Play all audios:
ABSTRACT Rechargeable batteries with high durability over wide temperature is needed in aerospace and submarine fields. Unfortunately, Current battery technologies suffer from limited
operating temperatures due to the rapid performance decay at extreme temperatures. A major challenge for wide-temperature electrolyte design lies in restricting the parasitic reactions at
elevated temperatures while improving the reaction kinetics at low temperatures. Here, we demonstrate a temperature-adaptive electrolyte design by regulating the dipole-dipole interactions
at various temperatures to simultaneously address the issues at both elevated and subzero temperatures. This approach prevents electrolyte degradation while endowing it with the ability to
undergo adaptive changes as temperature varies. Such electrolyte favors to form solvation structure with high thermal stability with rising temperatures and transits to one that prevents
salt precipitation at lower temperatures. This ensures stably within a wide temperature range of ‒60 −55 °C. This temperature-adaptive electrolyte opens an avenue for wide-temperature
electrolyte design, highlighting the significance of dipole-dipole interactions in regulating solvation structures. SIMILAR CONTENT BEING VIEWED BY OTHERS HIGH-ENTROPY ELECTROLYTES FOR
PRACTICAL LITHIUM METAL BATTERIES Article 06 July 2023 RATIONAL DESIGN OF ANTI-FREEZING ELECTROLYTES FOR EXTREMELY LOW-TEMPERATURE AQUEOUS BATTERIES Article 09 May 2024 HIGH ENTROPY LIQUID
ELECTROLYTES FOR LITHIUM BATTERIES Article Open access 27 January 2023 INTRODUCTION Rechargeable batteries operating under extreme conditions are often required to have exceptional
durability across a wide range of temperatures1,2. Yet, the temperature range of current battery technology is rather limited due to the rapid performance decay at elevated /subzero
temperatures3,4,5. Electrolyte, acting as the “blood” to connect all the components in the battery, plays a vital role in determining the electrochemistry performance, especially at extreme
temperatures. The viscosity and desolvation energy of the electrolyte dramatically increase at low temperatures, slowing down ion migration and subsequently leading to decreases in battery
output voltage and capacity6,7. While, at elevated temperatures, electrolytes vigorously decompose at the interphase of electrodes despite of the improved ion migration kinetics, posing a
serious threat to the long-term cycling stability and safety of batteries8,9,10. Broadening the operating temperatures of batteries faces intrinsic trade-offs and limitations. Taking ethers
or carboxylate esters as an example, they could serve as co-solvents in low-temperature electrolytes due to their low viscosity and freezing point, these solvents, however, significantly
deteriorates the chemical stability at high temperatures11,12. Vice versa, to ensure high thermal and chemical stability at elevated temperatures, solvents such as phosphates and fluorides
are introduced to the electrolytes13,14, while these solvents have poor capability to dissolve salts and compatibility with other solvents, leading to significant voltage polarization at low
temperatures. Clearly, conventional electrolytes with cocktail recipe struggle to meet the demands at elevated and subzero temperatures concurrently15,16. Exploring of new electrolyte that
has “_temperature-adaptive_” feature to simultaneously address the issues arising from both high and low temperatures is, therefore, expected to boost the electrochemical performance under
extreme conditions. Solvation structure of electrolytes plays a vital role of impacting the battery performance. There are numerous studies demonstrating that the understanding of solvation
structures, although mostly conducted at ambient temperature, have achieved tremendous progress in the past years. In general, the formation of solvation structure is balanced among the
intricate interplay among ion-ion, ion-solvent (ion-dipole), and solvent-solvent (dipole-dipole) interactions17,18. Through optimizing the ion-dipole interaction, the most commonly presented
one in the solvation structure, electrolytes with weak solvation19,20 or highly concentrated salt21,22 have been explored with success to some extent. As temperature being changed, however,
the degree of salt dissociation and the solvating capability of solvents both vary accordingly, which inevitably affects the solvation structure and, subsequently, the electrolyte
properties as a whole23. Unfortunately, such a substantial temperature dependence of solvation structures has long been neglected, especially the effect of dipole-dipole interaction on the
solvation structure at high and/or low temperatures, if any, remains elusive24. Considering its much higher temperature sensitivity than ion-solvent interactions25, we argue that
understanding the underlying dipole-dipole interaction mechanism could help us to identify suitable solvents for different purpose, but more importantly, we may be able to design temperature
responsive electrolyte through further manipulating the dipole-dipole interaction rather than the common ion-dipole interaction, which potentially opens another window for wide-temperature
electrolyte design. Through regulating the dipole-dipole interactions at various temperatures, we indeed explore an electrolyte by dissolving NaPF6 in 2-methyltetrahydrofuran (MeTHF),
tetrahydrofuran (THF) and anisole (AN) dilute (denoted as SMTA), which demonstrates great temperature-adaptive feature in this work. Specifically, by adjusting the interactions between
anti-solvents (AN) and co-solvents (MeTHF and THF), SMTA electrolyte achieves high thermal stability under elevated temperature and synchronously, fast kinetics at subzero temperature. We
find that, at high temperature, AN shows strong interaction with MeTHF and thus suppresses parasitic reactions, while at low temperatures, AN exhibits strong interaction with THF and thereby
inhibits salt precipitation. Such temperature-adaptive feature enables hard carbon anodes working stably with high capacity in SMTA electrolyte within a wide temperature range of ‒60–55 °C.
Furthermore, the enhancement of the performance on a series of electrolytes validates the universality and effectiveness of this concept. Our finding offers a approach to the development of
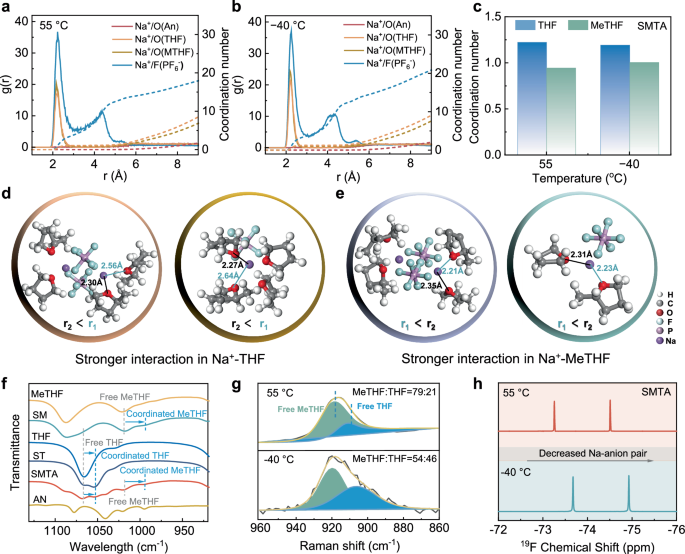
wide-temperature electrolytes. RESULTS ELECTROLYTE SOLVATION STRUCTURE The solvation structure of Na+ in SMTA electrolyte was firstly analyzed by molecular dynamics (MD) simulation. In the
equilibrated SMTA system at various temperatures, the radial distribution functions (RDFs) of Na+ with O atoms and Na+ with F atoms (Fig. 1a, b and Supplementary Fig. 1) reveal that THF,
MeTHF, and PF6- enter the primary solvation sheath of Na+. Notably, the coordination number (CN) of Na+-AN remains 0 among all temperature range, indicating that AN does not participate in
the primary solvation structure. With temperature dropping from 55 °C to −40 °C, the average CN of Na+ with O atoms in THF decreases from 1.22 to 1.19 while that of Na+ with MeTHF increases
from 0.94 to 1.0 (Fig. 1c). The RDFs and CN results implies that as temperature drops, the solvation structure experiences a decrease in the proportion of THF and a corresponding increase in
the proportion of MeTHF. In addition, the steady-state distance (_r_) between Na+ and solvent molecules exhibited significant variations (Fig. 1d, e and Supplementary Fig. 2). The _r_
between Na+ and O atom in MeTHF (denoted as _r_1) and that between Na+ and O atom in THF (denoted as _r_2) were calculated at various temperatures (Fig. 1d, e and Supplementary Fig. 2),
where shorter distance indicates stronger interaction26,27. In the solvation structure of SMTA, the values of _r_1 (2.17 Å at 25 °C and 2.56 Å at 55 °C) are significantly higher than _r_2
(2.09 Å at 25 °C and 2.30 Å at 55 °C), while those of _r_1 are smaller than _r_1 at −40 °C (Fig. 1e). These results indicate a stronger interaction between Na+ and THF at 55 °C and a
stronger interaction between Na+ and MeTHF at −40 °C. Infrared spectroscopy (IR) analysis was carried out to further obtain a comprehensive understanding of the Na+-solvent complexes in the
SMTA system (Fig. 1f). Taking SMTA electrolyte at 25 °C as an example, direct observation of coordination peaks corresponding to Na+-THF (1052 cm–1) and Na+-MeTHF (993 cm–1) show that both
THF and MeTHF are involved in the primary solvation sheath. Meanwhile, no change in the characteristic peak of the C-O-C stretching vibration at 1040 cm–1 in the AN solvent is observed. IR
observations demonstrate that the absence of coordination between Na+ and AN, which is in good accordance with MD calculation results (Fig. 1a, b and Supplementary Fig. 1). Raman spectra
reveal that with temperature decreasing from 55 °C to −40 °C (Fig. 1g and Supplementary Fig. 3), the proportion of free MeTHF and THF molecules changes from 79:21 to 54:46. The variation of
anions in solvation structure as function of temperature was further analyzed by 19F nuclear magnetic resonance (NMR). With temperature dropping from 55 °C to −40 °C, the F peaks in PF6-
undergoes an upfield shift resulting from an increase in electron cloud density around F- (Fig. 1h). Such change could be attributed to a reduction in the interaction force between Na+ and
the anions, facilitating the dissociation of salts, inhibiting the formation of unstable clusters, and ultimately enhancing conductivity of electrolyte at low temperatures. Both simulation
and experimental results confirm that the solvation structure transforms from a THF-dominated configuration at high temperatures to a MeTHF-dominated solvated structure at low temperatures.
DIPOLE-DIPOLE INTERACTION IN SOLVENT-ANTISOLVENT The molecular structures of three solvents are illustrated in Fig. 2a. The letters (from A to L) in Fig. 2a represent the hydrogen atoms at
their respective positions in each molecular structure, and their 1H NMR chemical shifts are listed in Supplementary Table 1. The variations in the solvation structure of the SMTA system
with temperatures were investigated through temperature-dependent one dimensional (1D) 1H NMR tests (Fig. 2b). The continuous shifts of all 1H nuclei in THF and MeTHF as the temperature
decreases indicate the continuous change of the solvation structure in response to temperature. Specifically, with temperature dropping (Fig. 2b and Supplementary Fig. 4), all characteristic
peaks of THF shifts upfield, and the extent of this shift gradually decreases compared to the solvation at room temperature, indicating a weakening of the interaction between Na+ and THF.
On the contrary, the shift in all characteristic peaks of MeTHF continues to increase compared to that at room temperature, suggesting a strengthening of the interaction between Na+ and
MeTHF. We further use two-dimensional (2D) 1H-1H correlation spectroscopy (COSY) to reveal proton coupling between various molecules (Fig. 2c, d and Supplementary Fig. 5). Specifically, the
hydrogen atoms at A and B sites in THF primarily interact with the hydrogen atoms at C and D sites in MeTHF. Only the hydrogen atom at J site in the methoxy group of AN interacts with both
MeTHF and THF molecules, resulting in a strong and directional coupling effect. At 55 °C and 25 °C (Fig. 3c and Supplementary Fig. 5), no coupling signals of AN with other MeTHF protons is
observed except for α-H (hydrogen on carbon directly connected to the functional group) proton coupling (J, D), indicating a highly directional and stable intermolecular interaction. In
sharp contrast, at −40 °C, new coupling peaks emerge at positions (J, C), (J, F), and (J, G), suggesting a reduction in directional interaction (Fig. 2d). We collected diffusion-ordered
spectroscopy (DOSY) to elucidate the strength of interactions among distinct solvates. At 25 °C, compared to the SMT electrolyte, the 1H DOSY NMR spectrum of the SMTA electrolyte
demonstrates a decreased overlap in the diffusion dimension (D) of the 1H DOSY NMR spectra for THF and MeTHF. This observation indicates that, the dipole-dipole interactions between THF and
MeTHF molecules are significantly weakened (Supplementary Figs. 6 and 7) in the presence of AN. The peak integrals are fitted to the Stejskal-Tanner equation to calculate the D
(Supplementary Figs. 8 and 9). Noted that “Dbefore” represents the D of molecules in the SMT electrolyte without AN, whereas “Dafter” denotes the D of molecules in the SMTA electrolyte after
the introduction of AN. A higher ratio of Dbefore /Dafter indicates a stronger interaction between AN and the solvent. The calculated Dbefore /Dafter values of MeTHF are 1.08, 1.10, and
1.14 at 55, 25, and -40 °C (Fig. 2e, f), respectively, while those of THF are 1.01, 1.05, and 1.34, respectively. 1H DOSY NMR results illustrate that AN possesses stronger binding affinity
towards MeTHF at high temperatures (Supplementary Figs. 8 and 9) and stronger affinity towards THF at low temperatures. Overall, both 1D and 2D NMR results show that the solvation change is
enabled by the varieties of dipole-dipole interactions between AN-MeTHF and AN-THF. As the temperature rises, the AN solvent exhibits stronger dipole-dipole interactions with MeTHF, leading
to the formation of a solvent structure dominated by THF. At low temperatures, however, the interaction between AN and THF becomes stronger, resulting in the formation of a solvent structure
primarily consisted of MeTHF (Fig. 2g and Supplementary Fig. 10). PHYSICOCHEMICAL AND ELECTROCHEMICAL PROPERTIES OF SMTA ELECTROLYTE Changes in the intermolecular interactions, accompanied
by alterations in solvation structure, lead to significant variations in both physical and electrochemical properties of the electrolyte across a wide temperature range. For electrolytes,
the most significant challenges are severe parasitic reactions at high temperatures and salt precipitation at low temperatures. We chose 1.0 M NaPF6 dissolved in MeTHF and THF (1:1 in
volume, denoted as SMT) as a control sample to illustrate the effect of AN anti-solvent. We can clearly see that the color of SMT electrolyte darkens after storage at 55 °C. Such a poor
chemical stability is mainly attributed to the decomposition of cyclic ether solvents in SMT at high temperature to form alkyl species, as evidenced by 1H NMR results (Fig. 3b and
Supplementary Figs. 11-12). Additionally, the 1H DOSY-NMR spectrum of the SMT electrolyte at 55 °C reveals the appearance of a methoxy peak at a chemical shift of 3.28 ppm (Supplementary
Fig. 6), further confirming the decomposition and ring-opening of cyclic solvents. On the contrary, such decomposition process is evidently inhibited in SMTA system (Fig. 3a, c, and
Supplementary Figs. 11-12). With temperature dropping, both SMT and SMTA shows good chemical stability after long-term storage, nevertheless, salt readily precipitates at −60 °C in the SMT
electrolyte (Fig. 3a), which inevitably impairs the ion diffusion kinetics at low temperatures. Fortunately, the addition of AN to electrolyte significantly inhibits the salt precipitation
at low temperatures. The SMTA electrolyte has a low freezing point of −77.5 °C, allowing to maintain a high conductivity even at low temperatures (Fig. 3d). The conductivity and viscosity of
the SMTA system at different temperatures were measured (Fig. 4e). The SMTA electrolyte exhibited an impressive conductivity of 2.4 mS cm–1 and a low viscosity of 3.88 cP at –40 °C. Even at
–60 °C, the SMTA electrolyte retains a conductivity of 1.57 mS cm–¹, which is sufficient to support battery operating at such an extremely low temperature. Evidently, the addition of AN not
only enhances the high-temperature stability of the electrolyte but also lowers its freezing point at low temperatures, thereby enabling SMTA as an wide-temperature electrolyte. The
electrochemical performance of SMTA electrolyte was investigated in HC | |Na half cells. The high ionic conductivity of the SMTA electrolyte at low temperatures favors fast Na+ transport
kinetics. As revealed in cyclic voltammetry (CV) results, CV curves exhibit excellent reversibility and no significant change in redox potentials over a wide temperature range (Supplementary
Fig. 13). The galvanostatic charge/discharge (GCD) curves of HC | |Na cells equipped with SMTA electrolyte at a specific current of 100 mA g-1 across a wide temperature range were shown in
Fig. 3f. HC anodes exhibit high specific capacities over a wide temperature range. At 55 °C, 25 °C, 0 °C, −20 °C, −40 °C, −50 °C, and −60 °C, the discharge capacities are 301.4 mAh g–1,
293.1 mAh g–1, 265.4 mAh g–1, 246.8 mAh g–1, 225.3 mAh g–1, 180.8 mAh g–1, and 112.9 mAh g–1, respectively. The Na+ insertion/extraction process in HC anodes, which is composed of adsorption
(slope region in GCD curve) and insertion/deposition (plateau region in GCD curve) process, is highly reversible even at −50 °C with SMTA electrolyte. After 200 cycles, the capacity
retention is as high as 99.3% (Supplementary Fig. 14). As for SMT electrolyte, on the contrary, HC only delivers a capacity of 57.5 mAh g-1, with complete vanishing of insertion/deposition
process28. The low ionic conductivity as a result of salt precipitation leads to sluggish kinetics (Fig. 3g and Supplementary Fig. 15) in SMT, preventing the effective insertion of Na+29,30.
In addition to the improved kinetics at low temperatures, the cycling stability of HC operated in SMTA electrolyte is also significantly improved compared with those in SMT electrolyte
especially at high temperatures. At 55 °C, the capacity retention of HC cycled in SMTA electrolyte is 73.5% after 200 cycles (Fig. 3h) while that of HC in SMT only remains 33.1% after 150
cycles. HC anode also shows high stability after extended cycling in SMTA electrolyte. The capacity retentions of HC after 700 cycles are 80.1% and 87.0%, respectively, at 25 °C and −40 °C
(Fig. 3i and Supplementary Fig. 16). The role of AN ensures a rapid kinetic process and excellent cycle stability in SMTA electrolyte across wide temperatures. Given that the desolvation
process is crucial for the kinetics of Na+ storage in HC anodes31, we calculated the desolvation energies of solvation structures in SMTA electrolyte at 55 °C and –40 °C by density
functional theory (DFT). As evidenced in Fig. 3j, ΔE (-THF) is significantly higher than ΔE (-MeTHF) at 55 °C, but becomes smaller with temperature dropping to –40 °C. Such an observation
indicates that the binding force between Na+ and THF solvents is stronger than that between Na+ and MeTHF at high temperature but weaker at low temperature. The leapfrog improvement in
interfacial dynamics is well demonstrated by temperature-dependent electrochemical impedance spectroscopy (EIS) measurements and the fitted results according to the classical Arrhenius law
(Fig. 3j and Supplementary Fig. 17). The activation energy of Na+ in SMTA electrolyte through SEI transport (_E_a, SEI) and charge transfer process (_E_a, ct) is 14.1 kJ mol–1 and 17.7 kJ
mol–1, respectively. These _E_a values are relatively low, and temperature has minimal influence on them, emphasizing the significance of intermolecular interactions between AN and solvents
in the SMTA electrolyte in promoting charge transfer processes at the electrode interface. MECHANISM OF DIPOLE-DIPOLE INTERACTIONS To understand the underline mechanism for high-temperature
instability, we investigated a series of solvents and electrolytes after being stored at various temperatures (Supplementary Fig. 18). No obvious changes is observed in all the solvents
after storage at high temperatures, however, the addition of NaPF6 salt leads to significant discoloration in the SM electrolyte (1.0 M NaPF6 in MeTHF), even stored at an ambient environment
of 25 °C. 1H-NMR spectra (Supplementary Figs. 19-20) show that new alkyls peaks (-CH, -CH2, -OCH3, etc) emerges in SM electrolyte after two weeks storage at 25 °C, confirming that the
structural decomposition of MeTHF is the root cause of the degradation in high-temperature performance of the electrolyte. The electrostatic potential (ESP) of these three molecular
structures was calculated by DFT to analyze their surface charge distributions (Fig. 4a). Unlike THF, which exhibits high structural symmetry and low ring strain, the presence of a methyl
group in MeTHF leads to a highly asymmetrical charge distribution. The electron-withdrawing effect of oxygen (O) enhances the positive charge on the α-carbon adjacent to the neighboring O
atom, leading to an increase in its chemical reactivity and the enhancement of the acidity of α-H. In SMT electrolyte, the reaction between NaPF6 and trace amounts of water produces the
[PF₅OH]⁻ anion. Subsequently, the nucleophilic [PF₅OH]⁻ anion selectively attacks the positively charged α-carbon atom within the O-C bond of MeTHF (Fig. 4b), leading to the formation of an
active intermediate with an anionic chain end. This process weakens the bond energy of the C-O bond, ultimately resulting in the ring-opening decomposition of MeTHF. Continuous addition of
anions to the monomers via the reaction facilitates the growth of the polymer chain. The addition of AN significantly improves the chemical stability of MeTHF molecules. As evidenced in 2D
1H-1H COSY results, the methoxyl group of AN shows a strong and directional interaction with the α-H in MeTHF at high temperatures, reducing the acidity of the α-H and diminishing the
positive charge on the α-C atom. The interactions between AN and MeTHF, therefore, significantly enhances the stability of the O-C bond and stabilizes the MeTHF structure. At low
temperatures, all electrolytes display high chemical stability (Supplementary Fig. 21), thus salt precipitation and freezing primarily influence the low-temperature performance. As revealed
by 2D NMR (Fig. 2c, d), this transition hinders the formation of ordered clusters, thus promoting an increase in entropy. From thermodynamic point of view, rising entropy potentially favors
the formation of a homogeneous solution, thus prevents salt precipitation and stabilizes the liquid-phase system (Fig. 4c)32. Overall, the temperature adaptive feature of SMTA electrolyte is
realized by manipulating dipole-dipole interactions among solvents (Fig. 5). At elevated temperatures, thermal motion among molecules significantly intensifies. Due to the presence of
methoxy and methyl side chains, AN and MeTHF possess considerable steric hindrance, which slows down molecules movements and increases the frequency of molecular collisions between them,
thus leading to an enhancement of their interactions. The strong interactions between AN and MeTHF inhibits the parasitic decomposition of MeTHF and promotes the formation of stable
complexes. While at sub-zero temperatures, on the contrary, the thermal motion of all molecules is dramatically hindered, thus instead of the steric hindrance, polarization effect among
molecules become the primary factor influencing interactions. The greater difference in polarizability is, the stronger the intermolecular interactions become. The molecular polarizability
of AN, MeTHF, and THF is 13.05, 9.81 and 7.94, respectively33. The symmetrical structure of THF renders it less sensitive to polarization changes at low temperatures. The large difference in
polarizability between AN and THF leads to an enhanced dispersive force between them, leading to less directional interactions between AN and MeTHF. Consequently, the solvation structure is
less ordered, the system entropy increases, and thus the solubility of salt is enhanced. DISCUSSION To validate the effectiveness of this concept, we selected dimethoxybenzene (DMB),
1,3,5-trimethoxybenzene (TMB) and cyclopentyl methyl ether (CME) as antisolvents due to their identical structure to AN, particularly in their possession of methoxy groups as active
functionalities. The specific molecular structures are depicted in Supplementary Fig. 22. Simultaneously, THF and MeTHF remained as solvents. Three electrolytes, SMT-DMB (1 M NaPF6 in THF:
MeTHF: Dimethoxybenzene with a volume ratio of 17:17:6), SMT-TMB (1 M NaPF6 in THF: MeTHF: Trimethoxybenzene with a volume ratio of 17:17:6) and SMT-CME (1 M NaPF6 in THF: MeTHF: Cyclopentyl
methyl ether with a volume ratio of 17:17:6), were prepared and subsequently tested in HC | |Na half cells. They demonstrated significantly improved performance compared to SMT, especially
under room and low temperature conditions (Supplementary Figs. 23-24). The obtained results not only validate the significant feasibility of this strategy but also offer a practical approach
to enhancing the temperature adaptability of electrolytes by regulating the strength of dipole-dipole interactions. Although the composition and properties of the solid electrolyte
interphase (SEI) play a significant role in the electrochemical performance of batteries, our findings in this work confirm that the physicochemical properties of the electrolyte itself have
a more profound impact on battery performance under extreme temperatures. We utilized X-ray photoelectron spectroscopy (XPS) to investigate the interfacial chemistry at different
temperatures (25 °C and –40 °C) and etching depths of HC electrode after cycling. The components of the SEI in SMT and SMTA electrolytes exhibited good uniformity at various depths,
resulting in the formation of a predominantly inorganic-rich SEI composed of NaF/Na2CO3. These results suggest that there are no significant differences in the interfacial components formed
at different temperatures. (Supplementary Figs. 25–28). The superior electrochemical performance of the SMTA electrolyte at both high and low temperatures is not primarily attributed to the
SEI, but rather results from improvements in the physicochemical properties of the electrolyte itself. In summary, we found that regulating dipole-dipole interactions could endow solvation
structure with temperature adaptability, enabling high-performance sodium-ion batteries to operate stably within a wide temperature range. Such a temperature-adaptive feature could
simultaneously meet the demands both at high and low temperatures. In the SMTA electrolyte, AN shows strong interaction with MeTHF and stabilizes the α-H in molecular at high temperatures,
thereby suppressing parasitic reactions and enhancing thermal stability. At low temperatures, on the other hand, AN exhibits strong interaction with THF, thus inhibiting salt precipitation
and improve kinetics. Leveraging this advantage, we have ensured that hard carbon anodes maintain high capacity and competitive cycling stability even across an extensive temperature range
(− 60 °C–55 °C). Our work elucidates the significance of dipole-dipole interactions in regulating the solvation structure and points out a direction for the development of wide-temperature
rechargeable batteries. METHODS MATERIALS NaPF6, THF and MeTHF were purchased from Dodo chem and used as received. Anisole (AN) was purchased from Aladdin Reagent Co., Ltd. and used as
received. 1,3-Dimethoxybenzene was purchased from Maclin Inc. and was used after being dried over a molecular sieve. 1,3,5-Trimethoxybenzene and Cyclopentyl methyl ether were purchased from
Maclin Inc. and used as received. All the solvents mentioned above were stored in aluminum-plastic bottles. A pipette with a polypropylene tip was used to transfer the solvents into 5 ml
glass bottles, which were then placed on a magnetic stirrer to dissolve the salt at room temperature. MTA solution was prepared by THF: MeTHF: AN with a volume ratio of 17:17:6. MT solution
was prepared by MeTHF: THF with a volume ratio of 1:1. MA solutions were prepared by MeTHF: AN with a volume ratio of 37:13. TA solutions were prepared by THF: AN with a volume ratio of
37:13. MA solution was prepared by MeTHF: AN with a volume ratio of 37:13. SM or ST electrolytes were prepared by dissolving 1 M NaPF6 in MeTHF or THF, respectively. SMA electrolyte was
prepared by dissolving 1 M NaPF6 in MeTHF: AN with a volume ratio of 9:1. STA electrolyte was prepared by dissolving 1 M NaPF6 to THF: AN with a volume ratio of 37:13. SMTA electrolyte was
prepared by dissolving 1 M NaPF6 to THF: MeTHF: AN with a volume ratio of 17:17:6. SMT-DMB electrolyte was prepared by dissolving 1 M NaPF6 to THF: MeTHF: Dimethoxybenzene with a volume
ratio of 17:17:6. SMT-TMB electrolyte was prepared by dissolving 1 M NaPF6 to THF: MeTHF: 1,3,5-Trimethoxybenzene with a volume ratio of 17:17:6. SMT-CME electrolyte was prepared by
dissolving 1 M NaPF6 to THF: MeTHF: Cyclopentyl methyl ether with a volume ratio of 17:17:6. All the preparation procedure were carried out in an argon-filled glove box with H2O and O2
levels <0.01 ppm. Hard carbon materials were purchased from Kuraray Company. Sodium metal purchased from Sinopharm Group Chemical Reagent Co., LTD. (99.5%). ELECTROCHEMICAL TESTS HC
electrodes were prepared by blending 80 wt.% HC, 10 wt.% acetylene black and 10 wt.% polyvinylidene fluoride binders to form a homogeneous slurry. The as-prepared slurry was pasted uniformly
onto a carbon-coated Al foil and then dried at 120 °C overnight in a vacuum oven. All electrodes were cut into circular pieces with a diameter of 12 mm. The average loading mass is ∼5 mg
cm−2. Remove the sodium block from kerosene and trim off the oxidized layer to expose the fresh, metallic-sheen sodium. Roll it into a uniform, 1-2 mm thick sheet using an iron rod. Cut the
rolled sheet into 14 mm diameter disks with a hole punch in an Ar-filled glovebox. CR2025 coin-cells were used to prepare HC | |Na cells. A 19 mm diameter porous glass fibre (GF/D) was used
as separators. All the cells were assembled in an Ar-filled glovebox with oxygen and moisture levels less than 0.01 ppm. The electrochemical performances were carried out on
CT-4008Tn-5V50mA-HWX battery tester. The room-temperature electrochemical tests were carried out at a constant temperature of 25 °C in an environmental chamber. The high temperature
electrochemical tests were performed at a constant temperature of 55 °C in the temperature and humidity chamber. All low-temperature data were collected from these cells inside SCICOOLING
freezers (BTC-SG7503-02F) for −60 ∼55 °C tests. Before working at LT, the cells were cycled at RT for 5 cycles with a specific current of 100 mA/g and then stored at the targeted temperature
for 1 h before testing. The operation voltage range of HC | |Na cell was 0.001 ∼ 2.0 V. The HC | |Na cell was operated at a constant specific current of 100 mA g−1. CV experiments were
applied to investigate the electrode kinetics at a scan rate of 0.5 mV s−1 within the same voltage range on a Princeton PMC CHS08A electrochemical workstation. CHARACTERIZATION The ionic
conductivities of electrolytes were tested by a conductivity measuring meter (INESA DDS-307, Leici) within the temperature range from −60 to 25 °C. The DSC measurements were carried out in a
DSC 214 (NETZSCH) differential scanning calorimeter from 25 to −120 °C with a cooling rate of 5 °C min−1. An investigation of solid electrolyte interphase on hard carbon cycled in SMTA
electrolyte was characterized via a high sensitivity Kratos AXIS Supra X-ray photoelectron spectrometer (XPS) with Al Kα radiation (1486.6 eV). The sputtering with a power of 4 kV × 140 μA
on a 3 mm × 3 mm surface was conducted via Ar ions, with a sputtering rate on Ta2O5 calibrated to be 10 nm min−1. All values of binding energy were referenced to the C 1 s peak of carbon
located at 284.8 eV. Before the XPS characterizations, the cycled HC electrodes were washed three times to remove residual salts by the corresponding solvents, and dried at 50 °C in glovebox
for 3 h to totally remove the solvents. The glovebox is connected to the vacuum transfer chamber of the XPS system to avoid the sample exposure to air. NMR (Bruker AVANCE NEO, 600 MHz)
techniques were deployed to reveal the electrolyte solvation structures. samples were measured at 233.1 ± 0.1 K, 298.1 ± 0.1 K, and 328.1 ± 0.1 K without rotation and with 4 dummy scans
prior to 16 scans. Acquisition parameters were set as follows: FID size = 64 K, spectral width = 24.5044 ppm, receiver gain = 4, acquisition time = 2.22 s, relaxation delay = 1 s, and FID
resolution = 0.3 Hz. Deuterated acetone was placed in an external coaxial insert and then in the NMR tube with the samples. The 1H chemical shifts were referenced to acetone-_d__6_ at 2.05
ppm (at high temperatures, Dimethyl-_d__6_ sulfoxide at 2.50 ppm) and TMS was used as an internal reference. The Raman spectra of the electrolytes were recorded using a spectrometer (XploRA
INV, Horiba), excited by a 532 nm laser. The electrolyte samples were cooled down to the target temperature with a nitrogen coolant and controller. COMPUTATIONAL DETAILS MD simulations were
carried out by a Materials Studio software, reversion 201834. The MD simulation package Forcite was used for all the simulations with COMPASS II force field35. The model for SMTA electrolyte
contains *NaPF6, *THF molecules, *MeTHF molecules and *AN molecules. The obtained box is a 39.33 × 39.33 × 39.33 Å3 cube. The corrected charge simulations for molecules were performed using
DMol3 packge36, based on Density Functional Theory (DFT). In these simulations, the pure density functional method M06-L was employed, which is a widely-used formulation within the
meta-generalized gradient approximation(m-GGA) method37. The electronic energy was considered self-consistent when the energy change was smaller than 10−5 eV, while the tolerance convergence
in ionic was 10−5 eV, too. Furthermore, the van der Waals correction of Grimme’s DFT-D3 model was adopted38. The simulation temperature was set at 298, 233 and 328 K, respectively. The
Ewald summation method was used for the electrostatic interactions between the permanent charges with either permanent charges or induced dipole moments with K = 63 vectors39. Multiple
timestep integration was employed with an inner timestep of 0.5 fs (bonded interactions), a central time step of 1.5 fs for all non-bonded interactions within a truncation distance of 8.0 Å
and an outer timestep of 3.0 fs for all non-bonded interactions between 7.0 Å and the non-bonded truncation distance of 14–16 Å. The reciprocal part of Ewald was calculated every 3.0 fs. A
Nose‒Hoover thermostat was used to control the temperature with the associated frequencies of 10‒2 and 0.1 × 10‒4 fs. A Berendsen barostat was used to control the pressure with a decay
constant of 0.1 ps40. The atomic coordinates were saved every 2 ps for post-analysis. Each system will be subjected to an Annealing process from 300 K to 500 K to help the mixture disperse
more evenly. Subsequently, initial equilibration runs of ~5 ns were performed in an NPT ensemble to obtain the equilibrium box size that is used in the follow-up equilibration and production
runs of ~1 ns performed in the NVT ensemble. DATA AVAILABILITY The data supporting the findings of this study are available within the article and its Supplementary Information files. All
the relevant data within this paper and its Supplementary Information file are available from the corresponding author upon request. REFERENCES * Lin, X. et al. Rechargeable battery
electrolytes capable of operating over wide temperature windows and delivering high safety. _Adv. Energy Mater._ 10, 2001235 (2020). Article CAS Google Scholar * Sun, Y., Li, J.-C., Zhou,
H. & Guo, S. Wide-temperature-range sodium-metal batteries: From fundamentals and obstacles to optimization. _Energy Environ. Sci._ 16, 4759–4811 (2023). Article CAS Google Scholar *
Liu, Q. & Wang, L. Fundamentals of electrolyte design for wide-temperature lithium metal batteries. _Adv. Energy Mater._ 13, 2301742 (2023). Article CAS Google Scholar * Chen, L.,
Wu, H., Ai, X., Cao, Y. & Chen, Z. Toward wide-temperature electrolyte for lithium–ion batteries. _Battery Energy_ 1, 20210006 (2022). Article CAS Google Scholar * Zhang, L., Liu, Y.,
You, Y., Vinu, A. & Mai, L. Nasicons-type solid-state electrolytes: The history, physicochemical properties, and challenges. _Interdiscip. Mater._ 2, 91–110 (2023). Google Scholar *
Su, X. et al. Liquid electrolytes for low-temperature lithium batteries: Main limitations, current advances, and future perspectives. _Energy Storage Mater._ 56, 642–663 (2023). Article
Google Scholar * Zhang, G. et al. A monofluoride ether-based electrolyte solution for fast-charging and low-temperature non-aqueous lithium metal batteries. _Nat. Commun._ 14, 1081 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar * Wang, Y. et al. Revitalising sodium–sulfur batteries for non-high-temperature operation: A crucial review. _Energy Environ. Sci._
13, 3848–3879 (2020). Article CAS Google Scholar * Yang, C., Xin, S., Mai, L. & You, Y. Materials design for high-safety sodium-ion battery. _Adv. Energy Mater._ 11, 2000974 (2021).
Article CAS Google Scholar * Huang, Y. The discovery of cathode materials for lithium-ion batteries from the view of interdisciplinarity. _Interdiscip. Mater._ 1, 323–329 (2022). Google
Scholar * Wang, Z. et al. Co-intercalation-free ether-based weakly solvating electrolytes enable fast-charging and wide-temperature lithium-ion batteries. _ACS Nano_ 17, 18103–18113 (2023).
Article CAS PubMed Google Scholar * Zhang, Y. et al. Electrolyte design for lithium-ion batteries for extreme temperature applications. _Adv. Mater._ 36, 2308484 (2024). Article CAS
Google Scholar * Zheng, X. et al. Toward high temperature sodium metal batteries via regulating the electrolyte/electrode interfacial chemistries. _ACS Energy Lett._ 7, 2032–2042 (2022).
Article CAS Google Scholar * Yang, C. et al. Engineering a boron-rich interphase with nonflammable electrolyte toward stable li||ncm811 cells under elevated temperature. _Adv. Mater._ 36,
2307220 (2024). Article CAS Google Scholar * Liao, Y. et al. Electrolyte degradation during aging process of lithium-ion batteries: Mechanisms, characterization, and quantitative
analysis. _Adv. Energy Mater._ 14, 2304295 (2024). Article CAS Google Scholar * Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. _Chem. Rev._ 104, 4303–4418
(2004). Article CAS PubMed Google Scholar * Chen, K. et al. Correlating the solvating power of solvents with the strength of ion-dipole interaction in electrolytes of lithium-ion
batteries. _Angew. Chem. Int. Ed._ 62, e202312373 (2023). Article ADS CAS Google Scholar * Hou, R., Guo, S. & Zhou, H. Atomic insights into advances and issues in low-temperature
electrolytes. _Adv. Energy Mater._ 13, 2300053 (2023). Article CAS Google Scholar * Fan, X. et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents.
_Nat. Energy_ 4, 882–890 (2019). Article ADS CAS Google Scholar * Ma, T. et al. Optimize lithium deposition at low temperature by weakly solvating power solvent. _Angew. Chem. Int. Ed._
61, e202207927 (2022). Article ADS CAS Google Scholar * Cao, X. et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize li
depletion and pulverization. _Nat. Energy_ 4, 796–805 (2019). Article ADS CAS Google Scholar * Chen, S. et al. High-voltage lithium-metal batteries enabled by localized
high-concentration electrolytes. _Adv. Mater._ 30, 1706102 (2018). Article Google Scholar * Wang, Z. & Zhang, B. Weakly solvating electrolytes for next-generation lithium batteries:
Design principles and recent advances. _Energy Mater. Devices_ 1, 9370003 (2023). Article Google Scholar * Xiao, P. et al. Insights into the solvation chemistry in liquid electrolytes for
lithium-based rechargeable batteries. _Chem. Soc. Rev._ 52, 5255–5316 (2023). Article CAS PubMed Google Scholar * Chen, X., Zhang, X. Q., Li, H. R. & Zhang, Q. Cation−solvent,
cation−anion, and solvent−solvent interactions with electrolyte solvation in lithium batteries. _Batteries Supercaps_ 2, 128–131 (2019). Article CAS Google Scholar * Guo, D., Wang, J.,
Lai, T., Henkelman, G. & Manthiram, A. Electrolytes with solvating inner sheath engineering for practical na–s batteries. _Adv. Mater._ 35, 2300841 (2023). Article CAS Google Scholar
* Zhao, Y. et al. Strong solvent and dual lithium salts enable fast-charging lithium-ion batteries operating from −78 to 60 °c. _J. Am. Chem. Soc._ 145, 22184–22193 (2023). Article CAS
PubMed Google Scholar * Dong, R. et al. Elucidating the mechanism of fast na storage kinetics in ether electrolytes for hard carbon anodes. _Adv. Mater._ 33, 2008810 (2021). Article CAS
Google Scholar * Li, X. et al. Electrolyte modulators toward polarization-mitigated lithium-ion batteries for sustainable electric transportation. _Adv. Mater._ 34, 2107787 (2022). Article
CAS Google Scholar * Nagmani, Kumar, A. & Puravankara, S. Optimizing ultramicroporous hard carbon spheres in carbonate ester-based electrolytes for enhanced sodium storage in
half-/full-cell sodium-ion batteries. _Battery Energy_ 1, 20220007 (2022). Article Google Scholar * Lu, Z. et al. Step-by-step desolvation enables high-rate and ultra-stable sodium storage
in hard carbon anodes. _Proc. Natl. Acad. Sci._ 119, e2210203119 (2022). Article CAS PubMed PubMed Central Google Scholar * Yang, C. et al. Entropy-driven solvation toward
low-temperature sodium-ion batteries with temperature-adaptive feature. _Adv. Mater._ 35, e2301817 (2023). Article PubMed Google Scholar * Joshi, S. S., Aminabhavi, T. M. & Shukla, S.
S. Densities and viscosities of binary liquid mixtures of anisole with methanol and benzene. _J. Chem. Eng. Data_ 35, 187–189 (1990). Article CAS Google Scholar * Yan, H. et al.
Molecular model construction of low-quality coal and molecular simulation of chemical bond energy combined with materials studio. _Energy Fuels_ 35, 17602–17616 (2021). Article CAS Google
Scholar * Rocco, E. The compass future: Compass ii. _Prog. Part. Nucl. Phys._ 67, 288–293 (2012). Article ADS CAS Google Scholar * Delley, B. DMol3 DFT studies: From molecules and
molecular environments to surfaces and solids. _Comput. Mater. Sci._ 17, 122–126 (2000). Article CAS Google Scholar * Grimme, S. Semiempirical GGA-type density functional constructed with
a long-range dispersion correction. _J. Comput. Chem._ 27, 1787–1799 (2006). Article CAS PubMed Google Scholar * Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and
accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. _J. Chem. Phys._ 132, 154104 (2010). Article ADS PubMed Google Scholar *
Toukmaji, A. Y. & Board, J. A. Ewald summation techniques in perspective: A survey. _Comput. Phys. Commun._ 95, 73–92 (1996). Article ADS CAS Google Scholar * Chen, J. et al.
Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. _Nat. Energy_ 5, 386–397 (2020). Article ADS CAS Google
Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Key Research and Development Program of China (2022YFB3803400, [Y.Y.]), and National Natural Science
Foundation of China (Grant No. 52172234, [Y.Y.]). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan
University of Technology, Hubei, Wuhan, 430070, P. R. China Meilong Wang, Luming Yin, Xiaowei Liu, Chao Yang, Wenxi Hu, Jingjing Xie, Ruitao Sun & Ya You * College of Chemical and
Biological Engineering, Zhejiang University, Hangzhou, Zhejiang Province, 310027, China Mengting Zheng & Jun Lu * International School of Materials Science and Engineering, School of
Materials Science and Microelectronics, Wuhan University of Technology, Hubei, Wuhan, 430070, P. R. China Jin Han & Ya You Authors * Meilong Wang View author publications You can also
search for this author inPubMed Google Scholar * Luming Yin View author publications You can also search for this author inPubMed Google Scholar * Mengting Zheng View author publications You
can also search for this author inPubMed Google Scholar * Xiaowei Liu View author publications You can also search for this author inPubMed Google Scholar * Chao Yang View author
publications You can also search for this author inPubMed Google Scholar * Wenxi Hu View author publications You can also search for this author inPubMed Google Scholar * Jingjing Xie View
author publications You can also search for this author inPubMed Google Scholar * Ruitao Sun View author publications You can also search for this author inPubMed Google Scholar * Jin Han
View author publications You can also search for this author inPubMed Google Scholar * Ya You View author publications You can also search for this author inPubMed Google Scholar * Jun Lu
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.Y. and J.L. conceived the idea. M.W., L.Y., C.Y., W.H., and R.S. characterized materials
and tested electrochemical performance. X.L. conducted and analyzed the calculations. M.W., L.Y., M.Z., Y.Y., and J.L. wrote the paper. J.X., J.H., Y.Y., and J.L. supervised the research.
M.W., L.Y., M.Z., and X.L. contributed equally to this work. CORRESPONDING AUTHORS Correspondence to Ya You or Jun Lu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is
available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0
International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s)
and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material
derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, M., Yin, L., Zheng, M. _et al._ Temperature-responsive solvation enabled by dipole-dipole interactions towards wide-temperature sodium-ion
batteries. _Nat Commun_ 15, 8866 (2024). https://doi.org/10.1038/s41467-024-53259-5 Download citation * Received: 30 May 2024 * Accepted: 04 October 2024 * Published: 14 October 2024 * DOI:
https://doi.org/10.1038/s41467-024-53259-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative