Play all audios:
ABSTRACT Eukaryotic Argonaute proteins (eAgos) utilize short nucleic acid guides to target complementary sequences for RNA silencing, while prokaryotic Agos (pAgos) provide immunity against
invading plasmids or bacteriophages. The Sir2-domain associated short pAgo (SPARSA) immune system defends against invaders by depleting NAD+ and triggering cell death. However, the molecular
mechanism underlying SPARSA activation remains unknown. Here, we present cryo-EM structures of inactive monomeric, active tetrameric and active NAD+-bound tetrameric SPARSA complexes,
elucidating mechanisms underlying SPARSA assembly, guide RNA preference, target ssDNA-triggered SPARSA tetramerization, and tetrameric-dependent NADase activation. Short pAgos form
heterodimers with Sir2-APAZ, favoring short guide RNA with a 5′-AU from ColE-like plasmids. RNA-guided recognition of the target ssDNA triggers SPARSA tetramerization via pAgo- and
Sir2-mediated interactions. The resulting tetrameric Sir2 rearrangement aligns catalytic residue H186 for NAD+ hydrolysis. These insights advance our understanding of Sir2-domain associated
pAgos immune systems and should facilitate the development of a short pAgo-associated biotechnological toolbox. SIMILAR CONTENT BEING VIEWED BY OTHERS TARGET SSDNA ACTIVATES THE NADASE
ACTIVITY OF PROKARYOTIC SPARTA IMMUNE SYSTEM Article 06 November 2023 NUCLEIC ACID MEDIATED ACTIVATION OF A SHORT PROKARYOTIC ARGONAUTE IMMUNE SYSTEM Article Open access 06 June 2024
STRUCTURAL INSIGHTS INTO MECHANISMS OF ARGONAUTE PROTEIN-ASSOCIATED NADASE ACTIVATION IN BACTERIAL IMMUNITY Article Open access 13 June 2023 INTRODUCTION Argonaute proteins are ubiquitous
across all domains of life1,2,3. Eukaryotic Argonaute proteins (eAgos) use small oligonucleotides as guides to recognize complementary target RNAs, thus participating in gene silencing
pathways or defending against invading viruses4,5. Prokaryotic Ago proteins (pAgos) function as prokaryotic immune systems against invading plasmids and viruses2,3,6,7,8,9,10. pAgos are
phylogenetically classified into three major clades (long-A, long-B, and short pAgos)2,6. eAgos and long pAgos typically share a conserved bilobed architecture, with the N-terminal lobe
comprising the N and PAZ (PIWI-Argonaute-Zwille) domains and the C-terminal lobe containing an RNase H-like fold P-element-induced wimpy testis (PIWI) domain and a Rossmann-like fold MID
(Middle) domain3. The PAZ and MID domains in eAgos bind to guide RNAs (gRNAs) at their 3′ and 5′ ends, respectively, while the PIWI domain is responsible for cleaving the target RNA3.
However, short pAgos, constituting over 60% of all pAgos, contain only MID and catalytically inactive PIWI domains6. Short pAgos typically associate with APAZ (analog of PAZ)-containing
proteins fused with nuclease and NADase family members, such as Mrr, Toll/interleukin-1 receptor (TIR), and Silent information regulator 2 (Sir2)2,3,6,7, which compensate for inactive PIWI
endonuclease activity and defend against the invading bacteriophages and plasmids. Sir2 proteins are NAD+-dependent deacetylases found in organisms ranging from bacteria to eukaryotes11 that
function in various biological processes. Sir2 proteins typically catalyze protein deacetylation or ADP-ribosylation in monomeric form using NAD+ as a cofactor11,12,13. Recent bioinformatic
analyses revealed that Sir2-domain-containing proteins function as NADases in bacterial anti-phage systems, such as Thoeris14, DSR (defense-associated sirtuins)15 and short pAgo6,7,16,17
defense systems, directly depleting NAD+ to trigger cell death. Approximately, half of the short pAgos identified to date are associated with Sir2 domains6. The short pAgo/Sir2-APAZ (SPARSA)
system from _Geobacter sulfurreducens_ depletes NAD+ via its Sir2 domain upon RNA-guided detection of invading bacteriophage or plasmid DNA in vivo17. However, the molecular mechanism
underlying the activation of SPARSA NADase activity remains unknown. Here, we present cryo-EM structures of _G. sulfurreducens_ SPARSA in its inactive monomeric and active tetrameric states.
Structural analysis, combined with in vitro biochemical and in vivo plasmid targeting analyses, revealed that SPARSA preferentially targets invading ColE1-like plasmids. This targeting
triggers the tetramerization of SPARSA and the subsequent tetramerization-dependent activation of Sir2 NADase activity, resulting in depletion of the essential coenzyme NAD+ and the
initiation of cell death or dormancy of the infected cells. RESULTS TARGET SSDNA TRIGGERS OLIGOMERIZATION-DEPENDENT ACTIVATION OF SPARSA NADASE ACTIVITY The _G. sulfurreducens_ SPARSA system
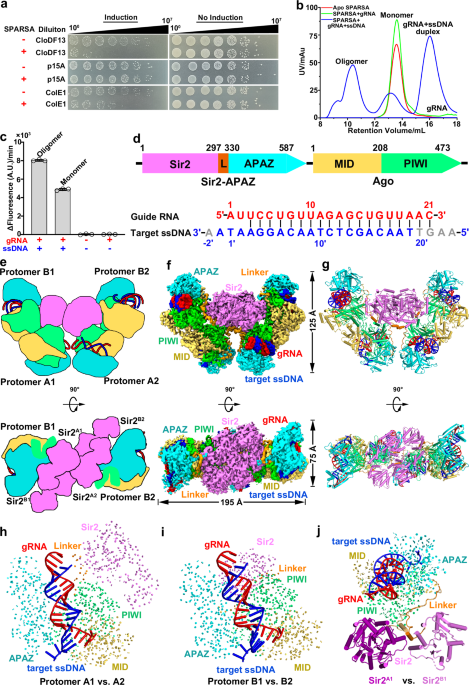
was previously shown to interfere with invading plasmids in vivo17. Consistent with this observation, our in vivo analysis revealed the SPARSA system interferes with the transformation of
plasmids specifically containing ColE1-like origins of replication (ori), including CloDF13 and ColE1 ori regions (Fig. 1a). To explore the underlying molecular mechanisms, we co-expressed
_G. sulfurreducens_ pAgo and Sir2–APAZ proteins in _Escherichia coli_ and purified them from the cell lysate. As observed in previously17, pAgo and Sir2–APAZ formed a stable heterodimeric
complex, termed the monomeric SPARSA complex (Supplementary Fig. 1a). A gRNA and a complementary ssDNA target to the purified monomeric SPARSA complex resulted in detection of an additional
peak during size exclusion chromatography (SEC). This peak was absent from apo SPARSA or in the presence of gRNA alone, indicating that binding to target ssDNA triggers the oligomerization
of the SPARSA complex (Fig. 1b). Notably, the oligomeric SPARSAgRNA–target ssDNA complex showed pronounced NADase activity, whereas moderate NADase activity was observed for the monomeric
SPARSAgRNA–target ssDNA complex (Fig. 1c). The activity detected from the monomeric SPARSAgRNA–target ssDNA complex might have been due to the conversion of this complex into an oligomeric
state during its prolonged incubation with ε-NAD+ at 37 °C, as transformation from a monomeric to oligomeric form was observed during incubation at 37 °C (Supplementary Fig. 1b). We did not
observe any detectable NADase activity for monomeric SPARSA, even in the presence of gRNA, demonstrating the essential role of target ssDNA in the activation of SPARSA NADase activity. Taken
together, our findings demonstrate that target ssDNA binding triggers oligomerization of the SPARSAgRNA complex, leading to the activation of NADase activity. THE ACTIVE SPARSAGRNA–SSDNA
COMPLEX ASSEMBLES INTO A TETRAMERIC STRUCTURE To gain structural insights into the dependence of SPARSA activation on its oligomeric state, we performed cryo-EM studies of the oligomeric
SPARSAgRNA–ssDNA complex. 3D classification revealed the presence of two distinct states within this sample: monomeric and tetrameric SPARSAgRNA-ssDNA complexes at a resolution of 2.6 Å and
3.4 Å, respectively (Fig. 1d–g, Supplementary Figs. 2, 3 and Table 1). The tetrameric SPARSA complex adopts bowl-shaped architecture comprising a ‘dimer of dimers,’ with each protomer bound
to a gRNA–target ssDNA duplex. The tetramer is approximately 195 Å long, 75 Å wide and 125 Å in height, respectively (Fig. 1f). Protomer A1 or A2 interact with protomer B1 or B2,
respectively, to form a dimer unit with a buried interface of ~1100 Å2, which is facilitated by pAgo– and Sir2–mediated interactions. Subsequently, two of these dimer units come together to
form a tetramer via Sir2-mediated interactions, creating a total buried interface of ~1980 Å2. Therefore, active SPARSA assembles into a tetrameric structure comprising a ‘dimer of dimers.’
THE SIR2 NADASE DOMAIN IS REARRANGED UPON SPARSA TETRAMERIZATION The monomeric SPARSAgRNA-ssDNA complex, as characterized by 3D classification, adopts a bilobed architecture with
gRNA–targeted ssDNA bound in the positively charged channel between lobes (Supplementary Fig. 4). One lobe contains a conserved pAgo subunit with MID and inactive PIWI domains, while the
other consists of APAZ and Sir2 domains; density of the Sir2 domain was not well defined, pointing to the flexibility of the Sir2 domain upon target ssDNA binding (Supplementary Fig. 4).
Structural comparisons among the four protomers revealed similarities between protomers A1 and A2, and between protomers B1 and B2 (Fig. 1h, i and Supplementary Fig. 5), while protomers A1
and B1, as well as protomers A2 and B2, show distinct conformations of Sir2 and its associated linker domains within SPARSA (Fig. 1j). These findings indicate that the Sir2 domains are
rearranged during SPARSA tetramerization upon target ssDNA binding. As we failed to observe the Sir2 domain of the monomeric SPARSAgRNA–ssDNA complex during 3D classification, we sought to
gain overall insights into the SPARSA complex by analyzing the composition of protomer A in the tetrameric SPARSAgRNA–ssDNA complex. The structure of protomer A generally resembles _Thermus
thermophilus_ Ago (PDB 4NCA, RMSD 3.33 Å over 484 residues), which belongs to the long-A pAgos family, but with a distinct Sir2 domain, highlighting the conservation of Ago architecture
(Supplementary Fig. 6a). However, structural and sequence alignment revealed that SPARSA lacks the four conserved catalytic acidic residues (DEDX) responsible for target DNA cleavage within
the PIWI domain (Supplementary Fig. 6b)18, indicating that SPARSA lacks the ability to cleave target ssDNA. Further structural comparisons revealed that the Sir2 domain of SPARSA comprises a
helical module and a Rossmann fold module (Supplementary Fig. 6c). Both the overall structure and its catalytic sites resemble the Sir2 domain within ThsA (PDB 6LHX, RMSD of 2.63 Å over 200
residues)19, which directly hydrolyzes NAD+ (Supplementary Fig. 6d), more than it resembles eukaryotic Sir2 protein yHst2 (PDB 1SZC, RMSD of 2.84 Å over 201 residues)20, which requires both
an acetyl-lysine–containing peptide substrate and an NAD+ cofactor for protein deacetylation (Supplementary Fig. 6e). Notably, the channel for binding peptide substrates is blocked by the
presence of α10 in Sir2 (Supplementary Fig. 6e), suggesting that the Sir2 domain of SPARSA cannot function as an NAD+-dependent deacetylase. Collectively, these results indicate that the
short pAgo of SPARSA lacks target DNA cleavage ability but is fused with an additional Sir2 domain that functions as an NAD+ hydrolase rather than an NAD+-dependent deacetylase. SPARSA
PREFERENTIALLY BINDS TO GUIDE RNAS CONTAINING 5′-AU DINUCLEOTIDES In our determined tetrameric SPARSAgRNA-ssDNA complex, a 21-nt gRNA and a traceable 20-nt segment of the 25-nt complementary
ssDNA target interact extensively with the SPARSA complex, excluding the Sir2 domain (Fig. 2a, b and Supplementary Fig. 3b). The nucleotide 5′-A1 is specifically recognized by residues D164
and F158, whereas the nucleotide 5′-U2 is specifically recognized by R190, highlighting a preference for 5′-AU dinucleotides in the gRNA (Fig. 2a, b, black inset and Supplementary Fig. 3b).
The 5′-phosphate (5′-P) group of the gRNA is stabilized by residues I182MID and H166MID, along with a divalent cation in the MID domain (Fig. 2b, black inset and Supplementary Fig. 3b). The
divalent cation is stabilized by residues N434PIWI and M473PIWI, effectively neutralizing the negative charge of the 5′-P. The 2′-OH groups of nucleotides at positions 4, 12, and 14 in the
gRNA are specifically recognized by residues K396PIWI, Y240PIWI, and H310PIWI through hydrogen-bond interactions (Fig. 2a and Supplementary Fig. 3b), indicating the preference for RNA over
ssDNA as a guide. These structural insights provide a clear explanation for SPARSA′s preference for small RNAs with 5′-AU dinucleotides, especially those containing 5′-P in vivo17. A PAGO
SENSOR LOOP DETECTS SSDNA BINDING AND INITIATES SPARSA TETRAMERIZATION Upon binding of RNA-guided SPARSA to ssDNA, the first four nucleotides (A-2′, A-1′, T1′ and A2′) at the 3′ end of the
target ssDNA splay outwards and are stabilized by hydrophobic interactions (Y530APAZ, F532APAZ and Y359PIWI) and a hydrogen-bond interaction via S358PIWI in the MID, PIWI, and APAZ domains
(Fig. 2a). To investigate how SPARSA senses gRNA–target ssDNA duplex binding for its activation, we performed cryo-EM analysis of the apo SPARSA complex (Supplementary Fig. 7a–f). While the
low resolution of the map did not allow us to perform manual tracing of each residue, fitting the SPARSA structure predicted by AlphaFold221 into this map revealed a loop region in the PIWI
domain clashing with nucleotides G13 and C14 of the gRNA–target ssDNA duplex (Supplementary Fig. 7g, h). Upon gRNA–target ssDNA duplex binding, this loop undergoes substantial conformational
changes, directly interacting with nucleotides G13 and C14 of the gRNA–target ssDNA duplex (Fig. 2b, red inset). Mutating either the entire sensor loop (D261–S276) into a GGS linker (Δloop)
or residues N272 and Y274 on its tip into alanine (N272A, Y274A double mutant) significantly reduced the NADase activity of SPARSA (Fig. 2c). Consistent with this, the Δloop and the N272A,
Y274A double mutant exhibited reduced ability to interfere with plasmid transformation in an in vivo functional assay (Fig. 2d). These findings demonstrate the essential role of this loop in
sensing the gRNA–target ssDNA duplex for SPARSA activation. TETRAMERIZATION IS ESSENTIAL FOR ACTIVATING THE NADASE ACTIVITY OF SPARSA In the tetrameric SPARSA complex, protomers A1 and B1,
as well as protomers A2 and B2, form a dimer unit through pAgo-to-pAgo and Sir2-to-Sir2 interfaces (Fig. 3a). This pAgo-to-pAgo interface is maintained through hydrogen-bond interactions
involving residues H104, P175 and T253 from the adjacent pAgo subunits (Fig. 3a, black inset). The Sir2-to-Sir2 interfaces exhibit a head-to-tail arrangement formed by several
hydrogen-bonding interactions in Sir2A1 and Sir2B1, which we refer to as the Sir2A1-to-Sir2B1 interface (Fig. 3a, green inset). Mutating residues in either interface (H104 in the
pAgo-to-pAgo interface and N7, E8, or R296-Q297 in the Sir2A1-to-Sir2B1 and Sir2A2-to-Sir2B2 interfaces) to alanine significantly reduced the NADase activity of SPARSA (Fig. 3b),
highlighting the importance of these interfaces in the dimer units of SPARSA for SPARSA activation. Two SPARSA dimer units further assemble into a tetramer in back-to-back configuration
though their Sir2A1-to-Sir2A2, Sir2A1-to-Sir2B2, and Sir2A2-to-Sir2B1 interfaces (Fig. 3c). The Sir2A1-to-Sir2A2 interface is stabilized by six pairs of hydrogen-bond interactions (D153A1,A2
and G156A2,A1; H158A1,A2 and L159A2,A1; Y168A1,A2 and Q192A2,A1), resulting in a buried interface of ~1240 Å2. The Sir2A2-to-Sir2B1 interface, with a buried interface of ~750 Å2, is
stabilized by three hydrogen-bonding interactions involving residues N175, K210, and N245 in Sir2A2, along with residues H249, N244, and N175 in Sir2B1. Mutating the residues involved in
Sir2A1-to-Sir2A2 (H158 or Y168) or Sir2A1-to-Sir2B2 and Sir2A2-to-Sir2B1 (N244) interactions into alanine significantly reduced the NADase activity of SPARSA (Fig. 3b), highlighting their
critical role in SPARSA activation. Notably, both the R296A, Q297A double mutations in the Sir2A1-to-Sir2B1 and Sir2A2-to-Sir2B2 interfaces and the Y168A mutation in the Sir2A1-to-Sir2A2
interface disrupted the assembly of SPARSA tetramer, resulting in smaller complexes compared with wild-type SPARSA (Fig. 3d). Collectively, these findings highlight the crucial role of the
pAgo-to-pAgo and Sir2-to-Sir2 interfaces in SPARSA tetramerization and demonstrate the indispensable role of SPARSA tetramerization in activating its NADase activity. SPARSA TETRAMERIZATION
ALIGNS THE CATALYTIC RESIDUES OF SIR2 FOR NAD+ HYDROLYSIS To elucidate the molecular basis of SPARSA’s tetramerization-dependent NAD+ hydrolysis activity, we incubated the substrate (1 mM
NAD+) with a catalytically inactive SPARSA(N142A)gRNA–ssDNA complex on ice for 5 min, followed by cryo-EM sample preparation to capture the NAD+ bound structure (Supplementary Fig. 8a). We
then determined the 3.4-Å cryo-EM structure of the SPARSAgRNA–ssDNA–NAD+ complex (Fig. 4a and Supplementary Fig. 8b–d), which resembles the structure of the tetrameric SPARSAgRNA–ssDNA
complex (RMSD 0.35 Å). A previous study showed that SPARSA binds to endogenous NAD+ at a ~1:1 ratio17, however, we observed NAD+ densities only in the cleft between the helical and Rossmann
fold modules in the Sir2 domain of protomers A1 and A2 (Fig. 4a, b), while the flexibility of the helical module of Sir2 likely results in the absence of NAD+ densities in protomers B1 and
B2. The helical modules of protomers A1 and A2 are stabilized by protomers B1 and B2, whereas the helical modules of protomers B1 and B2 are exposed to the solvent, suggesting their
flexibility. Notably, only the adenosine monophosphate (AMP) moiety of NAD+ could be confidently modeled, suggesting the flexibility of the nicotinamide ribose of NAD+ (Fig. 4b). This aligns
with previous reports, suggesting the conformational flexibility of nicotinamide ribose groups in the NAD+ catalytic pocket13,22 (Supplementary Fig. 8e). NAD+ adopts the active
configuration only in the presence of the acetyl-lysine-containing peptide substrate13. However, the channel for binding such peptide substrates is blocked by α10, located exactly in the
position of the substrate peptide, potentially compensating for the absence of the substrate peptide (Fig. 4c and Supplementary Fig. 6e). Superimposing the structure of Sir2 domain in SPARSA
with that of the yHst2-NAD+ complex suggested that the NAD+ is positioned for binding in an active conformation (Fig. 4c). In this binding position, the 3′-OH of the adenine ribose and the
phosphate group are stabilized by direct hydrogen bonds through residues S227 and R229, respectively (Fig. 4c, red inset). Mutating of either S227 or R229 into alanine significantly
decreased the NADase activity of SPARSA (Fig. 4d). A cofactor binding loop (residues G25–A37) critical for NAD+ binding is involved in forming a binding pocket (C pocket) to accommodate the
nicotinamide moiety and enable subsequent nucleophilic attack (Fig. 4c, inset)13. The A26L or A37F mutations nearly abolished the NADase activity of SPARSA (Fig. 4d), highlighting the
importance of this cofactor binding loop. The catalytic residues N142 and H186, which are crucial for NAD+ catalysis (Fig. 4d and Supplementary Fig. 8a), are essentially superimposed with
the corresponding N116 and H135 residues in active yHst2 (Fig. 4c, inset), suggesting that these catalytic residues are well positioned for catalysis. To investigate why the NADase activity
of SPARSA requires Sir2 tetramerization, whereas the Sir2 NAD+-dependent deacetylase can function in monomeric form, we superimposed the active tetrameric SPARSAgRNA–ssDNA–NAD+ complex onto
the inactive monomeric counterpart (PDB 8JL0, RMSD of 1.18 Å over 216 residues)23 (Fig. 4e). Despite the similarity of overall architecture, this comparison revealed substantial
conformational changes, with up to 9 Å shift in the α10 helix and a loop carrying catalytic H186, termed the catalytic loop. These changes precisely positioned H186, aligning it with the
catalytic residues N142 for catalysis (Fig. 4c, inset). The dramatic alterations in the α10 helix and catalytic loop arise from tetrameric interactions between adjacent Sir2 domains,
particularly involving the catalytic loop (Q192A2) and the α9 helix (Y168A1) in the Sir2A1-to-Sir2A2 interface (Fig. 4e, inset). The Y168A mutation disrupted tetramer assembly, resulting in
the loss of NADase activity (Fig. 3b, d). Notably, the α9A1, α10A2 helix, and catalytic loop that are critical for tetramerization and NAD+ hydrolysis in SPARSA are absent from the Sir2
NAD+-dependent deacetylase yHst2, which functions as a monomer (Fig. 4c and Supplementary Fig. 6e), suggesting that the tetramerization of the Sir2 domain in SPARSA is essential for
optimally positioning the catalytic resides for subsequent catalysis. Taken together, our results indicate that tetramerization of Sir2 domain in SPARSA activates its NADase activity by
inducing conformational changes of the catalytic loop and aligning the catalytic residues for NAD+ hydrolysis. DISCUSSION Short pAgos, linked to TIR, Sir2 NADase domains and Mrr nuclease
domains, confer bacterial immunity, with approximately half associated with Sir2 domains2,6. Here, we investigated the structure and function of the bacterial short pAgo/Sir2-APAZ (SPARSA)
immune complex in both its inactive and active forms, revealing the molecular mechanisms underlying target ssDNA-triggered SPARSA tetramerization and tetramerization-dependent NADase
catalytic activation. Sir2 proteins typically catalyze protein deacetylation, relying on both their substrate peptide and the cofactor NAD+, and function in a monomeric state24,25. However,
our results reveal that the monomeric apo SPARSA and SPARSAgRNA complexes lack NADase activity, whereas the tetrameric SPARSAgRNA–ssDNA complex possesses this activity (Fig. 1c).
Additionally, in contrast to TIR-domain-associated pAgo (SPARTA) complex, whose TIR-APAZ subunit contains an inhibitory peptide responsible for repressing its NADase activity in the absence
of target ssDNA26,27,28,29,30,31, the Sir2-APAZ of SPARSA lacks the inhibitory C-terminal peptide (Supplementary Fig. 9a). Two recent studies revealed structures of monomeric SPARSA
structures and assumed that the monomeric form represents the active state23,32. However, our structural and biochemical data demonstrate that tetrameric SPARSA is in its active form. These
different conclusions might be due to different conditions used in the experiments. In the previous studies, the complex were prepared at 4 °C but its NADase activity was tested at 37 °C,
and a less favorable long 42-nt gRNA was used; in the current study, we prepared the complex at 37 °C and used the in vivo preferred short 21-nt gRNA17. We demonstrated that the
SPARSAgRNA–ssDNA complex remains monomeric and is NADase-inactive at 4 °C, while conditions of 37 °C lead to tetramerization, activating the NADase activity of SPARSA (Supplementary Fig.
9b–d). The intact NAD+ in the catalytic pocket in previously determined monomeric SPARSAgRNA–ssDNA complexes also point to the inactive nature of its monomeric states23. Disrupting the
tetrameric interface abolished NADase activity, further demonstrating the essential role of oligomerization in the NADase activity of SPARSA. Recent studies on Sir2-containing proteins,
including ThsA33 and DSR234,35, revealed the essential role of Sir2 oligomerization in NADase activation (Supplementary Fig. 9e, f). These findings suggest that the oligomerization of the
Sir2 domain is critical for its NADase activity. SPARTA and SPARSA both belong to the short pAgos defense system and deplete NAD+ to counter the defense16,17. Despite sharing some conserved
characteristics, there are several unique features distinguishing these two systems. One obvious difference is their oligomer pattern: the X-shaped SPARTA system is formed by interactions
mediated its two-fold symmetric Ago proteins26,27,28,29,30,31,32,36, but the Ago proteins in SPARSA adopts a reverse configuration compared to those in SPARTA, resulting in an H-shape
(Supplementary Fig. 9g). Additionally, the TIR domains in SPARTA arrange into a two-stranded, parallel, head-to-tail rearrangement (Supplementary Fig. 9h), whereas the Sir2 domains in SPARSA
adopt a two-stranded, tail-to-tail rearrangement (Fig. 3c). Furthermore, the binding mode of the NAD+ molecules is also different with the effector domains. In SPARTA, the NAD+ binding
sites span across two adjacent TIR domains31 (Supplementary Fig. 9h), while in SPARSA, NAD+ is bound to individual Sir2 domains (Fig. 4c). SPARSA immune systems preferentially target
plasmids with a ColE1-like ori, including CloDF13 and ColE1 ori regions17 (Fig. 1a); they employ two RNAs to initiate replication, generating an R-loop with an ssDNA region during
replication37. ColE1-like plasmids might produce both potential guide RNA and target ssDNA, providing an explanation for why SPARSA prefers to target such plasmids. Based on our structural,
in vitro biochemical, and in vivo plasmid targeting studies, we propose a mechanistic model for the bacterial SPARSA-mediated immune response to invading DNA (Fig. 5). The short pAgo subunit
interacts with the Sir2-APAZ subunit to form an inactive apo SPARSA complex. The short pAgo subunit acquires small RNAs from invading phages or ColE1-like plasmids to form a SPARSAgRNA
binary complex. This complex recognizes and binds to the complementary target ssDNA region within the plasmid ori or phage DNA, triggering conformational changes in the sensor loop of short
pAgo and the release of the Sir2 domain. These conformational changes facilitate the formation of pAgo–pAgo dimer interfaces and the rearrangement of the Sir2 domain. Tetramerization of the
Sir2 domain induces conformational changes in the catalytic loop of Sir2, optimally positioning the catalytic residue H186 for NAD+ hydrolysis into NAM and ADPR, eventually leading to the
death of the infected cells and protecting the entire population from invasion. In summary, our findings provide mechanistic insights into the Sir2-domain-associated pAgo immune system
SPARSA, advancing our understanding of ssDNA targeting by RNA-guided pAgo and its associated tetramerization-dependent activation of Sir2 NADase activity. These findings provide a molecular
basis for developing SPARSA-based biotechnological tools in the future. METHODS BACTERIAL STRAINS _Escherichia coli_ DH5α, BL21-CodonPlus (DE3) RIL, and BL21-AI cells were used for plasmid
reconstruction, protein expression and in vivo plasmid interference assay, respectively. PROTEIN EXPRESSION AND PURIFICATION The full-length _Escherichia coli_ codon-optimized genes of
_Geobacter sulfurreducens_ SPARSA system were synthesized by Sangon Biotech (Shanghai). _sir2-APAZ_ and _ago_ were subcloned into the pRSFDuet-1 vector (Novagen), in which a N-terminal
hexahistidine tag was fused with _sir2-APAZ_. All constructs were verified by Sanger-sequencing and expressed in _E. coli_ BL21-CodonPlus (DE3) RIL strains. For cultivation, a single colony
was selected and grown overnight in LB media supplemented with 30 μg/mL kanamycin, and 34 μg/mL chloramphenicol. Then the culture was diluted in fresh LB media and cultured at 37 °C until
the OD600 reached 0.6. Proteins expression was then induced with 0.5 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) and cultured at 16 °C for another 20 h. Cells were harvested and
resuspended in a lysis buffer containing 20 mM Tris-HCl, 500 mM NaCl, 20 mM imidazole, 1 mM DTT, pH 7.5. Then the cell mixture was sonicated on ice for 30 min, followed by centrifugation at
32,914 × _g_ for 30 min to collect the supernatant. This was then applied to 5 mL HisTrap Fast flow column (Cytiva Life Sciences), incubated at 4 °C for 1 h. The column was washed with 10
column volumes (CV) of lysis buffer, and the protein was eluted using 20 mL of elution buffer (20 mM Tris-HCl, 500 mM NaCl, 300 mM imidazole, pH 7.5). The elution was then diluted in heparin
binding buffer (20 mM Tris-HCl, 100 mM NaCl) and loaded onto a 5 mL HiTrap Heparin column (Cytiva Life Sciences) pre-equilibrated with the same buffer. The proteins were eluted by a linear
NaCl gradient ranging from 100 mM to 1 M over 20 CVs. The fractions collected were concentrated using 30 kDa molecular mass cut-off concentrators (Merck Millipore, UFC903024) and further
purified using Superdex 200 Increase 10/300 GL column (Cytiva Life Sciences) pre-equilibrated with running buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM DTT, 2 mM MgCl2, pH 7.5). The pooled
fractions were then concentrated, flash frozen in liquid nitrogen, and stored at −80 °C for further use. All mutants were generated through site-directed mutagenesis and purified by the same
method as described above. IN VITRO ASSEMBLY OF SPARSAGRNA AND SPARSAGRNA-SSDNA COMPLEXES To assemble SPARSAgRNA complex, the purified SPARSA complex was mixed with 5′-P guide RNA
(5′P-AUUCCUGUUAGAGCUGUUAAC-3′) in a molar ration of 1:1.2. The mixture was then incubated on ice for 30 min and loaded onto a Superdex 200 Increase 10/300 GL column (Cytiva Life Sciences)
pre-equilibrated with running buffer. Fractions containing the SPARSAgRNA complex were pooled, flash frozen in liquid nitrogen, and stored at −80 °C. For the assembly of SPARSAgRNA-ssDNA
complex, the purified SPARSA complex was mixed with 5′-P guide RNA at a molar ration of 1:1.2 and incubated at 37 °C for 15 min, followed by the addition of target ssDNA
(5′-AAGTTAACAGCTCTAACAGGAATAA-3′) to the mixture in a final molar ration of 1:1.2:1.2 (SPARSA: gRNA: ssDNA). The entire mixture was then incubated at 37 °C for another 30 min before
undergoing further purification over a Superdex 200 increase 10/300 GL column (Cytiva Life Sciences). Fractions containing the SPARSAgRNA-ssDNA oligomer were pooled, flash frozen in liquid
nitrogen, and stored at −80 °C. CRYO-EM SAMPLE PREPARATION AND DATA ACQUISITION 3.0 μL of a ~ 2 mg/mL solution of purified SPARSA, SPARSAgRNA or SPARSAgRNA-ssDNA complex was applied onto
glow-discharged UltrAuFoil 300 mesh R1.2/1.3 grids (Quantifoil), respectively. The grids were blotted for 2 s at 100% humidity and flash frozen in liquid ethane using a FEI Vitrobot Mark IV
(FEI). Images were collected on a Titan Krios electron microscope (FEI) operated at an acceleration voltage of 300 kV, equipped with a Gatan K3 Summit detector with a physical pixel size of
1.1 Å. The defocus during image collection ranged from −1.0 µm to −2.5 µm. Images were dose-frasctionated into 32 frames with a total accumulated dose of 50 electrons per Å2. CRYO-EM DATA
PROCESSING Image processing was conducted by RELION 3.138 and cryoSPARC v3.139. Motion correction was performed with MotionCor240. Contrast transfer function (CTF) parameters were estimated
by Ctffind441. Auto-picked particles using the Laplacian-of-Gaussian method were extracted and underwent two rounds of 2D classification to remove junk particles and several rounds of
heterogeneous refinement in cryoSPARC v3.1, utilizing an initial model generated within cryoSPARC v3.1 as a reference. Particles corresponding to the best class with the highest-resolution
features were selected and subjected to non-uniform refinement in cryoSPARC v3.1. These particles then underwent CTF refinement followed by another round of non-uniform refinement,
generating the final reconstitutions. All resolutions were estimated by applying a soft mask around the protein density and the Fourier shell correlation (FSC) = 0.143 criterion in cryoSPARC
v3.1. Local resolution estimates were calculated from two half data maps in cryoSPARC v3.1. Further details related to data processing and refinement are summarized in Supplementary Table
1. ATOMIC MODEL BUILDING AND REFINEMENT All atomic models were built de novo according to the bulky residues and PSIPRED secondary prediction42, then refined by COOT v0.9.543 interactively.
All models were then refined against combined maps using Phenix.real_space_refine v1.19.244 by applying geometric and secondary structure restraints. All structural figures were prepared in
Pymol v2.4.2 (http://www.pymol.org) and Chimera X45. IN VITRO NADASE ASSAY NADase assays were carried out in a final volume of 50 μL, containing 10 mM MES, pH 6.5, 75 mM NaCl, 2 mM MgCl2,
and 50 μM ε-NAD+ (Sigma Aldrich, N2630). Typical reactions involved 1 μM of the purified SPARSA complex or its variants, 1.5 μM RNA guide, and 1.5 μM DNA target. To evaluate the enzymatic
activity in different states of aggregation, 1 μM of either the purified SPARSA, SPARSAgRNA or SPARSAgRNA-ssDNA complex in monomeric and oligomeric states were used. The reaction buffer
containing 10 mM MES, pH 6.5, 75 mM NaCl, 2 mM MgCl2, and 50 μM ε-NAD+ and reactions were performed at 37 °C for 5 min. Absorbance was measured using 96-well microplates (Corning, 3690) at
an excitation wavelength of 300 nm and an emission wavelength of 410 nm, utilizing Synergy H1 multimode reader (Biotek). CELL VIABILITY ANALYSIS ASSAY _E. coli_ BL21-AI strain cells were
heat-shock transformed with pRSFDuet-1 plasmid encoding either SPARSA or its variants, along with pCDFDuet-1 (ori: CloDF13), pACYCDuet-1 (ori: p15A) or pUC19 (ori: ColE1) plasmids to
evaluate the effect of different ori regions). The transformed cells were grown in liquid LB media supplemented with 2% glucose and corresponding antibiotics (30 μg/mL kanamycin, 50 μg/mL
streptomycin, 50 μg/mL ampicillin, or 34 μg/mL chloramphenicol) at 37 °C overnight. Subsequently, the culture was diluted 1:100 in fresh LB media supplemented with 2% glucose and
corresponding antibiotics and cultured at 37 °C until the OD600 reached 0.6. Then the cells were collected and resuspended in fresh LB media without glucose. Resuspended cells were serially
diluted, and aliquots were spotted on LB plates containing antibiotics and inducer (0.4% L-Arabinose and 0.25 mM IPTG) or glucose (2%). REPORTING SUMMARY Further information on research
design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY The data supporting the findings of this study are available from the corresponding
authors upon request. The cryo-EM density maps have been deposited in the Electron Microscopy Data Bank (EMDB) under accession number EMD-39027 (monomeric SPARSAgRNA–target ssDNA complex);
EMD-39028 (tetrameric SPARSAgRNA–target ssDNA complex); EMD-39030 (tetrameric SPARSA(N142A)gRNA–target ssDNA bound to NAD+ complex) and EMD-39031 (apo SPARSA complex). The atomic coordinates
have been deposited in the Protein Data Bank (PDB) with accession number 8Y7Z (monomeric SPARSAgRNA–target ssDNA complex); 8Y80 (tetrameric SPARSAgRNA–target ssDNA complex) and 8Y82
(tetrameric SPARSA(N142A)gRNA–target ssDNA bound to NAD+ complex). Source data are provided with this paper. REFERENCES * Bobadilla Ugarte, P., Barendse, P. & Swarts, D. C. Argonaute
proteins confer immunity in all domains of life. _Curr. Opin. Microbiol._ 74, 102313 (2023). Article CAS PubMed Google Scholar * Koopal, B., Mutte, S. K., Swarts, D. C. A long look at
short prokaryotic Argonautes. _Trends Cell Biol_. 33, 605–618 (2023). * Swarts, D. C. et al. The evolutionary journey of Argonaute proteins. _Nat. Struct. Mol. Biol._ 21, 743–753 (2014).
Article CAS PubMed PubMed Central Google Scholar * Kuhn, C. D. & Joshua-Tor, L. Eukaryotic Argonautes come into focus. _Trends Biochem. Sci._ 38, 263–271 (2013). Article CAS
PubMed Google Scholar * Sheu-Gruttadauria, J. & MacRae, I. J. Structural foundations of RNA silencing by Argonaute. _J. Mol. Biol._ 429, 2619–2639 (2017). Article CAS PubMed PubMed
Central Google Scholar * Ryazansky, S., Kulbachinskiy, A. & Aravin, A. A. The expanded universe of prokaryotic Argonaute proteins. _mBio_ 9, e01935–18 (2018). Article PubMed PubMed
Central Google Scholar * Makarova, K. S., Wolf, Y. I., van der Oost, J. & Koonin, E. V. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a
novel system of defense against mobile genetic elements. _Biol. Direct_ 4, 29 (2009). Article PubMed PubMed Central Google Scholar * Zeng, Z. F. et al. A short prokaryotic Argonaute
activates membrane effector to confer antiviral defense. _Cell Host Microbe_ 30, 930–943 (2022). Article CAS PubMed Google Scholar * Chen, Y., Zeng, Z. F., She, Q. X. & Han, W. Y.
The abortive infection functions of CRISPR-Cas and Argonaute. _Trends Microbiol._ 31, 405–418 (2023). Article CAS PubMed Google Scholar * Song, X. M. et al. Catalytically inactive long
prokaryotic Argonaute systems employ distinct effectors to confer immunity via abortive infection. _Nat. Commun._ 14, 6970 (2023). Article ADS CAS PubMed PubMed Central Google Scholar
* North, B. J. & Verdin, E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. _Genome Biol._ 5, 224 (2004). Article PubMed PubMed Central Google Scholar * Gallego-Jara, J.
et al. Bacterial sirtuins overview: an open niche to explore. _Front Microbiol._ 12, 744416 (2021). Article PubMed PubMed Central Google Scholar * Yuan, H. & Marmorstein, R.
Structural basis for sirtuin activity and inhibition. _J. Biol. Chem._ 287, 42428–42435 (2012). Article CAS PubMed PubMed Central Google Scholar * Ofir, G. et al. Antiviral activity of
bacterial TIR domains via immune signalling molecules. _Nature_ 600, 116–120 (2021). Article ADS CAS PubMed Google Scholar * Garb, J. et al. Multiple phage resistance systems inhibit
infection via SIR2-dependent NAD+ depletion. _Nat. Microbiol._ 7, 1849–1856 (2022). Article CAS PubMed Google Scholar * Koopal, B. et al. Short prokaryotic Argonaute systems trigger cell
death upon detection of invading DNA. _Cell_ 185, 1471–1486.e1419 (2022). Article CAS PubMed PubMed Central Google Scholar * Zaremba, M. et al. Short prokaryotic Argonautes provide
defence against incoming mobile genetic elements through NAD+ depletion. _Nat. Microbiol._ 7, 1857–1869 (2022). Article CAS PubMed Google Scholar * Sheng, G. et al. Structure-based
cleavage mechanism of Thermus thermophilus Argonaute DNA guide strand-mediated DNA target cleavage. _Proc. Natl. Acad. Sci. USA_ 111, 652–657 (2014). Article ADS CAS PubMed Google
Scholar * Ka, D., Oh, H., Park, E., Kim, J. H. & Bae, E. Structural and functional evidence of bacterial antiphage protection by Thoeris defense system via NAD(+) degradation. _Nat.
Commun._ 11, 2816 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Zhao, K., Harshaw, R., Chai, X. & Marmorstein, R. Structural basis for nicotinamide cleavage and
ADP-ribose transfer by NAD(+)-dependent Sir2 histone/protein deacetylases. _Proc. Natl. Acad. Sci. USA_ 101, 8563–8568 (2004). Article ADS CAS PubMed PubMed Central Google Scholar *
Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. _Nucleic Acids Res._ 50, D439–D444
(2022). Article CAS PubMed Google Scholar * Avalos, J. L., Bever, K. M. & Wolberger, C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity
of a Sir2 enzyme. _Mol. Cell_ 17, 855–868 (2005). Article CAS PubMed Google Scholar * Zhen, X. et al. Structural basis of antiphage immunity generated by a prokaryotic
Argonaute-associated SPARSA system. _Nat. Commun._ 15, 450 (2024). Article ADS CAS PubMed PubMed Central Google Scholar * Feldman, J. L., Dittenhafer-Reed, K. E. & Denu, J. M.
Sirtuin catalysis and regulation. _J. Biol. Chem._ 287, 42419–42427 (2012). Article CAS PubMed PubMed Central Google Scholar * Bheda, P., Jing, H., Wolberger, C. & Lin, H. The
substrate specificity of sirtuins. _Annu. Rev. Biochem._ 85, 405–429 (2016). Article CAS PubMed Google Scholar * Guo, L. et al. Auto-inhibition and activation of a short
Argonaute-associated TIR-APAZ defense system. _Nat. Chem. Biol._ 20, 512–520 (2024). * Shen, Z. et al. Oligomerization-mediated activation of a short prokaryotic Argonaute. _Nature_ 621,
154–161 (2023). Article ADS CAS PubMed PubMed Central Google Scholar * Gao, X. et al. Nucleic-acid-triggered NADase activation of a short prokaryotic Argonaute. _Nature_ (2023). *
Finocchio, G. et al. Target DNA-dependent activation mechanism of the prokaryotic immune system SPARTA. _Nucleic Acids Res_. 52, 2012–2029 (2024). * Zhang, J. T., Wei, X. Y., Cui, N., Tian,
R. & Jia, N. Target ssDNA activates the NADase activity of prokaryotic SPARTA immune system. _Nat. Chem. Biol._ 20, 503–511 (2023). Article PubMed Google Scholar * Ni, D., Lu, X.,
Stahlberg, H. & Ekundayo, B. Activation mechanism of a short argonaute-TIR prokaryotic immune system. _Sci. Adv._ 9, eadh9002 (2023). Article CAS PubMed PubMed Central Google Scholar
* Wang, X. et al. Structural insights into mechanisms of Argonaute protein-associated NADase activation in bacterial immunity. _Cell Res._ 33, 699–711 (2023). Article ADS CAS PubMed
PubMed Central Google Scholar * Manik, M. K. et al. Cyclic ADP ribose isomers: production, chemical structures, and immune signaling. _Science_ 377, eadc8969 (2022). Article CAS PubMed
Google Scholar * Zhang, J.-T. et al. Structural basis for phage-mediated activation and repression of bacterial DSR2 anti-phage defense system. _Nat. Commun._ 15, 2797 (2024). Article ADS
CAS PubMed PubMed Central Google Scholar * Yin, H. et al. Insights into the modulation of bacterial NADase activity by phage proteins. _Nat. Commun._ 15, 2692 (2024). Article ADS CAS
PubMed PubMed Central Google Scholar * Guo, M. et al. Cryo-EM structure of the ssDNA-activated SPARTA complex. _Cell Res._ 33, 731–734 (2023). Article CAS PubMed PubMed Central
Google Scholar * Masukata, H., Dasgupta, S. & Tomizawa, J. Transcriptional activation of ColE1 DNA synthesis by displacement of the nontranscribed strand. _Cell_ 51, 1123–1130 (1987).
Article CAS PubMed Google Scholar * Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. _J. Struct. Biol._ 180, 519–530 (2012). Article CAS
PubMed PubMed Central Google Scholar * Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination.
_Nat. Methods_ 14, 290–296 (2017). Article CAS PubMed Google Scholar * Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron
microscopy. _Nat. Methods_ 14, 331–332 (2017). Article CAS PubMed PubMed Central Google Scholar * Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from
electron micrographs. _J. Struct. Biol._ 192, 216–221 (2015). Article PubMed PubMed Central Google Scholar * Buchan, D. W., Minneci, F., Nugent, T. C., Bryson, K. & Jones, D. T.
Scalable web services for the PSIPRED Protein Analysis Workbench. _Nucleic Acids Res._ 41, W349–W357 (2013). Article PubMed PubMed Central Google Scholar * Emsley, P. & Cowtan, K.
Coot: model-building tools for molecular graphics. _Acta Crystallogr. D Biol. Crystallogr._ 60, 2126–2132 (2004). Article ADS PubMed Google Scholar * Adams, P. D. et al. PHENIX: a
comprehensive Python-based system for macromolecular structure solution. _Acta Crystallogr. D Biol. Crystallogr._ 66, 213–221 (2010). Article ADS CAS PubMed PubMed Central Google
Scholar * Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. _Protein Sci._ 30, 70–82 (2021). Article CAS PubMed Google Scholar
Download references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (Grant No. 32270050 to N.J.), Guangdong and Shenzhen Natural Science
Foundation (Grant No. 2314050005743 and JCYJ20220530114409022 to N.J.), National Natural Science Foundation of China (Grant No. 32400024 to J.T.Z.), National Natural Science Foundation of
China (Grant No. 32300025 to N.C.), and the Guangdong Provincial Science and Technology Innovation Council Grant (2017B030301018). N.J. is an investigator of SUSTech Institute for Biological
Electron Microscopy. We thank the staff at Southern University of Science and Technology (SUSTech) Cryo-EM Center for assistance in data collection on the SUSTech Titan KRIOS cryo-electron
microscope. AUTHOR INFORMATION Author notes * These authors contributed equally: Ning Cui, Jun-Tao Zhang. AUTHORS AND AFFILIATIONS * Department of Biochemistry, School of Medicine, Southern
University of Science and Technology, Shenzhen, China Ning Cui, Jun-Tao Zhang, Zhuolin Li, Xin-Yang Wei & Ning Jia * Department of Chemistry, Southern University of Science and
Technology, Shenzhen, China Jie Wang * Shenzhen Key Laboratory of Cell Microenvironment, Guangdong Provincial Key Laboratory of Cell Microenvironment and Disease Research, Southern
University of Science and Technology, Shenzhen, China Ning Jia * Key University Laboratory of Metabolism and Health of Guangdong, Institute for Biological Electron Microscopy, Southern
University of Science and Technology, Shenzhen, China Ning Jia Authors * Ning Cui View author publications You can also search for this author inPubMed Google Scholar * Jun-Tao Zhang View
author publications You can also search for this author inPubMed Google Scholar * Zhuolin Li View author publications You can also search for this author inPubMed Google Scholar * Xin-Yang
Wei View author publications You can also search for this author inPubMed Google Scholar * Jie Wang View author publications You can also search for this author inPubMed Google Scholar *
Ning Jia View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.J. conceived the study. N.C., Z.L., and X.Y.W. undertook biochemical studies on
sample preparation and purification, biochemical assays. N.C. and Z.L. performed in vivo plasmid interference studies. J.T.Z. and X.Y.W. performed cryo-EM data collection, data processing,
structure refinement and data analysis. J.W. contributed to the helpful discussions regarding this project. N.J. and J.T.Z. wrote the manuscript with input from other authors. CORRESPONDING
AUTHOR Correspondence to Ning Jia. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks
Wenyuan Han, and the other, anonymous, reviewer(s) for their contribution tso the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING
SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which
permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a
link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or
parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Cui, N., Zhang, JT., Li, Z. _et al._ Tetramerization-dependent activation of the Sir2-associated short prokaryotic Argonaute immune system. _Nat Commun_ 15, 8610 (2024).
https://doi.org/10.1038/s41467-024-52910-5 Download citation * Received: 23 April 2024 * Accepted: 23 September 2024 * Published: 04 October 2024 * DOI:
https://doi.org/10.1038/s41467-024-52910-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative