMF shows similar dynamics as the membrane potential and is maintained at a higher level at pH 6 than pH 7. In line with the low electrical capacitance of lipid bilayers and the
relatively high buffer capacity of the internal medium the ΔΨ is formed faster than the ΔpH and is initially the main component of the PMF at pH 7. PMF FROM L-MALATE DECARBOXYLATION FUELS
THE TRANSPORT OF GLUTAMATE Next, we used the ΔΨ and ∆pH formed by L-malate decarboxylation to drive the accumulation of L-glutamate and D-lactose. We overexpressed and purified GltP of _E.
coli_ and co-reconstituted the protein with MleP in vesicles composed of _E. coli_ polar lipids:egg PC 3:1 (Fig. 5a). Both proteins were co-reconstituted at relatively high LPR (250 each) to
assure a high reconstitution efficiency37,38. Cryo-TEM shows the size-distribution and the predominantly unilamellar nature of the vesicles (Supplementary Fig. 17a). Estimation of the
incorporation efficiency of GltP and MleP in the same vesicles was not possible by SDS-PAGE, because both proteins migrate similarly. Individually, they were reconstituted with an efficiency
of 60 and 50%, respectively (Fig. 5b & Supplementary Fig. 1a, b). MleS, NAD+, Mn2+, sodium-L-lactate plus pyranine were encapsulated in the MleP-GltP vesicles. Co-incorporation of GltP
did not significantly affect the performance of the L-malate decarboxylation pathway (Supplementary Fig. 19a). Addition of L-glutamate leads to a small drop in the pH gradient, which is in
agreement with 3H+ symported with L-glutamate by GltP (Supplementary Fig. 19b). Figure 5c shows the uptake of L-glutamate, driven by the PMF that is generated by L-malate decarboxylation.
After 40–50 min a steady state is reached, which lasts at least 4 hours; the accumulation level ([Glu]IN/[Glu]OUT) is ~140 (based on a specific internal volume of 3 µL/mg of lipid39) (Fig.
5c & Supplementary Fig. 20). The uptake of L-glutamate is sigmoidal, because it takes some time to generate the PMF. When the transport reaction is initiated 30 min after the start of
the L-malate decarboxylation (i.e., pre-formed PMF), there is no delay and the initial glutamate uptake increases linearly with time (compare blue and orange lines, Fig. 5c). There is no
transport of L-glutamate when succinate instead of L-malate is used (Fig. 5c, inset). The rate of L-glutamate uptake depends on the L-glutamate concentration with a _K_mapp = 33 μM
(Supplementary Fig. 20). In line with the effect on the PMF (Figs. 3d and 4c), the rate of glutamate transport driven by the L-malate decarboxylation at pH 6 is not substantially different
from that at pH 7 but the accumulation level is higher at pH 6 (Supplementary Fig. 21), which is in agreement with the higher driving force. The accumulation level of ~140 matches the ΔpH
(0.5 pH units) and ΔΨ (−20 mV) at pH 7 obtained 50 min after L-malate addition (Fig. 4d) and the H+/glutamate¯ stoichiometry of 3. Equation 2 yields a [Glu]IN/[Glu]OUT of ~150-fold for the
PMF generated by L-malate decarboxylation, indicating a good correspondence between the generated driving force and the formed glutamate gradient via GltP. $$2\Delta \Psi
-3\frac{{{{\rm{RT}}}}}{{{{\rm{F}}}}}2.3\Delta
{{{\rm{pH}}}}=\frac{{{{\rm{RT}}}}}{{{{\rm{F}}}}}2.3{{{\rm{log}}}}\left(\frac{{\left[{{{{\rm{Glu}}}}}^{-}\right]}_{{{{\rm{IN}}}}}}{{\left[{{{{\rm{Glu}}}}}^{-}\right]}_{{{{\rm{OUT}}}}}}\right)$$
(2) Accumulated L-glutamate leaves the vesicles when ΔΨ and ΔpH are dissipated by the action of the ionophores valinomycin and nigericin (Fig. 5d). Addition of valinomycin (a highly
selective K+ ionophore) leads to a transient pH increment and dissipation of the membrane potential, which results in a small efflux of glutamate. The total PMF is dissipated upon subsequent
addition of nigericin (an ionophore that exchanges K+ for H+), and efflux of glutamate to equilibration levels is observed. Since the ΔpH acts three times as driving force, whereas the ∆Ψ
acts twice (See Eq. 2), the dissipation of ∆Ψ has less effect on the steady state levels of glutamate than ΔpH dissipation. In line with this, lower but sustained levels of glutamate are
observed when valinomycin was present from the beginning of the experiment (Supplementary Fig. 22b). The power of the L-malate decarboxylation pathway is not only demonstrated by the high
levels of L-glutamate uptake, but also by the maintenance of large solute gradients for hours. This is especially clear when L-glutamate accumulation driven by the L-malate decarboxylation
is compared with the transport driven by a K+ diffusion potential together with an acetic acid diffusion potential, which is the generic approach to study PMF-dependent transport
processes40,41 (Fig. 5e). Dilution of MleP-GltP vesicles, containing Na-acetate, into a solution with a lower concentration of Na-acetate establishes an inward H+ gradient as a consequence
of the outward passive diffusion of acetic acid (Fig. 5e). The ΔpH is proportional to the in/out ratio of the acetate concentration (See Methods). Along with a negative-inside ΔΨ, generated
by valinomycin-mediated outward K+ diffusion, the two gradients yield a transient PMF (Fig. 5e) that we used as benchmark for the PMF from the L-malate decarboxylation pathway. With an
artificially-imposed pH gradient of 0.5 (alkaline inside) and ΔΨ varying from 0 to −100 mV, we determined the dependence of L-glutamate transport on the driving force (Supplementary Fig.
23b, c). The maximal rate is higher than with L-malate decarboxylation but L-glutamate leaks out after 10 min, because the ΔΨ and ΔpH are transient (Fig. 5f and Supplementary Fig. 23b).
Moreover, the acetate gradient yields a ΔpH ≈ 0.4 that decreases slowly in the absence and rapidly in the presence of ΔΨ (inside negative) (Supplementary Fig. 23d). Thus, the transient
nature of diffusion potentials and the interdependence of ΔΨ on ΔpH and vice versa prohibit thermodynamic analyzes of transport reactions as exemplified here by Eq. 2. By contrast, the
L-malate decarboxylation pathway yields smaller gradients but they can be kept constant for hours (Figs. 3 and 4). By comparing the initial rate of L-glutamate uptake driven by the L-malate
decarboxylation with the initial rates of glutamate uptake driven by diffusion potentials (Supplementary Fig. 23b–c), we estimate that the driving force from the L-malate decarboxylation is
comparable to a ΔpH of 0.5 (by acetate diffusion) and a membrane potential of ~−40 mV (by valinomycin-mediated potassium diffusion) and thus a PMF of −70 mV, which is in line with direct
measurements of ΔpH and ΔΨ by the fluorometric probes pyranine and DiSC3(5) (Fig. 4d). PMF FROM L-MALATE DECARBOXYLATION FUELS THE TRANSPORT OF LACTOSE We also co-reconstituted the L-malate
decarboxylation pathway with _E. coli_ lactose permease, LacY25, which functions as a H+/galactoside symporter (Fig. 6a). The (co-)reconstitution protocol for LacY-MleP42,43,44 differs from
the one we used for GltP-MleP, but we obtained mostly unilamellar vesicles as shown by cryo-TEM (Supplementary Fig. 17b). We find that co-reconstitution mediated by
octyl-β-D-galactopyranoside (OG), and detergent removal via rapid dilution, generated the largest L-malate dependent D-lactose uptake (Supplementary Fig. 24). The ΔpH formed by L-malate
decarboxylation was comparable with and without LacY in the vesicles (see Supplementary Fig. 25 and Fig. 3d). D-lactose uptake reached its maximal level after 2 hours (Fig. 6c);
[lactose]IN/[lactose]OUT ~ 20 (or 75 mV). A slight reduction in accumulation level was observed at later times, presumably due to a decrease in PMF or as a result of an uncoupled lactose
efflux. Since lactose is taken up with 1 proton the accumulation is much lower than for glutamate, which is symported with 3 protons; the [lactose]IN/[lactose]OUT gradient of 75 mV is in
line with a PMF of −70 mV (see Eq. 3). $$\Delta \Psi -\frac{{{{\rm{RT}}}}}{{{{\rm{F}}}}}2.3\Delta
{{{\rm{pH}}}}=\frac{{{{\rm{RT}}}}}{{{{\rm{F}}}}}2.3{{{\rm{log}}}}\left(\frac{{\left[{{{\rm{Lactose}}}}\right]}_{{{{\rm{IN}}}}}}{{\left[{{{\rm{Lactose}}}}\right]}_{{{{\rm{OUT}}}}}}\right)$$
(3) Thus, the L-malate decarboxylation pathway forms a PMF that is stable on the timescale of hours and allows the accumulation of lactose and L-glutamate to a point of good correspondence
between the generated PMF and the established solute gradient from the active transport. COUPLING OF L-MALATE-DEPENDENT LACTOSE TRANSPORT TO CARBOHYDRATE METABOLISM Next, we encapsulated
β-galactosidase (LacZ), hexokinase (HK) plus glucose-6-phosphate dehydrogenase (G6P-DH) in MleP-LacY vesicles with L-malate decarboxylation pathway. These enzymes catalyze the hydrolysis of
D-lactose into galactose and glucose, phosphorylation of glucose to glucose-6-phosphate (G6P, entry point for glycolytic pathway) and formation of 6-phosphoglucono-δ-lactone (pentose
phosphate pathway) plus NADPH (Fig. 6d). The reduction of NADP+ to NADPH was used to monitor the activity of the overall pathway (Fig. 6e). We ran the L-malate decarboxylation for 30 min to
pre-form a PMF and then added D-lactose, which elicits an increase in NADPH fluorescence that is not observed in vesicles without LacY (Fig. 6e). The NADPH fluorescence is reduced in the
presence non-hydrolysable substrates methyl-β-D-thiogalactoside (TMG) and thiodigalactoside (TDG) (Fig. 6e, f), which act as low (_K_D ≈ 1 mM) and high (_K_D = 30–50 µM) affinity competitive
inhibitors of lactose transport, respectively45,46,47. NADPH production is dependent on the D-lactose concentration with a half saturation constant (_K_mapp) of ~0.2 mM), which is close to
the apparent _K_m for LacY (Fig. 6g, h and Table 1). In our design of the reaction network, the maximal levels of NADPH formed are determined by the amount NADP+ encapsulated in the
vesicles; additional control experiments are shown in Supplementary Fig. 26a. Finally, NADPH was also formed in the absence of L-malate decarboxylation albeit at a lower rate. Since lactose
is internally hydrolyzed, an out-to-in lactose gradient is maintained to facilitate the uptake of lactose. Furthermore, the galactose formed upon hydrolysis of lactose by LacZ is a substrate
of LacY and enables lactose/galactose exchange, which is even faster than lactose-H+ symport driven by the PMF (Supplementary Fig. 26b)45,48. Yet, the fastest metabolism of lactose is
observed when a PMF is formed by the L-malate decarboxylation pathway and used to drive the uptake of lactose. We thus show full functionality of a reaction network involving (i) PMF
generation by electrogenic transport and decarboxylation of L-malate; (ii) PMF consumption by lactose-H+ symport; (iii) metabolism of lactose to galactose plus 6-phosphoglucono-δ-lactone;
and (iv) reduction of NADP+ to NADPH. DISCUSSION We have developed a minimal PMF-generating system in lipid vesicles that enables the efficient uptake of nutrients via secondary active
transporters. We show that transport of the amino acid L-glutamate and the sugar lactose, mediated by GltP and LacY, respectively, reach maximal accumulation levels in good agreement with
the generated driving force. Furthermore, we have shown coupling of this chemiosmotic system to the initial steps of lactose metabolism and the formation of NADPH. This work is part of our
efforts to construct synthetic cells from molecular components and the development of pathways for long-term fueling of energy requiring processes. In comparison to strategies to feed
synthetic cells with building blocks by diffusion via pore-forming toxins49, nanopores50,51 or low-selectivity channels52, the L-malate decarboxylation pathway and ion-linked transporters
allow the accumulation of nutrients against their concentration gradient. This is an essential feature of living cells, not only to transport molecules in but also to pump metabolic end
products out. Additionally, the components of the system presented here are proteins that can be expressed and regenerated from their genetic units by an encapsulated
transcription-translation machinery, contributing to the construction of an autonomous synthetic cell53,54. Besides the sustained chemiosmotic transport of nutrients, our study offers
kinetic and mechanistic insights into the two protein components of the L-malate decarboxylation pathway (Figs. 1 and 2). Particularly, the association of the half turnover electrical
signals (Fig. 1g, h) with the presence of a highly conserved positively charged residue in the binding pocket of 2HCT family members55,56 suggests that the preferred species transported by
MleP are the monoprotonated forms of L-malate (HMal−) and L-lactate (L-lactic acid). Thus, we conclude that MleP generates a membrane potential by mono-anionic L-malate/L-lactic acid
antiport or at a much lower rate by mono-anionic L-malate uniport. The L-malate decarboxylation pathway is a particularly useful and robust system for the provision of metabolic energy in
the form of a PMF, because: (i) The L-malate decarboxylation pathway is constituted of only two proteins: an integral membrane protein that works as an electrogenic MleP and a soluble enzyme
that catalyzes the decarboxylation of L-malate to L-lactate (MleS). Decarboxylation pathways involving an electrogenic exchange or antiport reaction are arguably the simplest mechanisms to
generate an electrochemical proton gradient across the membrane. (ii) MleP exchanges structurally related substrates, L-malate and L-lactic acid, which have in common the 2-hydoxycarboxylate
unit. The direction of transport is determined by the substrate concentration gradients and not by the orientation of MleP in the membrane57,58. Indeed, we show that an inside negative
potential is formed when L-malate is taken up in exchange for internal L-lactate, whereas a positive potential is formed for L-malate exit in exchange for external L-lactate (Fig. 1). This
is a technical advantage over primary transporters that facilitate H+ translocation in a light-dependent process59,60, a redox reaction61 or ATP hydrolysis62. (iii) MleP and MleS are more
active at pH 6 (Figs. 1 and 2) than at pH 7 or higher, implying a built-in mechanism for pH homeostasis. Thus, more protons are taken up by the decarboxylation pathway when the internal pH
decreases, _e.g_. as a result of a lower external pH or import of protons via secondary active transporters such as GltP and LacY. The relative simplicity and versatility of the pathway to
generate a PMF and to maintain the internal pH relatively constant is an advantage for the further integration of metabolic modules in synthetic cells. Indeed, we show successful integration
of the L-malate decarboxylation pathway with a metabolic network for the formation of precursors of the glycolytic (glucose-6P) and pentose phosphate pathway (6-phosphoglucono-δ-lactone)
from the internalized lactose. The main function of the pentose phosphate pathway is to provide the cell with precursors for nucleotide synthesis and reducing power (NADPH)63. Hence, the
here presented system constitutes a platform for the integration of other essential functions like redox or ATP/ADP homeostasis64,65. What are the limitations of the L-malate decarboxylation
pathway? First, L-lactic acid (present in low amounts at pH 7 but in rapid equilibrium with L-lactate) is highly membrane permeable66,67,68, which in our system results in competition
between external L-lactic acid with L-malate. This reduces the exchange rate and, hence, limits the generation of PMF. Second, the decrease in activity in MleP and MleS at pH > 6 may
impose a limit on the capacity of the L-malate decarboxylation pathway to generate a PMF at alkaline pH values. The PMF in living cells fluctuates and responds to changes in the external
medium69,70. PMF values in cells have been reported to fall within the range −100 to −270 mV, depending on the specific organism and conditions71. The PMF from the L-malate decarboxylation
pathway in liposomes at pH 7 was maximally ≈ −100 mV, i.e., 8 min after L-malate addition and reached ≈ −120 mV after 12 min at pH 6 (Fig. 4d, e). Subsequently, the PMF decreased to ~−70 mV
at pH 7, mostly as a consequence of the partial dissipation of the membrane potential (Fig. 4d). Hence, at longer times, the contribution of ΔpH to the PMF increased relative to that of the
ΔΨ. We used in most of our studies a pH of 7; the PMF is higher at lower pH values reflecting the pH regulation of MleP and MleS. The accumulation of L-glutamate and D-lactose nicely follows
the transients in the PMF, suggesting the energy from the PMF is actively transformed into a substrate gradient by the symporters and, remarkably, we obtain H+/solute stoichiometries of ~3
for GltP and ~1 for LacY. These values nicely match the mechanistic stoichiometries of these proteins. In older literature, the relationship between the magnitude of the PMF and lactose
accumulation has been found condition-dependent, which has led to the suggestion that the mechanistic stoichiometry of LacY (and other transporters) may vary. Our estimates match the
mechanistic stoichiometry, which may be due to the fact that we work at relatively low PMF and close to pH 7, where leak pathways may be less prominent72,73. The internal L-glutamate
concentration reaches 1 mM, when the external concentration is only 20 µM. For biosynthesis, the cellular amino acid concentrations are typically in the low millimolar range; the glutamate
concentrations are typically higher because this amino acid also serves a role as compatible solute, and its oxidation can feed oxidative phosphorylation with reducing equivalents for in
vitro protein synthesis74,75,76,77,78. Reaching intravesicular concentrations in the GltP vesicles similar to those in _E. coli_ would require an external concentration of L-glutamate of
0.6–0.8 mM. We also note that, at 20 µM of L-glutamate, GltP operates at <40% of its maximal rate. A reference value for the intracellular concentration of lactose is not available,
because in cells the disaccharide is quickly broken down to galactose plus glucose, which are further metabolized via the Leloir and glycolytic pathway79, respectively. We have determined
the uptake of lactose at an external concentration of 50 μM, which is well below the _K_m of LacY (0.5 mM, Table 1). Hence, a much faster uptake is feasible at higher substrate
concentration. We also notice slow lactose efflux at higher internal lactose concentrations (at _t_ > 2 h, Fig. 6c), which is possibly linked with H+:lactose uncoupling events that are
more prominent at alkaline pH values73,80. Indeed, uncoupling of solute-H+ symport has been reported as strategy to prevent unconstrained accumulation of solutes81,82. In summary: we show
sustainable and long-term accumulation of an amino acid and metabolism of a sugar, driven by a PMF-generating pathway that could be implemented in various types of synthetic cells and used
to sustain far-from-equilibrium metabolism. A synthetic cell requires 20 amino acids (but also other nutrients), which could be taken up by separate amino acid transporters. To limit the
number of transporters to be reconstituted and later on to be produced by in-vesicle synthesis and membrane insertion, we envisage the use of a broad specificity di-/tripeptide transporter
such as DtpT together with luminal peptidases to supply the cell with all amino acids41,83,84. Similarly, the integration of H+:ribonucleoside symporters along with ribonucleoside kinases or
H+:nucleotide symporters can provide building blocks for the synthesis of DNA and RNA85,86. Co-reconstitution of the L-malate decarboxylation pathway along with Na+/H+ antiporters is a
strategy to fine-tune the pH homeostasis or convert the PMF into a SMF. This would extend the possibilities of building block carriers to symporters driven by a Na+ electrochemical gradient,
e.g., as present in mammalian cells. Given the importance of the PMF (and SMF) as energy carrier in all organisms from all kingdoms of life, the here-developed chemiosmotic network may
inspire other studies of molecular and cellular processes that require electrochemical ion gradients. METHODS PLASMID CONSTRUCTION FOR THE EXPRESSION OF MLEP AND MLES The plasmids bearing
the _mleP_ and _mleS_ genes were constructed following the method from87 as follows: The _mleP_ and _mleS_ genes were amplified from the genome of _L. lactis_ IL1403 by PCR with primers
_mleP_clic_fw_ and _mleP_clic_rev_ for MleP, and _mleS_clic_fw_ and _mleS_clic_rev_ for MleS (Supplementary Table 2), using Phusion HF DNA polymerase (Thermo Fisher Scientific, Inc.). This
yielded plasmids pNZ_clic_MleP and pNZ_clic_MleS. Both genes are under the nisin A-inducible _P_nis promoter, and the proteins have a TEV cleavable 10 His-tag at the C-terminus. Both
pNZ_clic_MleP and pNZ_clic_MleS were transformed into _L. lactis_ NZ9000. EXPRESSION OF _L. LACTIS_ MLEP AND MLES Expression of _L. lactis_ MleP and MleS was performed in a batch culture as
follows: _L. lactis_ NZ9000 cells transformed with pNZ_clic_MleP or pNZ_clic_MleS were grown in 3 L of rich media (2% (w/v) Gistex, 65 mM sodium phosphate pH 7, 1% (w/v) glucose)
supplemented with 5 µg mL−1 chloramphenicol at 30 °C (without stirring) after inoculation with 50 mL of an overnight pre-culture. At an optical density (OD600) of 0.5, the expression was
induced with 0.05% (v/v) of culture supernatant from a nisin A-producing strain. The strains were grown for an additional 2 hours and then harvested by centrifugation (6000 × _g_, 4 °C, 15
minutes), washed once, resuspended in ice-cold 100 mM potassium phosphate pH 7 to an OD600 ≈ 100, frozen in liquid nitrogen, and stored at −80 °C. EXPRESSION OF _E. COLI_ GLTP GltP was
produced from plasmid pBad24‐GltP. Expression was performed in _Escherichia coli_ MC1061 cells, grown in LB broth at 37 °C and shaken at 200 rpm. Ampicillin was added to the cultures to a
final concentration of 100 µg mL−1. At an optical density at 600 nm of 0.8–1.0, L‐arabinose was added to a final concentration of 0.01% (w/v) and the temperature was switched to 25 °C. Five
hours after induction, the cells were harvested (6268 × _g_, 10 min, 4 °C, Beckman JLA 9.1000 rotor), washed once, and resuspended in ice-cold 20 mM Tris‐HCl pH 8 to an OD600 ≈ 150, frozen
in liquid nitrogen, and stored at −80 °C. EXPRESSION OF _E. COLI_ LACY _E. coli_ BL21 cells transformed with the plasmid pT7C3H-lacY (containing the coding region of LacY and the protein
tagged with 10× His at the C-terminus) were grown in LB medium supplemented with 100 µg mL−1 of ampicillin at 37 °C and stirring at 150 rpm. Induction of protein expression was performed at
30 °C at an OD600 of 0.5 with 0.4 mM of isopropyl β-D-1-thiogalactopyranoside for a period of 3 h. Cells were harvested by centrifugation (6000 × _g_, 4 °C, 15 minutes), washed once,
resuspended in ice-cold 100 mM potassium phosphate pH 7 to an OD600 ≈ 100, frozen in liquid nitrogen, and stored at −80 °C. PURIFICATION OF _L. LACTIS_ MLES _L. lactis_ cells overexpressing
MleS were thawed and lysed at 30 kPsi in a high-pressure homogenizer (HPL6, Maximator) in the presence of 100 µg mL−1 DNAse, 2 mM MgSO4 and 1 mM PMSF. After lysis, 5 mM of sodium-EDTA was
added. Cell debris was removed by centrifugation (15 min, 22,000 × _g_, 4 °C) and the supernatant was centrifuged for 90 min at 125,000 × _g_ and 4 °C. Protein concentration in the cell
lysate was determined by the bicinchoninic acid assay (BCA) (ThermoFisher Scientific Protein Assay kit) using BSA as standard, the cell lysate was frozen in liquid nitrogen and stored at −80
°C. Ni2+-Sepharose resin (Cytiva) was washed with milliQ water and equilibrated with buffer A (200 mM NaCl, 50 mM potassium phosphate pH 7.5) plus 10 mM imidazole. Lysate was thawed on ice
and incubated with Ni2+-Sepharose resin (0.5 ml column volume per 50 mg of total protein content) for 1 h with gentle mixing at 4 °C. The suspension was transferred to a glass chromatography
column (Bio-Rad). The resin was washed with 20 column volumes of buffer A plus 50 mM imidazole. Protein was eluted with buffer A containing 500 mM of Imidazole, and the protein
concentration was determined from absorbance measurements at 280 nm using a NanodropTM (ThermoFisher Scientific). Fractions with the highest protein concentration were used for
size-exclusion chromatography on a Superdex 200 Increase 10/300 GL column (GE Healthcare) in 100 mM NaCl, 50 mM potassium phosphate pH 7.0. Protein was supplemented with 10% glycerol,
aliquoted, flash-frozen in liquid nitrogen, and stored at −80 °C. PURIFICATION OF MLEP AND LACY _L. lactis_ cells overexpressing MleP or _E. coli_ cells overexpressing LacY were thawed and
lysed at 30 kpsi (_L. lactis_) or 20 kPsi (_E. coli_) in a high-pressure homogenizer (HPL6, Maximator) in the presence of 100 µg mL−1 DNAse, 2 mM MgSO4 plus 1 mM PMSF. After lysis, 5 mM of
sodium-EDTA was added. Cell debris was removed by centrifugation (15 min, 22,000 × _g_, 4 °C) and the supernatant was centrifuged for 90 min at 205,000 × _g,_ and 4 °C. Supernatant was
discarded, and the pellet of cell membranes was resuspended in ice-cold potassium phosphate pH 7 to a total protein concentration of 10 mg mL−1 (Determined by the BCA assay). Resuspended
membranes were flash-frozen in liquid nitrogen and stored at −80 °C. Membrane vesicles containing 20 mg of total protein were thawed on ice, and solubilized for one hour with
_n_-dodecyl-β-D-maltoside (DDM) [0.5% (w/v) for MleP, 1.0% for LacY] in 200 mM NaCl, 50 mM potassium phosphate pH 7.5. Non-solubilized membranes were removed by centrifugation (25 min,
270,000 × _g_, 4 °C). Ni2+-Sepharose resin (Cytiva) was washed with milliQ water, equilibrated with buffer A (200 mM NaCl, 50 mM potassium phosphate pH 7.5) supplemented with 10 mM imidazole
plus 0.03% (for MleP) or 0.05% (for LacY) (w/v) of DDM and added to the solubilized membrane vesicles. The suspension was nutated for 1 h and subsequently transferred to a Poly-Prep
chromatography column (Bio-Rad). The resin was washed with 20 column volumes of buffer A plus 50 mM imidazole and 0.03% (for MleP) or 0.05% (for LacY) (w/v) of DDM. Proteins were eluted with
buffer A plus 350 mM imidazole and 0.03% (for MleP) or 0.05% (for LacY) (w/v) of DDM. Protein concentration was determined from absorbance measurements at 280 nm using a NanodropTM
(ThermoFisher Scientific). 2 mM of β-mercaptoethanol was added to all the buffers for the purification of LacY. PURIFICATION OF GLTP _E. coli_ cells overexpressing GltP were thawed and lysed
at 25 kPsi (_E. coli_) in a high-pressure homogenizer (HPL6, Maximator) in the presence of 100 µg mL−1 DNAse, 2 mM MgSO4 plus 1 mM PMSF. Unbroken cells and cell debris were pelleted (30
min, 12,074 × _g_, 4 °C), and the supernatant was subjected to ultracentrifugation (150 min, 193,727 × _g_, 4 °C). Membrane pellets were resuspended in 20 mM Tris‐HCl pH 8, and stored at
‐80°C. The protein concentration in the membranes was determined using BCA method, with BSA as a standard. Proteins were solubilized from membrane vesicles in buffer B (300 mM NaCl, 50 mM
HEPES pH 8.0), containing 15 mM imidazole pH 8.0 plus 1% _n_‐decyl‐β‐maltoside (w/v) (DM), at a final protein concentration of 3 mg mL−1. After incubation on a rocking platform for 60 min,
the solution was centrifuged (30 min, 286,286 × _g_, 4 °C). Supernatants were incubated on a rotating platform for 60 min at 4 °C with Ni2+‐Sepharose slurry (Fast‐flow, GE Healthcare, bed
volume of 0.5 ml), pre‐equilibrated with buffer A. The mixture was loaded on a BioRad Poly‐Prep column, and unbound protein was allowed to flow through. Columns were washed with 20 column
volumes of buffer C (500 mM KCl, 0.15% DM (w/v), 50 mM MES pH 6.0), supplemented with 150 mM imidazole pH 6.0 and continued washed with 5 column volumes of buffer C, supplemented with 200 mM
imidazole pH 6.0. Protein was eluted from the column in three fractions of 350, 800, and 400 μL using buffer C, supplemented with 500 mM imidazole pH 6.0. The second elution fraction from
the affinity chromatography contained most of the purified protein. Protein concentration was determined from absorbance measurements at 280 nm using a NanodropTM (ThermoFisher Scientific).
LIPID AND VESICLE PREPARATION _E. coli polar lipids: egg PC (3:1). E. coli_ polar lipids were prepared by precipitation with acetone and then extraction with diethyl ether from a commercial
extract of _E. coli_ total lipids (Avanti Polar Lipids), according to ref. 88. _E. coli_ polar lipids and egg PC (Avanti Polar Lipids) dissolved in chloroform were mixed to a molar ratio of
3:1. _DOPE:DOPG:DOPC (1:1:2)_. Synthetic lipids 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dioleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (DOPG) and
1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) (Avanti Polar Lipids) were dissolved in chloroform to a concentration of 25 mg mL−1 and mixed in a molar ratio of 1:1:2. For the formation of
vesicles (liposomes), the chloroform in the lipid mixtures was evaporated in a rotary evaporator (Büchi Labortechnik AG), the lipids were washed with diethyl ether, and the lipid film was
hydrated and resuspended in 50 mM K-phosphate at pH 7 to a concentration of 20 mg mL−1. Lipid resuspension was facilitated by sonication with a tip sonicator at 70% amplitude, 15 s ON, 45 s
OFF for 16 cycles, while the suspension was kept cold in ice-water and subjected to three cycles of freezing (in liquid nitrogen)—thawing (in a water bath at room temperature).
Large-unilamellar vesicles (LUVs) were formed by extrusion of the lipid suspension through a 400 nm pore-size polycarbonate filter (Whatman, GE Healthcare). RECONSTITUTION OF MEMBRANE
PROTEINS RECONSTITUTION MEDIATED BY TRITON X−100 AND DETERGENT REMOVAL WITH POLYSTYRENE BEADS (MLEP, GLTP, AND MLEP PLUS GLTP) The protocol was adapted from ref. 89. Briefly, an aliquot (1
mL) of 20 mg mL−1 of a mixture _E. coli_ polar lipids:egg phosphatidylcholine (PC) 3:1 (mol ratio) or DOPE:DOPG:DOPC 1:1:2 (mol ratio) lipids were resuspended in 50 mM potassium phosphate pH
7.0 and 13× extruded through a 400 nm polycarbonate filter (Whatman, GE Healthcare) to form LUVs, diluted to a lipid concentration 4 mg mL−1 in 50 mM potassium phosphate pH 7.0, and
destabilized with 10% Triton X−100 by titration to OD540 = 0.6 × _R__sat_ (_R__sat_ is point of maximal OD540). Detergent-purified membrane protein (≈1 mg mL−1) was added to the lipid-Triton
X−100 mixture at the desired LPR ratio (w/w), and the protein-detergent-lipid mixture was incubated at room temperature for 30 min with gentle agitation. Detergent was removed by
consecutive additions of polystyrene beads (BioBeads SM-2, BioRad) with continuous and gentle agitation as follows: 25 mg mL−1 of polystyrene beads were added and incubated for 30 min at
room temperature. A second portion of 15 mg mL−1 was added and incubated for 60 min at 4 °C. A third portion of 20 mg mL−1 was added and incubated overnight at 4 °C. After the last addition
of 40 mg mL−1 the mixture was incubated for 2 h at 4 °C. Polystyrene beads were discarded, proteoliposomes were harvested by centrifugation (25 min, 270,000 × _g_, 4 °C) and resuspended to a
lipid concentration of 50 mg mL−1 in 50 mM potassium phosphate pH 7.0. RECONSTITUTION MEDIATED BY N-OCTYL-Β-D-GLUCOPYRANOSIDE (OG) AND RAPID DILUTION (MLEP, LACY AND MLEP PLUS LACY) The
protocol was adapted from refs. 42,43,44. Briefly, 1 mL of 20 mg mL−1 of _E. coli_ polar: egg PC 3:1 (mol ratio) lipids were resuspended in 50 mM potassium phosphate pH 7.0 plus 1 mM
dithiothreitol (DTT) and dissolved by adding OG to a final concentration of 1.8% (w/v) and incubation at room temperature for 2 h with agitation. DDM-purified membrane protein (≈1 mg mL−1)
was added, and the mixture was incubated on ice for 10 min. The protein-detergent-lipid mixture was diluted at least 100× in ice-cold 50 mM potassium phosphate pH 7.0 plus 1 mM DTT, and the
proteoliposomes were harvested by centrifugation (120 min, 205,000 × _g_, 4 °C). The pellet was resuspended in 50 mM potassium phosphate pH 7.0 plus 1 mM DTT to a lipid concentration of 10
mg mL−1, polystyrene beads (BioBeads SM-2, BioRad) were added (50 mg mL−1), and the mixture was incubated overnight at 4 °C with gentle agitation. Polystyrene beads were discarded,
proteoliposomes were harvested by centrifugation (25 min, 270,000 × _g_, 4 °C) and resuspended to a lipid concentration of 50 mg mL−1 in 50 mM potassium phosphate pH 7.0 plus 1 mM DTT. MleP
LPR 100 vesicles composed of _E. coli_ polar lipids: egg PC 3:1 (mol ratio) was the standard for the characterization of MleP by SSM measurements. This LPR yields about 60 MleP molecules per
vesicle of diameter 200 nm, which is sufficient for large and robust electrical signals even at very low concentrations of substrate. Previous studies have shown that the reconstitution
efficiency decreases with decreasing LPR37,38. Hence, we increased the LPR to 250 for experiments when multiple membrane proteins had to be reconstituted (MleP with GltP or LacY) and MleS
was encapsulated. We tested two different lipid compositions to demonstrate that, besides _E. coli_ polar lipids+egg PC (3:1), MleP also functions in synthetic lipid mixtures like
DOPE:DOPG:DOPC (1:1:2) (Fig. 1f and Supplementary Fig. 2), albeit less efficiently (Supplementary Fig. 2). SSM-BASED ELECTROPHYSIOLOGICAL MEASUREMENTS Liposomes or proteoliposomes were
adsorbed onto an SSM pre-formed on a gold sensor chip and transport currents were detected via capacitive coupling, as explained elsewhere28,29. Proteoliposomes with MleP in _E. coli_ polar:
egg PC lipids 3:1 (mol ratio) at the indicated LPR (w/w) were used for the encapsulation of sodium-L-lactate or sodium-L-malate in 100 mM potassium phosphate pH 7.0 by 5× freeze-thaw cycles
(flash-freezing in liquid nitrogen, thawing in an ice-water bath at ≈10 °C), followed by gentle mixing and 13× extrusion through a 200 nm polycarbonate filter (Whatman, GE Healthcare).
Extruded proteoliposomes were collected by centrifugation (25 min, 270,000 × _g_, 4 °C) and resuspended to a final lipid concentration of 5 mg mL−1 in non-activating (NA) solution (see
below) containing either L-malate or L-lactate. 10 µL of MleP proteoliposomes were applied onto an SSM, pre-formed on an octadecanethiol-functionalized gold sensor chip (3 mm diameter,
Nanion) by the addition of 1,2-diphytanoyl PC (15 mg mL−1 in _n_-decane) (Avanti Polar Lipids) and NA solution (see below). The adsorption of proteoliposomes to the SSM was accelerated by
centrifugation of the sensor chip at 2500 × _g_, 30 min at room temperature. The sensor chip with the proteoliposomes adsorbed on the SSM were loaded into the chamber of a SURFE2R N1 device
(Nanion) and a jump in the concentration of substrate was triggered via a software-controlled fast solution exchange protocol29 at room temperature. The solution exchange protocol consisted
of an initial perfusion of NA solution for 1 sec followed by the perfusion of activating solution (A) for 1 sec and a final perfusion of NA solution for 1 sec. The A solution contained the
substrate that initiates the transport. The composition of the NA solution was the same as that in the lumen of the proteoliposomes. Current signals obtained during the perfusion of A
solution were taken for quantification purposes (ON signals). For all the measurements, we used 100 mM potassium phosphate at the indicated pH. Ionic strength and osmolarity were made
similar for the A and NA solutions; acetate− and sulfate2− were used as non-transported anions to replace L-lactate− and L-malate2−, respectively. For pH dependence, proteoliposomes adsorbed
onto the SSM were incubated for 20 min in NA solution at the corresponding pH before the solution exchange for pH equilibration. Supplementary Table 3 summarizes the transport modes and
conditions of the solution exchange. For the L-malate dependence, the A solution was x mM Na-L-malate, (30−x) mM Na-sulfate plus 30 mM Na-acetate, while the NA solution was 30 mM
Na-L-lactate plus 30 mM of Na-sulfate. For the L-lactate dependence, the A solution was x mM Na-L-lactate, (30−x) mM Na-acetate plus 30 mM Na-sulfate, while the NA solution was 30 mM
Na-L-malate plus 30 mM of Na-acetate. L-MALATE EFFLUX ASSAYS For the efflux assays 20 mg of MleP proteoliposomes (lipid-to-protein ratio 400:1) were used. The vesicles were extruded 13 times
through a 200 nm pore-size polycarbonate filter, diluted to 6 mL in 100 mM potassium phosphate pH 7.0, and collected by centrifugation (25 min, 270,000 × _g_, 4 °C). The vesicles were
resuspended in as little solution as possible (<100 µL). To load the vesicles with substrate, 1 mM of 14C-L-malate was added (final specific activity: 600 MBq mmol−1). This suspension was
incubated at room temperature for 1 hour, followed by overnight incubation at 4 °C. The vesicles were diluted to a final concentration of 3.34 mg of lipid mL−1 in 100 mM potassium phosphate
pH 7.0 or sodium phosphate pH 7.0. Next, 0.2 µM valinomycin (stock solution of 100 µM in ethanol) was added to either dissipate any formed membrane potential, or to create a membrane
potential (inside negative) in vesicles resuspended in sodium phosphate buffer. 100 µL samples were taken at given time intervals, diluted in 2 ml of ice-cold quenching buffer (100 mM
potassium phosphate pH 7.0), and filtered over 0.45 µm pore size cellulose nitrate filters. The filters were washed with 2 mL quenching buffer. Radioactivity was quantified by liquid
scintillation counting using Ultima Gold MV scintillation fluid (PerkinElmer) and a Tri-Carb 2800TR scintillation counter (PerkinElmer). MLES ACTIVITY ASSAY L-malate decarboxylation activity
catalyzed by MleS was estimated from the H+ consumption with a pH combination microelectrode (BlueLine 16, SI Analytics) in a solution with low buffer capacity. 700 µL of reaction solution
containing 0.5 mM NAD+, 0.1 mM MnCl2, 100 mM KCl, and 2 mM potassium phosphate at pH 7.0 were incubated for 3 min at 30 °C. The enzyme was added at the concentration indicated in Fig. 2
(50–150 nM), and a temperature equilibration step of 3 min was performed. The decarboxylation reaction was initiated (_t_ = 0) by the addition of sodium-L-malate to a final concentration of
5 mM (or otherwise indicated) from a stock solution whose pH was adjusted to pH 7.0 with NaOH. For the pH dependence, 2 mM potassium phosphate was used at pH 6–8, while sodium-acetate was
used for experiments at pH 4 and 5, keeping the L-malate concentration at 5 mM and MleS concentration at 150 nM. For the L-malate dependence, the enzyme concentration was 150 nM. The
L-malate decarboxylation activity was calculated as the H+ consumption expressed in µmol H+ min−1 mg−1, using a titration curve with NaOH to calibrate the buffer capacity. The pH was
recorded during the progression of the reaction with the software MultiLab Pilot V5.21 (Xylem Inc.). ENCAPSULATION OF SOLUBLE COMPONENTS OF THE L-MALATE DECARBOXYLATION The protocol was
adapted from65. 2 mM sodium-L-lactate, 1.0 mM sodium-β-nicotinamide adenine dinucleotide (NAD+), 0.5 mM MnCl2, 0.1 mM HPTS, pyranine, (ThermoScientific), and 2.5 µM MleS was mixed with 10 mg
of proteoliposomes resuspended to a lipid concentration of 25 mg mL−1 in 50 mM potassium phosphate pH 7 to a final volume of 400 µL. Encapsulation of soluble components was performed by a
5× freeze-thaw cycle (flash-freezing in liquid nitrogen, and thawing in an ice-water bath at ≈10 °C) followed by gentle mixing and 13× extrusion through a 400 nm polycarbonate filter
(Whatman, GE Healthcare). External components were removed by gel filtration on a 22 cm long column with Sephadex G-75 (Sigma) pre-equilibrated with 2 mM sodium-L-lactate plus 50 mM
potassium phosphate pH 7.0. Proteoliposomes were washed twice with 60× volume dilution, followed by centrifugation (25 min, 270,000 × _g_, 4 °C) and resuspension to a final lipid
concentration of 125 mg mL−1. 2 mM of DTT was added to all buffers when vesicles with LacY were used. INTERNAL PH MEASUREMENTS The internal pH of the vesicles was determined from
fluorescence of encapsulated pyranine (trisodium-8-hydroxypyrene-1,3,6-trisulfonate, HPTS (ThermoFisher Scientific))90. Pyranine was encapsulated in proteoliposomes (along with the soluble
components of the L-malate decarboxylation pathway) at a concentration of 0.1 mM, as described in the previous section. Liposomes or proteoliposomes containing internal pyranine were diluted
50 times in an external solution (2 mM sodium-L-lactate plus 50 mM potassium phosphate pH 7, unless otherwise indicated) to a lipid concentration of 2.5 mg mL−1 in a quartz fluorescence
cuvette (105.250 QS, Hellma Analytics). To eliminate the fluorescence from traces of pyranine on the outside, 5 mM of the collisional quencher p-xylene-bis-pyridinium bromide (DPX) was
included in the external solution. The mixture was incubated for 10 min at 30 °C, and the L-malate decarboxylation reaction started by the addition of 10 mM (unless otherwise indicated) of
sodium-L-malate (from a 2 M stock pre-adjusted to pH 7 with NaOH). Where the effect of ionophores was to be determined, valinomycin and nigericin dissolved in dimethyl sulfoxide (DMSO) were
added to a concentration of 1 µM (lipid:ionophore ≈3000:1 (mol ratio)). Fluorescence measurements were performed in an FP-8300 spectrofluorometer (Jasco, Inc). Excitation spectra of pyranine
between 380 and 480 nm (λexc) at an emission wavelength (λem) of 512 nm were taken at intervals of 1 min. The ratio between the fluorescence intensities at λexc = 450 nm and 405 nm
(_F_450nm/_F_405nm) was calculated and interpolated in a calibration curve to obtain the pH values. For the calibration curve: Pyranine was diluted 1000 times in the solution of 50 mM
potassium phosphate at the indicated pH to a final concentration of 0.1 µM, and the excitation spectra were measured as described above. The pH of the buffer was measured right before
starting the fluorescence measurements with a pH microelectrode. _F_450nm/_F_405nm was plotted against the measured pH (Supplementary Fig. 10c), and the data were fitted to a logistic
equation of the form: $${{{\rm{y}}}}=\frac{{{{\rm{a}}}}}{1+{{{{\rm{e}}}}}^{-{{{\rm{k}}}}({{{\rm{x}}}}-{{{{\rm{x}}}}}_{{{{\rm{c}}}}})}}$$ (4) Where _y_ = F450/F405, _x_ = pH, _a_ = 3.741, _k_
= 2.223 and _x_c = 7.883. To calculate the pH from the fluorescence excitation spectra of pyranine we used the following equation:
$${{{\rm{pH}}}}=7.883-\frac{1}{2.223}{{{\rm{Ln}}}}\left(\frac{3.741}{({{{{\rm{F}}}}}_{450}/{{{{\rm{F}}}}}_{405})}-1\right)$$ (5) L-LACTATE QUANTIFICATION BY RP-HPLC MLES ACTIVITY IN SOLUTION
To measure L-lactate production by MleS in solution, the enzyme was diluted to a concentration of 50 nM in 0.5 mM NAD+, 0.1 mM MnCl2, 100 mM KCl plus 2 mM potassium phosphate pH 7.0 and
incubated at 30 °C for 3 min. The reaction was initiated by the addition of 5 mM sodium-L-malate (pre-adjusted to pH 7 with NaOH). 50 µL aliquots were taken at 0 s, 10 s, 30 s, 60 s, 2 min,
3 min, 5 min, 10 min, 20 min, and 30 min, and processed as described below. PRODUCTION OF L-LACTATE BY THE L-MALATE DECARBOXYLATION PATHWAY IN VESICLES MleP-proteoliposomes containing 2 mM
sodium-L-lactate, 1.0 mM NAD+, 0.5 mM MnCl2 plus 50 mM potassium phosphate pH 7.0 were diluted 50 times in external solution containing 2 mM sodium-L-lactate and 50 mM potassium phosphate pH
7.0 and incubated at 30 °C for 3 min. The L-malate decarboxylation pathway was initiated by the addition of 10 mM sodium-L-malate (from a 2 M stock pre-adjusted to pH 7 with NaOH), and 50
µL aliquots were taken at the indicated times over a period of 4 hours and processed as described below. A sample before the addition of L-malate was also taken. DERIVATIZATION AND ANALYSIS
The protocol for the analysis of L-lactate was adapted from ref. 91. L-malate decarboxylation in solution or in MleP-proteoliposomes was stopped by transferring 50 µL of samples into 20 µL
of quenching solution (7% perchloric acid plus 4.5 mM EDTA). The excess acid was neutralized by the addition of 15 µL of 1 M KOH plus 1 M KHCO3, and samples were incubated overnight at −20
°C. Samples were centrifuged at 16,000 × _g_ for 5 min at room temperature in a table top centrifuge and 10 µL of the supernatant was transferred to 20 µL of 5% (w/v) triethanolamine (TEA)
in acetonitrile (ACN) plus 90 µL of 90 mM tetra-_n_-butylammonium bromide in acetonitrile. 380 µL of derivatization reagent (10 mM 9-chloromethyl anthracene (9-CMA, ThermoFisher Scientific)
in acetonitrile) was added, and the reaction was run at 70 °C for 30 min. The solution 9-CMA was previously sonicated in a water bath for 15 min and filtrated through a PTFE filter.
Derivatized samples at room temperature were centrifuged for 5 min at 16,000 × _g_ and 10 µL of a 2× diluted supernatant was analyzed by RP-HPLC on a 1260 LC HPLC system (Agilent) composed
of a G1311B binary pump, G1329B autosampler, G1316A thermostated column compartment and a G1315C diode array detector, using a Shimadzu XR-ODS 3 × 75 mm C18 column. Samples were run with a
binary gradient between ACN and water as follows: start was at 30% ACN, 80% ACN at 10 min, 95% ACN from 11 to 15 min, 30% ACN from 16 min to the end (20 min), flow rate 0.9 µL min−1. The
column was kept at 40 °C, while samples in the autosampler were kept at 10 °C. Samples were analyzed by detection of absorbance at 365 nm. L-lactate derivative was detected based on the
retention time and quantified by interpolation of the peak area from a calibration curve (Supplementary Fig. 6). DETERMINATION OF MEMBRANE POTENTIAL IN VESICLES The membrane potential in
liposomes and proteoliposomes was estimated using 3,3’-dipropylthiadicarbocyanine iodide (DiSC3(5), Invitrogen) as a fluorescent probe. MEMBRANE POTENTIAL IN VESICLES WITH THE L-MALATE
DECARBOXYLATION PATHWAY MleP at LPR 250 (w/w) in _E. coli_ polar lipids:egg PC 3:1 (mol ratio) liposomes containing 2.5 µM MleS, 1 mM NAD+, 0.5 mM MnCl2, 2 mM sodium-L-lactate, (±0.1 mM
pyranine) plus 50 mM potassium phosphate pH 7.0 were diluted 650 times in 0.98 mL of 2 mM sodium-L-lactate (unless otherwise indicated) plus 50 mM potassium phosphate pH 7.0 to a final
concentration of lipids of 0.2 mg mL−1 in a cuvette for fluorescence measurements. The suspension was incubated at 30 °C for 3 min with constant stirring (700 rpm) using a glass-covered
magnetic bar. DiSC3(5) was added to a final concentration of 1 µM from a 1 mM stock in DMSO. After an equilibration time of 5 min, L-malate decarboxylation was initiated by addition of 10 mM
sodium-L-malate (from a 2 M stock pre-adjusted to pH 7.0 with NaOH) and the fluorescence quenching (_F_q) was followed for 30–60 min. Valinomycin was added to a final concentration of 50 nM
from a 50 µM stock in DMSO to dissipate the membrane potential, which is observed as an increment in fluorescence (_F_val). The percentage of fluorescence quenching (\(\Delta\)F %) was
calculated according to Eq. (6) and interpolated in a calibration curve (obtained for the same liposome samples) to estimate the magnitude of the L-malate-induced membrane potential.
$$\Delta {{{\rm{F}}}}(\%)=\frac{{{{{\rm{F}}}}}_{{{{\rm{q}}}}}-{{{{\rm{F}}}}}_{{{{\rm{Val}}}}}}{{{{{\rm{F}}}}}_{{{{\rm{Val}}}}}}$$ (6) CALIBRATION CURVE MleP at LPR 250 (w/w) in _E. coli_
polar lipids:egg PC liposomes containing 50 mM of potassium phosphate pH 7.0 were 650 times diluted in 0.98 mL of 50 mM potassium phosphate, 50 mM sodium phosphate or mixtures of these
buffers to obtain the desired K+ concentration on outside of the vesicles (Supplementary Table 4). DiSC3(5) was added to a final concentration of 1 µM from a 1 mM stock in DMSO and, after
equilibration, valinomycin was added to a final concentration of 50 nM from a 50 µM stock in DMSO. As a response to the negative inside membrane potential established from the diffusion of
K+ along its concentration gradient, the fluorescence was quenched to a final level (_F_q) depending on the magnitude of the K+ gradient. Nigericin was added in order to dissipate the
membrane potential causing a fluorescence dequenching (_F_Nig). The calibration curve was constructed by plotting the percentage of quenching (Fluorescence quenching %), calculated according
to Eq. (7) and using the imposed potassium diffusion potential. $$\Delta
{{{\rm{F}}}}(\%)=\frac{{{{{\rm{F}}}}}_{{{{\rm{q}}}}}-{{{{\rm{F}}}}}_{{{{\rm{Nig}}}}}}{{{{{\rm{F}}}}}_{{{{\rm{Nig}}}}}}$$ (7) The K+ diffusion potential was calculated according to Eq. (8),
$$\Delta \Psi=2.303\left(\frac{{{{\rm{RT}}}}}{{{{\rm{F}}}}}\right)\log \left(\frac{[{{{{{{\rm{K}}}}}}^{+}}]_{{{{{\rm{OUT}}}}}}}{[{{{{{{\rm{K}}}}}}^{+}}]_{{{{{\rm{IN}}}}}}}\right)$$ (8) where
_R_ is the gas constant, _T_ is the temperature in Kelvin, and _F_ is the Faraday constant. Data were fitted to an exponential equation (Supplementary Fig. 16b). The membrane potential was
calculated from fluorescence quenching curves using Eq. (9), $$\Delta \Psi \left({{{\rm{mV}}}}\right)=k{{{\rm{Ln}}}}\left(\frac{\Delta
{{{\rm{F}}}}\left(\%\right)-{y}_{0}}{{{{\rm{A}}}}}\right)$$ (9) where _A_ = 30.7, _k_ = 108.4, _y_0 = −30.9 are fitting parameters of the data in Supplementary Fig. 16b, and ΔF(%)
corresponds to the fluorescence quenching. Fluorescence measurements were performed in an FP8300 spectrofluorometer (JASCO) using a 10 × 4 mm QS cuvette (Hellma Analytics, 109.004 F) at a
temperature of 30 °C under constant stirring (700 rpm) with a glass-covered magnetic bar. Excitation and emission wavelengths were 662 and 680 nm, respectively. QUANTIFICATION OF L-GLUTAMATE
AND D-LACTOSE UPTAKE IN PROTEOLIPOSOMES Proteoliposomes with MleP, GltP, or LacY containing the soluble components of the L-malate decarboxylation pathway (2 mM sodium-L-lactate, 1.0 mM
NAD+, 0.5 mM MnCl2 plus 50 mM potassium phosphate pH 7.0) were 50 times diluted in external solution containing 2 mM sodium-L-lactate, 50 mM potassium phosphate pH 7.0 and 20 µM of
14C-radiolabelled sodium-L-glutamate (Perkin Elmer) (specific activity 15 mCi/mmol, 555 MBq/mmol) for GltP or 50 µM of 14C-radiolabelled D-lactose (Amersham) (specific activity 15.4
mCi/mmol, 570.4 MBq/mmol) for LacY and incubated for 5 min at 30 °C with continuous stirring. 10 mM of sodium-L-malate was added (from a 1 M stock adjusted to pH 7 with NaOH) to start the
L-malate decarboxylation. 100 µL samples were taken at indicated times before and after the addition of L-malate for up to 4 hours, diluted into 2 mL of ice-cold quenching solution (0.1 M
LiCl), and filtered over 0.45 µm pore size nitrocellulose filters to stop the transport. The filter was washed, and radioactivity was quantified by liquid scintillation counting using Ultima
Gold MV scintillation fluid (PerkinElmer) and a Tri-Carb 2800TR scintillation counter (PerkinElmer). As an alternative protocol, proteoliposomes were diluted in an external solution without
substrate, and the radiolabelled substrate was added 30 min after the addition of L-malate; here, the L-glutamate or D-lactose transport is initiated after the generation of the PMF. When
the effect of membrane potential or pH gradient dissipation was to be evaluated, valinomycin or nigericin was added to a final concentration of 1 µM from a 1 mM stock in DMSO. To determine
the L-glutamate and D-lactose transport driven by the ΔΨ and ΔpH from valinomycin-facilitated K+ diffusion and acetic acid diffusion potentials, respectively, the corresponding
proteoliposomes (MleP plus GltP or MleP plus LacY) were loaded with 2 mM sodium-L-lactate, 70 mM potassium acetate and 25 mM of potassium phosphate at pH 7.0. The external composition was 2
mM sodium-L-lactate, 22 mM sodium acetate, 25 mM sodium phosphate pH 7.0, 20 µM of radiolabelled L-glutamate for GltP (or 50 µM of radiolabelled D-lactose for LacY), 1 µM of valinomycin and
different ratios of potassium-D-gluconate and sodium-D-gluconate to establish an outward K+ concentration gradient, according to Table 3. In this manner, the internal concentrations of K+
([K+]IN) and acetate− ([AcO−]IN) are 109 mM and 70 mM, respectively. The transport was initiated by a 50-fold dilution of proteoliposomes into the external solution (pre-incubated for 3 min
at 30 °C). 100 µL samples were taken at the indicated time intervals and processed as described above for quantification of radioactivity. The driving forces from the pH gradient and
membrane potential were calculated, using Eqs. (10) and (8), respectively. $${{{\rm{Z}}}}\Delta {{{\rm{pH}}}}=2.303\left(\frac{{{{\rm{RT}}}}}{{{{\rm{F}}}}}\right)\log
\left(\frac{[{{{{{\rm{AcO}}}}}^{-}}]_{{{{\rm{IN}}}}}}{[{{{{{\rm{AcO}}}}}^{-}}]_{{{{\rm{OUT}}}}}}\right)$$ (10) FLUOROMETRIC DETERMINATION OF LACTOSE METABOLISM IN VESICLES ENZYMES
β-galactosidase (LacZ) from _E. coli_ (Sigma. G5635, lyophilized) was hydrated in 50 mM K-phosphate pH 7, 5 mM β-mercaptoethanol, 10 mM MgCl2 plus 10% glycerol to a final concentration of 10
mg mL−1 on ice until a translucid solution was obtained. Hexokinase (HK) from yeast (Roche, 11426365001) and glucose-6-phosphate dehydrogenase (G6P-DH) from yeast (Sigma, G7877) were
acquired as suspensions in 3.2 M of ammonium sulfate. An aliquot of every resuspension was centrifuged at 15,000 × _g_ for 10 min at 4 °C, and the supernatant was discarded. The pellet was
dissolved in 50 mM K-phosphate pH 7, 5 mM β-mercaptoethanol, 10 mM MgCl2 plus 10% glycerol to a final concentration of 8.5 mg mL−1 (HK) and 15 mg mL−1 (G6P-DH). Protein concentration was
determined from absorbance measurements at 280 nm using a NanodropTM (ThermoFisher Scientific). ENCAPSULATION Soluble components of the L-malate decarboxylation pathway (2.5 µM MleS, 1 mM
NAD+, 0.5 mM MnCl2 plus 2 mM Na-lactate) along with 7 µM β-galactosidase, 7 µM hexokinase, 4 µM G6P-DH, 10 mM MgATP, 2 mM NADP+ were encapsulated in MleP LPR 250 (w/w)-LacY LPR 200 (w/w) or
MleP LPR 250 vesicles with _E. coli_ polar lipids: egg PC 3:1 (mol ratio) via OG-mediated reconstitution and detergent removal by rapid dilution42,43. Encapsulation, washing and resuspension
was performed according to the protocol described in “Encapsulation of soluble components of the L-malate decarboxylation”. NADPH FLUORESCENCE MEASUREMENTS Proteoliposomes with encapsulated
components were diluted 40× in 50 mM K-phosphate pH 7, Na-lactate 2 mM, 1 mM DTT plus 2.5 mM EDTA, unless otherwise indicated, and the suspension was incubated for 5 min at 30 °C. 10 mM of
Na-malate was added from a 1 M stock and the mixture was incubated for 30 min to pre-form a PMF from the L-malate decarboxylation pathway. Then, D-lactose was added to a final concentration
of 100 µM from a 4 mM stock (40× dilution). For inhibition of lactose transport, TMG or TDG were added to a final concentration of 20 mM before the addition of D-lactose. Fluorescence
measurements were performed in a FP-8300 spectrofluorometer (Jasco, Inc). Emission spectra of NADPH between 380 and 500 nm (_λ_em) at an excitation wavelength (_λ_exc) of 350 nm were taken
at intervals of 1 min during the whole experiment. STATISTICS AND REPRODUCIBILITY Uncropped gels for the images presented in Figs. 1a, 2b, 3b, 5b, 6b can be found in Supplementary Figs. 1
and 9 and as Source Data. The band patterns observed in the SDS-polyacrylamide gels are representative from at least two independent experiments (different sample preparations). In
Supplementary Fig. 1a, c, three and two independent uncropped gels are presented for the analysis of MleP and LacY in liposomes, respectively, showing that images in Figs. 1a and 6b are
representative from independent sample preparations. Lanes 7 and 8 in Supplementary Fig. 1b and Lanes 8 and 9 in Supplementary Fig. 9 correspond to independent reconstitution samples and
show that images in Figs. 5b and 3b are representative of independent preparations. Independent replicates with different sample preparations are indicated by _n_ number in the Figure
legends, and the standard deviation (SD) is indicated when _n_ > 2. DATA ANALYSIS AND FIGURES DESIGN Data and statistical analysis were performed using OriginPro Lab v8.5. Cartoons in
Fig. 1b, g, 2a, 3a, 4a, 5a, e, 6a, d. were created with Biorender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. Figures were designed
with Adobe Illustrator. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All data in
this study are available within the main text, Supplementary Information and Source Sata files. Source data is available for Figs. 1–6, Supplementary Figs. 1–3 and 5–27, Table 1 and
Supplementary Table 1 in the associated source data file. Source data are provided with this paper. REFERENCES * Konings, W. N., Poolman, B. & van Veen, H. W. Solute transport and energy
transduction in bacteria. _Antonie Van Leeuwenhoek_ 65, 369–380 (1994). Article CAS PubMed Google Scholar * Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic
phosphorylation. _Biol. Rev. Camb. Philos. Soc._ 41, 445–502, (1966). Article CAS PubMed Google Scholar * Konings, W. N. The cell membrane and the struggle for life of lactic acid
bacteria. _Antonie Van Leeuwenhoek_ 82, 3–27 (2002). Article CAS PubMed Google Scholar * Konings, W. N., Poolman, B. & Driessen, A. J. Bioenergetics and solute transport in
lactococci. _Crit. Rev. Microbiol._ 16, 419–476 (1989). Article CAS PubMed Google Scholar * Konings, W. N. et al. The role of transport processes in survival of lactic acid bacteria.
Energy transduction and multidrug resistance. _Antonie Van Leeuwenhoek_ 71, 117–128 (1997). Article CAS PubMed Google Scholar * Dimroth, P. & Schink, B. Energy conservation in the
decarboxylation of dicarboxylic acids by fermenting bacteria. _Arch. Microbiol._ 170, 69–77 (1998). Article CAS PubMed Google Scholar * Poolman, B. et al. Malolactic fermentation:
electrogenic malate uptake and malate/lactate antiport generate metabolic energy. _J. Bacteriol._ 173, 6030–6037 (1991). Article CAS PubMed PubMed Central Google Scholar * Renault, P.,
Gaillardin, C. & Heslot, H. Role of malolactic fermentation in lactic acid bacteria. _Biochimie_ 70, 375–379 (1988). Article CAS PubMed Google Scholar * Lolkema, J. S., Poolman, B.
& Konings, W. N. Role of scalar protons in metabolic energy generation in lactic acid bacteria. _J. Bioenerg. Biomembr._ 27, 467–473 (1995). Article CAS PubMed Google Scholar *
Konings, W. N. Microbial transport: adaptations to natural environments. _Antonie Van Leeuwenhoek_ 90, 325–342 (2006). Article CAS PubMed Google Scholar * Strahl, H. & Hamoen, L. W.
Membrane potential is important for bacterial cell division. _Proc. Natl. Acad. Sci. USA_ 107, 12281–12286 (2010). Article ADS CAS PubMed PubMed Central Google Scholar * Schulze, R. J.
et al. Membrane protein insertion and proton-motive-force-dependent secretion through the bacterial holo-translocon SecYEG-SecDF-YajC-YidC. _Proc. Natl. Acad. Sci. USA_ 111, 4844–4849
(2014). Article ADS CAS PubMed PubMed Central Google Scholar * Prindle, A. et al. Ion channels enable electrical communication in bacterial communities. _Nature_ 527, 59–63 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar * Yang, C. Y. et al. Encoding membrane-potential-based memory within a microbial community. _Cell Syst._ 10, 417–423.e413 (2020).
Article CAS PubMed PubMed Central Google Scholar * Anantharam, V., Allison, M. J. & Maloney, P. C. Oxalate:formate exchange. The basis for energy coupling in Oxalobacter. _J. Biol.
Chem._ 264, 7244–7250 (1989). Article CAS PubMed Google Scholar * Marty-Teysset, C. et al. Proton motive force generation by citrolactic fermentation in Leuconostoc mesenteroides. _J.
Bacteriol._ 178, 2178–2185 (1996). Article CAS PubMed PubMed Central Google Scholar * Ilgu, H. et al. Insights into the molecular basis for substrate binding and specificity of the
wild-type L-arginine/agmatine antiporter AdiC. _Proc. Natl. Acad. Sci. USA_ 113, 10358–10363 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Romano, A., Trip, H.,
Lonvaud-Funel, A., Lolkema, J. S. & Lucas, P. M. Evidence of two functionally distinct ornithine decarboxylation systems in lactic acid bacteria. _Appl. Environ. Microbiol._ 78,
1953–1961 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Small, P. L. & Waterman, S. R. Acid stress, anaerobiosis and gadCB: lessons from Lactococcus lactis and
Escherichia coli. _Trends Microbiol._ 6, 214–216 (1998). Article CAS PubMed Google Scholar * Molenaar, D., Bosscher, J. S., ten Brink, B., Driessen, A. J. & Konings, W. N. Generation
of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. _J. Bacteriol._ 175, 2864–2870 (1993). Article CAS PubMed
PubMed Central Google Scholar * Wolken, W. A., Lucas, P. M., Lonvaud-Funel, A. & Lolkema, J. S. The mechanism of the tyrosine transporter TyrP supports a proton motive tyrosine
decarboxylation pathway in Lactobacillus brevis. _J. Bacteriol._ 188, 2198–2206 (2006). Article CAS PubMed PubMed Central Google Scholar * Abe, K. et al. Plasmid-encoded asp operon
confers a proton motive metabolic cycle catalyzed by an aspartate-alanine exchange reaction. _J. Bacteriol._ 184, 2906–2913 (2002). Article CAS PubMed PubMed Central Google Scholar *
Tolner, B., Ubbink-Kok, T., Poolman, B. & Konings, W. N. Cation-selectivity of the L-glutamate transporters of Escherichia coli, Bacillus stearothermophilus and Bacillus caldotenax:
dependence on the environment in which the proteins are expressed. _Mol. Microbiol._ 18, 123–133 (1995). Article CAS PubMed Google Scholar * Groeneveld, M., Duurkens, R. & Slotboom
D. J. On the mechanism of prokaryotic glutamate transporter homologues - Chapter 5. PhD Thesis thesis, University of Groningen, (2010). * Kaback, H. R. & Guan, L. It takes two to tango:
the dance of the permease. _J. Gen. Physiol._ 151, 878–886 (2019). Article CAS PubMed PubMed Central Google Scholar * Sobczak, I. & Lolkema, J. S. The 2-hydroxycarboxylate
transporter family: physiology, structure, and mechanism. _Microbiol. Mol. Biol. Rev._ 69, 665–695 (2005). Article CAS PubMed PubMed Central Google Scholar * Bandell, M., Ansanay, V.,
Rachidi, N., Dequin, S. & Lolkema, J. S. Membrane potential-generating malate (MleP) and citrate (CitP) transporters of lactic acid bacteria are homologous proteins. Substrate
specificity of the 2-hydroxycarboxylate transporter family. _J. Biol. Chem._ 272, 18140–18146 (1997). Article CAS PubMed Google Scholar * Schulz, P., Garcia-Celma, J. J. & Fendler,
K. SSM-based electrophysiology. _Methods_ 46, 97–103 (2008). Article CAS PubMed Google Scholar * Bazzone, A., Barthmes, M. & Fendler, K. SSM-based electrophysiology for transporter
research. _Methods Enzymol._ 594, 31–83 (2017). Article CAS PubMed Google Scholar * Caspritz, G. & Radler, F. Malolactic enzyme of Lactobacillus plantarum. Purification, properties,
and distribution among bacteria. _J. Biol. Chem._ 258, 4907–4910 (1983). Article CAS PubMed Google Scholar * Schumann, C. et al. Malolactic enzyme from Oenococcus oeni: heterologous
expression in Escherichia coli and biochemical characterization. _Bioengineered_ 4, 147–152 (2013). Article PubMed Google Scholar * Denayrolles, M., Aigle, M. & Lonvaud-Funel, A.
Cloning and sequence analysis of the gene encoding Lactococcus lactis malolactic enzyme: relationships with malic enzymes. _FEMS Microbiol. Lett._ 116, 79–86 (1994). Article CAS PubMed
Google Scholar * Beber, M. E. et al. eQuilibrator 3.0: a database solution for thermodynamic constant estimation. _Nucleic Acids Res._ 50, D603–D609 (2022). Article ADS CAS PubMed
Google Scholar * Barreto, J. & Lichtenberger, L. M. Vesicle acidification driven by a millionfold proton gradient: a model for acid influx through gastric cell membranes. _Am. J.
Physiol._ 262, G30–G34 (1992). CAS PubMed Google Scholar * Waggoner, A. S. The use of cyanine dyes for the determination of membrane potentials in cells, organelles, and vesicles.
_Methods Enzymol._ 55, 689–695 (1979). Article CAS PubMed Google Scholar * Ivkova, M. N., Pechatnikov, V. A. & Ivkov, V. G. Mechanism of fluorescent response of the probe diS-C3-(5)
to transmembrane potential changes in a lecithin vesicle suspension. _Gen. Physiol. Biophys._ 3, 97–117 (1984). CAS PubMed Google Scholar * Fang, G. et al. Manipulation of activity and
orientation of membrane-reconstituted di-tripeptide transport protein DtpT of Lactococcus lactis. _Mol. Membr. Biol._ 16, 297–304 (1999). Article CAS PubMed Google Scholar * Richard, P.,
Rigaud, J. L. & Graber, P. Reconstitution of CF0F1 into liposomes using a new reconstitution procedure. _Eur. J. Biochem._ 193, 921–925 (1990). Article CAS PubMed Google Scholar *
Bailoni, E. & Poolman, B. ATP recycling fuels sustainable glycerol 3-phosphate formation in synthetic cells fed by dynamic dialysis. _ACS Synth. Biol._ 11, 2348–2360 (2022). Article CAS
PubMed PubMed Central Google Scholar * Driessen, A. J., van Leeuwen, C. & Konings, W. N. Transport of basic amino acids by membrane vesicles of Lactococcus lactis. _J. Bacteriol._
171, 1453–1458 (1989). Article CAS PubMed PubMed Central Google Scholar * Fang, G., Konings, W. N. & Poolman, B. Kinetics and substrate specificity of membrane-reconstituted peptide
transporter DtpT of Lactococcus lactis. _J. Bacteriol._ 182, 2530–2535 (2000). Article CAS PubMed PubMed Central Google Scholar * Viitanen, P., Newman, M. J., Foster, D. L., Wilson, T.
H. & Kaback, H. R. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. _Methods Enzymol._ 125, 429–452 (1986). Article CAS PubMed Google
Scholar * Gaiko, O., Bazzone, A., Fendler, K. & Kaback, H. R. Electrophysiological characterization of uncoupled mutants of LacY. _Biochemistry_ 52, 8261–8266 (2013). Article CAS
PubMed Google Scholar * Lopez Mora, N. et al. The membrane transporter lactose permease increases lipid bilayer bending rigidity. _Biophys. J._ 120, 3787–3794 (2021). Article ADS CAS
PubMed PubMed Central Google Scholar * Olsen, S. G. & Brooker, R. J. Analysis of the structural specificity of the lactose permease toward sugars. _J. Biol. Chem._ 264, 15982–15987
(1989). Article CAS PubMed Google Scholar * Guan, L. & Kaback, H. R. Binding affinity of lactose permease is not altered by the H+ electrochemical gradient. _Proc. Natl. Acad. Sci.
USA_ 101, 12148–12152 (2004). Article ADS CAS PubMed PubMed Central Google Scholar * Tavoulari, S. & Frillingos, S. Substrate selectivity of the melibiose permease (MelY) from
Enterobacter cloacae. _J. Mol. Biol._ 376, 681–693 (2008). Article CAS PubMed Google Scholar * Veenhoff, L. M. & Poolman, B. Substrate recognition at the cytoplasmic and
extracellular binding site of the lactose transport protein of Streptococcus thermophilus. _J. Biol. Chem._ 274, 33244–33250 (1999). Article CAS PubMed Google Scholar * Noireaux, V.
& Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. _Proc. Natl. Acad. Sci. USA_ 101, 17669–17674 (2004). Article ADS CAS PubMed PubMed Central Google
Scholar * Krishnan, S. et al. Molecular transport through large-diameter DNA nanopores. _Nat. Commun._ 7, 12787 (2016). Article ADS CAS PubMed PubMed Central Google Scholar *
Fragasso, A. et al. Reconstitution of ultrawide DNA origami pores in liposomes for transmembrane transport of macromolecules. _ACS Nano_ 15, 12768–12779 (2021). Article CAS PubMed PubMed
Central Google Scholar * Garamella, J., Majumder, S., Liu, A. P. & Noireaux, V. An adaptive synthetic cell based on mechanosensing, biosensing, and inducible gene circuits. _ACS Synth.
Biol._ 8, 1913–1920 (2019). Article CAS PubMed Google Scholar * Gaut, N. J. & Adamala, K. P. Reconstituting natural cell elements in synthetic cells. _Adv. Biol. (Weinh.)_ 5,
e2000188 (2021). Article PubMed Google Scholar * Bailoni, E. et al. Minimal out-of-equilibrium metabolism for synthetic cells: a membrane perspective. _ACS Synth. Biol._ 12, 922–946
(2023). Article CAS PubMed PubMed Central Google Scholar * Kim, J. W. et al. Structural insights into the elevator-like mechanism of the sodium/citrate symporter CitS. _Sci. Rep._ 7,
2548 (2017). Article ADS PubMed PubMed Central Google Scholar * Bandell, M. & Lolkema, J. S. Stereoselectivity of the membrane potential-generating citrate and malate transporters
of lactic acid bacteria. _Biochemistry_ 38, 10352–10360 (1999). Article CAS PubMed Google Scholar * Drew, D. & Boudker, O. Shared molecular mechanisms of membrane transporters.
_Annu. Rev. Biochem_ 85, 543–572 (2016). Article CAS PubMed Google Scholar * Jardetzky, O. Simple allosteric model for membrane pumps. _Nature_ 211, 969–970 (1966). Article ADS CAS
PubMed Google Scholar * Lee, K. Y. et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. _Nat. Biotechnol._ 36, 530–535 (2018).
Article CAS PubMed Google Scholar * Berhanu, S., Ueda, T. & Kuruma, Y. Artificial photosynthetic cell producing energy for protein synthesis. _Nat. Commun._ 10, 1325 (2019). Article
ADS PubMed PubMed Central Google Scholar * Biner, O., Fedor, J. G., Yin, Z. & Hirst, J. Bottom-up construction of a minimal system for cellular respiration and energy regeneration.
_ACS Synth. Biol._ 9, 1450–1459 (2020). Article CAS PubMed PubMed Central Google Scholar * Uzdavinys, P. et al. Dissecting the proton transport pathway in electrogenic Na(+)/H(+)
antiporters. _Proc. Natl. Acad. Sci. USA_ 114, E1101–E1110 (2017). Article CAS PubMed PubMed Central Google Scholar * Ge, T. et al. The role of the pentose phosphate pathway in diabetes
and cancer. _Front. Endocrinol. (Lausanne)_ 11, 365 (2020). Article PubMed Google Scholar * Partipilo, M. et al. Minimal pathway for the regeneration of redox cofactors. _JACS Au_ 1,
2280–2293 (2021). Article CAS PubMed PubMed Central Google Scholar * Pols, T. et al. A synthetic metabolic network for physicochemical homeostasis. _Nat. Commun._ 10, 4239 (2019).
Article ADS PubMed PubMed Central Google Scholar * Deamer, D. W. Proton permeation of lipid bilayers. _J. Bioenerg. Biomembr._ 19, 457–479 (1987). Article CAS PubMed Google Scholar
* Redelmeier, T. E., Mayer, L. D., Wong, K. F., Bally, M. B. & Cullis, P. R. Proton flux in large unilamellar vesicles in response to membrane potentials and pH gradients. _Biophys. J._
56, 385–393 (1989). Article CAS PubMed PubMed Central Google Scholar * Rossignol, M., Thomas, P. & Grignon, C. Proton permeability of liposomes from natural phospholipid mixtures.
_Biochim. Biophys. Acta_ 684, 195–199 (1982). Article CAS PubMed Google Scholar * Krulwich, T. A., Sachs, G. & Padan, E. Molecular aspects of bacterial pH sensing and homeostasis.
_Nat. Rev. Microbiol._ 9, 330–343 (2011). Article CAS PubMed PubMed Central Google Scholar * Biquet-Bisquert, A. et al. Spatio-temporal dynamics of the proton motive force on single
bacterial cells. _bioRxiv_, https://doi.org/10.1101/2023.04.03.535353 (2023). * Kashket, E. R. The proton motive force in bacteria: a critical assessment of methods. _Annu. Rev. Microbiol._
39, 219–242 (1985). Article CAS PubMed Google Scholar * Lolkema, J. S. & Poolman, B. Uncoupling in secondary transport proteins. A mechanistic explanation for mutants of lac permease
with an uncoupled phenotype. _J. Biol. Chem._ 270, 12670–12676 (1995). Article CAS PubMed Google Scholar * Poolman, B., Knol, J. & Lolkema, J. S. Kinetic analysis of lactose and
proton coupling in Glu379 mutants of the lactose transport protein of Streptococcus thermophilus. _J. Biol. Chem._ 270, 12995–13003 (1995). Article CAS PubMed Google Scholar * Yue, K. et
al. Bottom-up synthetic biology using cell-free protein synthesis. _Adv. Biochem. Eng. Biotechnol._ 185, 1–20 (2023). PubMed Google Scholar * Gonzales, D. T., Suraritdechachai, S. &
Tang, T. D. Compartmentalized cell-free expression systems for building synthetic cells. _Adv. Biochem. Eng. Biotechnol._ 186, 77–101 (2023). PubMed Google Scholar * Garenne, D. et al.
Cell-free gene expression. _Nat. Rev. Methods Prim._ 1, 49 (2021). * Jewett, M. C., Calhoun, K. A., Voloshin, A., Wuu, J. J. & Swartz, J. R. An integrated cell-free metabolic platform
for protein production and synthetic biology. _Mol. Syst. Biol._ 4, 220 (2008). Article PubMed PubMed Central Google Scholar * Cai, Q. et al. A simplified and robust protocol for
immunoglobulin expression in Escherichia coli cell-free protein synthesis systems. _Biotechnol. Prog._ 31, 823–831 (2015). Article CAS PubMed PubMed Central Google Scholar * Holden, H.
M., Rayment, I. & Thoden, J. B. Structure and function of enzymes of the Leloir pathway for galactose metabolism. _J. Biol. Chem._ 278, 43885–43888 (2003). Article CAS PubMed Google
Scholar * Varela, M. F. & Wilson, T. H. Molecular biology of the lactose carrier of Escherichia coli. _Biochim Biophys. Acta_ 1276, 21–34 (1996). Article PubMed Google Scholar *
Henderson, R. K., Fendler, K. & Poolman, B. Coupling efficiency of secondary active transporters. _Curr. Opin. Biotechnol._ 58, 62–71 (2019). Article CAS PubMed Google Scholar *
Nelson, N., Sacher, A. & Nelson, H. The significance of molecular slips in transport systems. _Nat. Rev. Mol. Cell Biol._ 3, 876–881 (2002). Article CAS PubMed Google Scholar *
Hagting, A., Kunji, E. R., Leenhouts, K. J., Poolman, B. & Konings, W. N. The di- and tripeptide transport protein of Lactococcus lactis. A new type of bacterial peptide transporter. _J.
Biol. Chem._ 269, 11391–11399 (1994). Article CAS PubMed Google Scholar * Garai, P., Chandra, K. & Chakravortty, D. Bacterial peptide transporters: Messengers of nutrition to
virulence. _Virulence_ 8, 297–309 (2017). Article CAS PubMed Google Scholar * de Koning, H. P., Watson, C. J. & Jarvis, S. M. Characterization of a nucleoside/proton symporter in
procyclic Trypanosoma brucei brucei. _J. Biol. Chem._ 273, 9486–9494 (1998). Article PubMed Google Scholar * Haferkamp, I. et al. Tapping the nucleotide pool of the host: novel nucleotide
carrier proteins of Protochlamydia amoebophila. _Mol. Microbiol._ 60, 1534–1545 (2006). Article CAS PubMed PubMed Central Google Scholar * Geertsma, E. R. & Poolman, B.
High-throughput cloning and expression in recalcitrant bacteria. _Nat. Methods_ 4, 705–707 (2007). Article CAS PubMed Google Scholar * Newman, M. J. & Wilson, T. H. Solubilization
and reconstitution of the lactose transport system from Escherichia coli. _J. Biol. Chem._ 255, 10583–10586 (1980). Article CAS PubMed Google Scholar * Geertsma, E. R., Nik Mahmood, N.
A., Schuurman-Wolters, G. K. & Poolman, B. Membrane reconstitution of ABC transporters and assays of translocator function. _Nat. Protoc._ 3, 256–266 (2008). Article CAS PubMed Google
Scholar * Clement, N. R. & Gould, J. M. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. _Biochemistry_
20, 1534–1538 (1981). Article CAS PubMed Google Scholar * Pellegrini, D., Onor, M., Degano, I. & Bramanti, E. Development and validation of a novel derivatization method for the
determination of lactate in urine and saliva by liquid chromatography with UV and fluorescence detection. _Talanta_ 130, 280–287 (2014). Article CAS PubMed Google Scholar * Bazzone, A.,
Madej, M. G., Kaback, H. R. & Fendler, K. pH regulation of electrogenic sugar/H+ symport in MFS sugar permeases. _PLoS One_ 11, e0156392 (2016). Article PubMed PubMed Central Google
Scholar * Gendreau, S. et al. A trimeric quaternary structure is conserved in bacterial and human glutamate transporters. _J. Biol. Chem._ 279, 39505–39512 (2004). Article CAS PubMed
Google Scholar * Juers, D. H., Matthews, B. W. & Huber, R. E. LacZ beta-galactosidase: structure and function of an enzyme of historical and molecular biological importance. _Protein
Sci._ 21, 1792–1807 (2012). Article CAS PubMed PubMed Central Google Scholar * Morales, F. C. & Bianconi, M. L. Influence of the oligomeric state of yeast hexokinase isozymes on
inactivation and unfolding by urea. _Biophys. Chem._ 91, 183–190 (2001). Article CAS PubMed Google Scholar * Saliola, M. et al. Intracellular NADPH levels affect the oligomeric state of
the glucose 6-phosphate dehydrogenase. _Eukaryot. Cell_ 11, 1503–1511 (2012). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Gea
Schuurman-Wolters for assistance with the expression and purification of LacY, Ineke van’t Land-Kuper for the preparation of _E. coli_ polar lipids extract, and Marc C. A. Stuart for
providing assistance with the cryo-TEM analysis of vesicles. The research was funded by the NWO Gravitation program “Building a synthetic cell” (BaSyC) and by the European Union’s Horizon
2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no.: 860954. AUTHOR INFORMATION Author notes * These authors contributed equally: Miyer F. Patiño-Ruiz,
Zaid Ramdhan Anshari. AUTHORS AND AFFILIATIONS * Department of Biochemistry, Groningen Biomolecular Sciences and Biotechnology Institute, University of Groningen, Nijenborgh 4, 9747 AG,
Groningen, The Netherlands Miyer F. Patiño-Ruiz, Zaid Ramdhan Anshari, Bauke Gaastra, Dirk J. Slotboom & Bert Poolman Authors * Miyer F. Patiño-Ruiz View author publications You can also
search for this author inPubMed Google Scholar * Zaid Ramdhan Anshari View author publications You can also search for this author inPubMed Google Scholar * Bauke Gaastra View author
publications You can also search for this author inPubMed Google Scholar * Dirk J. Slotboom View author publications You can also search for this author inPubMed Google Scholar * Bert
Poolman View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.P.R. performed the SSM measurements, analysis of enzymatic decarboxylation
reaction in solution, pH, and membrane potential determination in the vesicles. M.P.R. and Z.R.A. performed the uptake of radiolabeled substrates and fluorescence measurements. B.G. cloned
and expressed the _mleS_ and _mleP_ genes and performed the radiolabel transport experiments with MleP. M.P.R., Z.R.A., B.G., D.J.S., and B.P. designed the research and analyzed the data.
M.P.R., Z.R.A., D.J.S., and B.P. wrote the paper. D.J.S. and B.P. supervised the research. CORRESPONDING AUTHOR Correspondence to Bert Poolman. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Kwanwoo Shin and the other, anonymous, reviewers for their contribution to the peer
review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SUPPLEMENTARY INFORMATION SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed
material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are
included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Patiño-Ruiz, M.F., Anshari, Z.R., Gaastra, B. _et al._
Chemiosmotic nutrient transport in synthetic cells powered by electrogenic antiport coupled to decarboxylation. _Nat Commun_ 15, 7976 (2024). https://doi.org/10.1038/s41467-024-52085-z
Download citation * Received: 17 April 2024 * Accepted: 27 August 2024 * Published: 12 September 2024 * DOI: https://doi.org/10.1038/s41467-024-52085-z SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative