Play all audios:
ABSTRACT The antimicrobial resistance crisis along with challenges of antimicrobial discovery revealed the vital necessity to develop new antibiotics. Many of the animal proline-rich
antimicrobial peptides (PrAMPs) inhibit the process of bacterial translation. Genome projects allowed to identify immune-related genes encoding animal host defense peptides. Here, using
genome mining approach, we discovered a family of proline-rich cathelicidins, named rumicidins. The genes encoding these peptides are widespread among ruminant mammals. Biochemical studies
indicated that rumicidins effectively inhibited the elongation stage of bacterial translation. The cryo-EM structure of the _Escherichia coli_ 70S ribosome in complex with one of the
representatives of the family revealed that the binding site of rumicidins span the ribosomal A-site cleft and the nascent peptide exit tunnel interacting with its constriction point by the
conservative Trp23-Phe24 dyad. Bacterial resistance to rumicidins is mediated by knockout of the SbmA transporter or modification of the MacAB-TolC efflux pump. A wide spectrum of
antibacterial activity, a high efficacy in the animal infection model, and lack of adverse effects towards human cells in vitro make rumicidins promising molecular scaffolds for development
of ribosome-targeting antibiotics. SIMILAR CONTENT BEING VIEWED BY OTHERS ANTIPROTOZOAL ACTIVITY OF DIFFERENT _XENORHABDUS_ AND _PHOTORHABDUS_ BACTERIAL SECONDARY METABOLITES AND
IDENTIFICATION OF BIOACTIVE COMPOUNDS USING THE EASYPACID APPROACH Article Open access 24 June 2022 MULTIMODAL BINDING AND INHIBITION OF BACTERIAL RIBOSOMES BY THE ANTIMICROBIAL PEPTIDES
API137 AND API88 Article Open access 10 May 2024 INTEGRATED GENOMICS AND PROTEOMICS ANALYSIS OF _PAENIBACILLUS PEORIAE_ IBSD35 AND INSIGHTS INTO ITS ANTIMICROBIAL CHARACTERISTICS Article
Open access 07 November 2022 INTRODUCTION The rapid growth of antibiotic resistance among bacterial pathogens, along with the challenges of novel antimicrobial discovery, requires
development of new anti-infective agents1. About half of conventional antibiotics, most of which are of natural origin, target the bacterial ribosome. An essential role of ribosomes in
protein biosynthesis and a low mutational propensity still make them an ideal target for antimicrobials2. It has been shown that many genetically encoded endogenous animal host-defense
peptides are enriched with proline and arginine residues and inhibit the translation process. Ribosome-targeting proline-rich antimicrobial peptides (PrAMPs) from animals can be divided into
two groups based on their origin: oncocin- and apidaecin-like peptides from insects, and cathelicidins from Cetartiodactyla mammals3. Cathelicidins are synthesized as prepropeptides
containing an _N_-terminal signal peptide, a conservative cathelin-like domain (CLD), and a _C_-terminal mature AMP, which is enzymatically cleaved off once the peptide is secreted4. To
date, two main mechanisms of translation inhibition have been described for PrAMPs: class I peptides (oncocins, Bac7-like cathelicidins) inhibit the elongation phase by sterically preventing
the accommodation of the first incoming aminoacyl-tRNA into the ribosomal A site5, whereas class II peptides (apidaecins, drosocin) prevent dissociation of the release factors during the
termination phase of translation6,7. In general, PrAMPs are characterized by a capacity to form multiple interactions within the nascent peptide exit tunnel (NPET) that significantly reduces
the risk of bacterial resistance due to single modifications or spontaneous mutations of the nucleotides in the 23S rRNA. Search for natural PrAMPs is an important step toward the design of
highly effective translation inhibitors. To develop PrAMP-based antibiotics by medicinal chemistry approaches, a battery of molecular scaffolds needs to be created and optimized for the
purpose of enhancing their efficiency and getting the best result. Intensive development of the genome projects worldwide8,9 allows fast identification of immune-related genes encoding
animal host-defense peptides. In particular, genome mining reveals a significant potential for the discovery of proline-rich cathelicidins due to the high conserved structure of the
cathelin-like domain and quite simple organization of the corresponding gene clusters. Here, we describe a family of relatively small proline-rich cathelicidins widespread among ruminant
species. These peptides, named rumicidins, inhibit the formation of the first peptide bond presumably due to interference with the accommodation of aminoacyl-tRNA into the A site of the
ribosome. Our cryo-EM data reveals that rumicidins bind to the bacterial ribosome in the NPET similar to other known PrAMPs but form unique interactions with the elements of the 50S
ribosomal subunit. The obtained data on the biological activity of rumicidins allows us to consider them as a quite safe and effective ribosome-targeting antimicrobials. RESULTS GENES
ENCODING FOR A FAMILY OF PROLINE-RICH CATHELICIDINS ARE WIDESPREAD AMONG RUMINANTS We used the TBLASTN program to identify cathelicidin genes in the whole-genome sequencing (WGS) database
using the conservative cathelin-like domain (CLD) fragment FTVKETVCPRTSPQPPEQCDFKE encoded by the nucleotide sequence located in the second exon of the cattle procathelicidin-3 (the Bac7
precursor). We used this sequence as a query against all Cetartiodactyla WGS projects deposited in NCBI. The hit DNA contigs were analyzed and the obtained database of translated
proline-rich cathelicidins was classified into structural families10: Bac4-, Bac5-, Bac6-, Bac7-, P9-, ChBac3.4-, prophenin-like peptides, and several additional ones. A widespread family of
relatively short AMPs (28-30 residues long) was chosen for further study. The discovered peptides are similar in length to cetacean Bac7-like peptides, but lack the conserved consensus
fragment (R/K)XX(R/Y)LPRPR required for strong binding of class I PrAMPs in the NPET11,12. We reasoned that the peptides belonging to this family might have unique binding site(s) and a
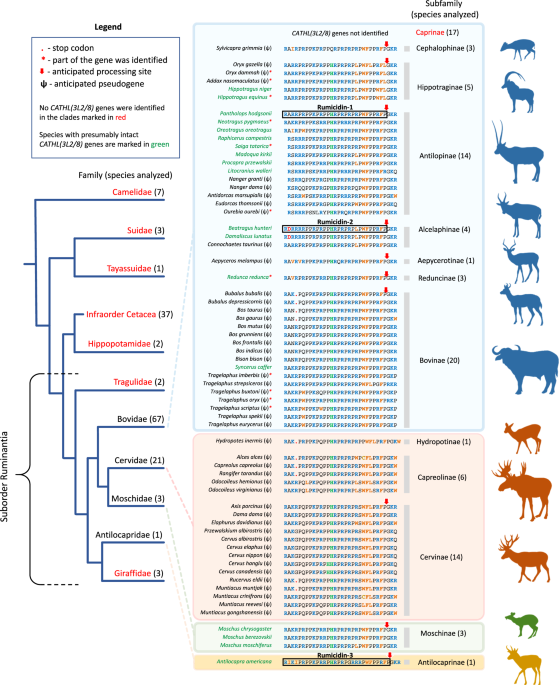
distinct mechanism of action. In this study, 147 Cetartiodactyla species were analyzed, and 65 of them were found to have these cathelicidin genes (Fig. 1). The genes were found only in
ruminants: in representatives of almost all subfamilies of bovids, as well as in musk deers, pronghorn, and cervids. Previously, in refs. 13,14, paralogous pseudogenes named _CATHL3L2_ and
_CATH8_ were described in the _Bos taurus_ genome, which encode preprocathelicidins belonging to this family. Therefore, the discovered ruminant genes we designated as _CATHL(3L2/8)_. Among
the found sequences, the major part belonged to pseudogenes (ψ), and only a quarter of the species (18 of 65) had presumably intact genes which displayed all characteristics of functional
cathelicidin genes (Supplementary Fig. 1, SUPPLEMENTARY DISCUSSION). Recently, the expression of one of _CATHL(3L2/8)_ genes has been shown in the musk deer _Moschus berezovskii_15. We also
performed de novo assembly of transcriptomes of several ruminant species and found target sequences (Supplementary Table 1). Analysis of ψ_CATHL(3L2/8)_ pseudogenes revealed different
variants of gene inactivation, including nonsense mutations (premature termination codon, indel) as well as start codon mutations (Supplementary Fig. 2 and Supplementary Data 1). The family
of PrAMPs encoded by _CATHL(3L2/8)_-like genes was named as rumicidins (RUMInant catheliCIDINS). In this study, we investigated three peptides: the rumicidin family consensus peptide
identical to the peptide from the Tibetan antelope chiru _Pantholops hodgsonii_ named rumicidin-1 and the most structurally distant orthologs identified in the genomes of the African
antelope hirola _Beatragus hunteri_ and the American pronghorn _Antilocapra americana_ and named as rumicidin-2 and rumicidin-3, respectively (Fig. 2a). Well-studied proline-rich
cathelicidins mini-ChBac7.5α, Bac7[1-22], and PR-39[1-22] were chosen as the reference class I PrAMPs (Fig. 2a). These truncated variants were shown to retain antibacterial activity of the
wild-type peptides16,17. PrAMPs used in this study were produced in _E. coli_ BL21 (DE3) cells using lactose-based autoinduction medium18. To facilitate the purification process and improve
the final yield, the recombinant peptides were obtained as fusion proteins with the _N_-terminal His-tag and thioredoxin A. After cleavage of the fusion proteins with cyanogen bromide, the
corresponding mature PrAMPs were purified by reversed-phase high-performance liquid chromatography (RP-HPLC) to reach a purity of ≥98%. Final yields of the recombinant cathelicidins and
their analogs ranged from 3 to 12 mg per 1 L of the culture medium. The obtained peptides were analyzed by Tricine-SDS-PAGE (Supplementary Fig. 3) and MALDI-TOF mass-spectrometry
(Supplementary Table 2). RUMICIDINS EFFECTIVELY INHIBIT THE ELONGATION STAGE OF PROTEIN SYNTHESIS IN BACTERIA Taking into account the previously reported data on binding of the PrAMPs to the
70S ribosome, we first tested the ability of rumicidins to inhibit protein biosynthesis in vitro (Fig. 2b). The experiment was carried out using _E. coli_ BL21(DE3) Star cell-free protein
synthesis system (CFPS, coupled transcription/translation system) expressing the enhanced green fluorescent protein (EGFP). Interestingly, high structure variability of the _N_-termini of
the studied peptides did not significantly alter the CFPS inhibition profile. We also tested the ability of rumicidins to suppress translation of the luciferase mRNA in vitro. It was shown
that all the peptides almost fully and rapidly inhibited the process at the concentration of 5 µM (Fig. 2c). To verify the proposed mode of action of rumicidins in live bacteria, we tested
their activity using an _E. coli_-based double-reporter system (Fig. 2d). As expected, rumicidins like Bac7[1-22] and erythromycin strongly induced biosynthesis of Katushka2S, but not of
RFP, which indicated that these peptides inhibited protein synthesis in vivo as well. To identify the step of translation specifically inhibited by rumicidins, we used a primer-extension
inhibition (toe-printing) assay. The addition of rumicidins as well as Bac7[1-22] at a concentration of 5 µM (5×MIC, minimum inhibitory concentration) to a PURExpress cell-free
transcription-translation system programmed with the ermCL mRNA19 resulted in the ribosome stalling at the AUG start codon (Fig. 2e). Similar patterns were shown by toe-printing with RST1
mRNA20 (Supplementary Fig. 4). To check an ability of rumicidins to influence formation of the first peptide bond, we compared the production of fMet-[14C]Val dipeptide on intact or
inhibited with rumicidin-1 or amicoumacin A ribosomal complexes (Supplementary Fig. 5). Antibiotic amicoumacin A is known for its multifaceted action on the ribosome with major effect on
translocation ensuring unaltered reactions of A-site binding and peptide bond formation21. The addition of 1 µM rumicidin-1 to the functional ribosomal complex severely impaired the reaction
resulting in essentially no dipeptide formed (7 ± 1%). At the same time, amicoumacin A containing ribosome complexes were as effective in the reaction of peptide bond formation as intact
complexes with the amount of dipeptide formed equal to 72 ± 7% and 77 ± 5%, respectively. Taken together, our results suggest that rumicidins display their antibacterial activity via
inhibition of protein biosynthesis. In particular, they block the first peptide bond formation and arrest elongation similar to other known class I PrAMPs. THE _N_-TERMINAL REGIONS OF
RUMICIDINS ARE NOT ESSENTIAL FOR TRANSLATION INHIBITION To verify roles of different structural elements of rumicidins both in translation inhibition and in antibacterial action, we
performed a structure-activity relationship (SAR) study of rumicidin-1 (Fig. 3). Surprisingly, the IC50 values of the truncated analogs [residues 1-16] and [residues 1-22] are 100- and
10-fold higher, respectively, compared to the full-length rumicidin-1 [residues 1-29], in distinction from Bac7, for which the first 16 residues retain a high efficiency of translation
inhibition11,22. Interestingly, the presence of the terminal extra (PR)3 fragment provides a significant inhibition effect of the analog 1-22 (IC50 of 7.2 ± 0.3 µM) as compared with that of
the analog 1-16 (IC50 of 44.6 ± 9.8 µM). Previous studies revealed that poly-PR peptides strongly bound to the polypeptide tunnels of both 70S and 80S ribosomes23. Notably, the deletion of
four _N_-terminal residues (RRIR) in the peptides Bac7[5-23] and Bac7[5-35] greatly diminished their antimicrobial activity against _E. coli_ strains ( ≥16-fold increase in MIC), albeit a
sufficient length was the case17. This highly cationic fragment is important for both high affinity binding of Bac7 to the ribosome and its penetration inside bacterial cells5. The effect of
_N_-terminal shortening of rumicidin-1 was not so marked: activities of the analogs 4-29 and 6-29 decreased only two-fold, while the MIC values of the analogs 9-29 and 11-29 against _E.
coli_ were ≥4-fold higher than those of the wild-type rumicidin-1. While both terminal analogs 1-22 and 9-29 were able to inhibit translation that was shown both in vitro (Fig. 2c;
Supplementary Fig. 6a) and in vivo (Fig. 2d), the latter peptide was as effective as the full-length rumicidin-1. This points to key role of contacts between the ribosome and _C_-terminal
residues of rumicidin-1 but not with _N_-terminal ones. As expected, the addition of the rumicidin-1 analog 9-29 led to a strong AUG toe-print signal comparable to that of the wild-type
peptide, whereas a weak band was observed for the _N_-terminal peptide analog 1-22 (Supplementary Fig. 4). Thus, the _N_-terminus is likely needed for cellular uptake rather than for the
ribosome binding, with the length of the terminus correlating with an antibacterial activity. Interestingly, the shortest _C_-terminal peptide analogs 13-29 and 15-29 were almost unable to
inhibit both bacterial translation and cell growth, which also indicated an important role of amino acid residues in the central part of the peptide for interaction with the ribosome.
However, the findings left open the question regarding the orientation of rumicidins in the NPET. RUMICIDINS COMBINE THE BINDING MODE OF SEVERAL KNOWN PRAMPS To determine the mode of binding
of rumicidins to the _E. coli_ 70S ribosome, we obtained a cryo-EM structure of rumicidin-2 bound to the functional mRNA-programmed ribosomal complex with fMet-tRNAfMet in the P site. This
peptide has a unique _N_-terminal part and the lowest homology with known PrAMPs among all representatives of this structural family. Focused refinement of the 50S subunit yielded a 1.95 Å
cryo-EM density map (Supplementary Fig. 7, Supplementary Table 3). As expected from structural studies of other PrAMPs, rumicidin-2 binds to the 70S ribosome within the exit tunnel of the
50S, which serves as the path for a nascent chain (Fig. 4a). A distinct density observed within the ribosomal exit tunnel could be unambiguously assigned to residues 12-27 of rumicidin-2
bound in an extended conformation. Rumicidin-2 enters the exit tunnel in a reversed orientation relative to a nascent polypeptide chain (Fig. 4b) and utilizes multiple hydrogen bonding,
stacking and van der Waals interactions to bottle it up (Fig. 4e, f). Our structure presents high-resolution details for the core regions of the 50S and well-resolved parts of the
rumicidin-2. The most pronounced variability, including the number and composition of amino acid residues, was observed for the _N_-terminal parts of PrAMPs. The _N_-terminal part of
rumicidin-2 consists of 11 amino acid residues, being the longest among all visualized PrAMPs. Although no density could be seen for this part of the peptide, presumably due to its mobility,
according to structure similarity with other peptides we assume that rumicidin-2 reaches into the A-tRNA binding pocket (Fig. 4c, d, Supplementary Fig. 8). The _C_-terminal parts of all
previously described oncocin-like peptides do not appear to make any specific contacts with the ribosome. Surprisingly, we have found that rumicidin-2 has a clear density protruding into the
depth of the exit tunnel interacting with its constriction point (Fig. 4f). The lower part of a spacer in a constriction site is formed by Trp23 and Phe24, which is a structural feature of
all rumicidins. Backbone-carbonyl oxygen of Pro22 forms a hydrogen bond with the Arg61 of the ribosome protein uL4 while the aromatic ring of Trp23 forms stacking interaction with nucleotide
A751 of helix H34 of 23S rRNA and, in addition, hydrogen bonds with the carbonyl oxygen of Lys90 of the ribosomal protein uL22 (Fig. 4f). Thus, our data suggest that the extended loop of
uL22 not only stabilizes the position of H34 backbone, facilitating the binding of peptide and 23S rRNA, but also interacts with rumicidin-2 directly. Phe24 stacks upon Thr65 of the
ribosomal protein uL4 via C-H⋯π-interactions, narrowing down the constriction of the exit tunnel together with the Trp23 (Fig. 4f). Interestingly, insect-derived class II PrAMP, Api137 has a
similar binding pattern despite the opposite orientation of the peptide in the tunnel6: Tyr7 stacks upon nucleotide A751 of H34 of 23S rRNA, while Arg10 forms a hydrogen bond with the
residue Arg61 of the ribosomal protein uL4. At the end of the _C_-terminal part, carbonyl oxygen of Pro25 forms hydrogen bonds with Arg67 (uL4), which rotates its side-chain towards the
backbone of rumicidin-2, although two alternative conformations of the Arg67 were observed in the structure. Due to high sequence resemblance with other oncocin-like peptides, the central
part of rumicidin-2 (residues 12-22) almost perfectly aligns with the residues 7-17 in Bac7[1-19]2,5 and thus makes similar contacts with the rRNA (Fig. 4d). His14 intercalates into the
A-site cleft (Fig. 4e), a hydrophobic pocket formed by the nucleobases of A2451 and C2452, and occupies similar position to Arg9 in Bac7 and Tyr6 in Onc11224 while forming H-bond with the
O2′ atom of the ribose G2505. Side-chain of Arg15 forms a hydrogen bond with the backbone phosphate of G2505 closely resembling a position of the Leu7 in Onc112. Multiple bonds are formed
between backbone amino/carbonyl groups and 23rRNA. Pro13 may form a hydrogen bond with U2585, although the latter is not sufficiently resolved. Backbone-carbonyl oxygen of Pro14 forms a
hydrogen bond with G2061, while both backbone oxygen and nitrogen of Arg15 form hydrogen bonds with U2506. In the upper tunnel, Arg17 stacks upon C2610, reaching the backbone phosphate of
U2609 identically to the Arg12 of Bac7 and Arg9 of Onc112, while backbone carbonyl oxygen forms a hydrogen bond with A2062. Clear density is observed for the side-chain of Arg19 and A2062
which interact by π-stacking identically to Arg16 of Bac7, implying an additional stabilization of the A2062 by this interaction. Contrary to other relatively well-defined arginine residues
in the central part, we have found no density in the map for the side-chain of Leu21. This lack of coordination may arise as Leu and Arg are used interchangeably at this location in
rumicidins (Supplementary Fig. 1). MECHANISMS OF BACTERIAL RESISTANCE TO RUMICIDIN-1 The selection of resistant strains makes it possible to shed light both on the key targets of the
antibiotic and on the mechanisms of protection against it in bacteria. At the first stage, we set a goal to obtain bacteria resistant to rumicidin-1 using the _E. coli_ SQ110LPTD strain
which has compromised outer membrane and lacks 6 of 7 chromosomal _rrn_ alleles encoding for rRNAs20. As a result, the final MIC value did not differ more than two-fold from the initial even
after 20 passages in the Mueller-Hinton broth (MHB) supplemented with rumicidin-1. Next, we applied _E. coli_ 1057 strain22 when performing resistance induction experiments (Supplementary
Fig. 9a). This strain carried two well-characterized mutations in _gyrA_ (S83L and D87N, Supplementary Fig. 10) causing a high-level fluoroquinolone resistance as well as a
higher-than-normal spontaneous mutation rate25. Notably, ≥ 8-fold increase in the MIC value was registered after several passages in the medium containing 0.9% NaCl subjected to selection by
PrAMPs. In this work, we identified _sbmA_ frameshift (Δ2 bp, the strain R1) in the case of rumicidin-1 (Supplementary Table 4, Fig. 5a). Earlier, we found the V102E substitution in SbmA of
the _E. coli_ 1057 strain after treatment by caprine mini-ChBac7.5Nα22. SbmA is a homodimer proton-driven transporter found amongst some classes of Proteobacteria, which is involved in the
uptake of non-ribosomal peptide antibiotics, bacteriocins, and PrAMPs26,27. Obviously, this transporter is under strong selective pressure when salt-containing media is used. Our previous
studies showed that in the absence of the salt in the medium PrAMPs were able to effectively act against strains with mutant SbmA22,28. Here, we found that rumicidin-1 also had the same MICs
of 1 µM against both BW25113 and its Δ_sbmA_ variant in salt-free MHB. Indeed, the absence of 0.9% NaCl in the media greatly affects both the dynamics and main molecular targets of
selective pressure under PrAMPs treatment (Supplementary Fig. 9a). We found no changes in MIC for mini-ChBac7.5Nα and 16-fold increase in MIC for rumicidin-1 (the strain R2). The
whole-genome sequencing of the strain R2 (Fig. 5a) showed the point mutation in the _macB_ gene [N470D] and the nonsense point mutation in the _rpoS_ gene [Q3stop] (Supplementary Table 4).
RpoS is a stress sigma factor that controls the expression of many genes implicated in the survival of the cell under suboptimum or stressful growth conditions29. Here, we found that _E.
coli_ BW25113 Δ_rpoS_ was even 2-fold more susceptible to PrAMPs as compared to the wild-type strain. MacB is an ABC transporter that collaborates with the MacA adapter protein and the TolC
exit duct and, in this way, provides an efflux of antibiotics out of the bacterial cell30. The MacB transmembrane domain lacks a central cavity through which antibiotics could be passed
inside the cell but conveys conformational changes upon ATP binding30. Indeed, the gene knockout of either _macA_ or _macB_ resulted in a 4-fold decrease in MIC for rumicidin-1. Perhaps,
MacAB-TolC can efflux PrAMPs from periplasmic space, while the N470D substitution in the MacB seems to increase the selectivity of the transporter to AMPs (Supplementary Fig. 11AND
SUPPLEMENTARY DISCUSSION). To test this hypothesis, we checked the activity of the BW25113 Δ_rpoS_ strains having additional plasmid-borne allele encoding either the wild-type MacB or the
mutant MacB[N470D]. The target genes were expressed using three variants of complementation plasmids: under the strong constitutive artificial promoter J2311931, or the inducible leaky
hybrid T5lac promoter, or the tightly regulated arabinose promoter (Supplementary Fig. 12). In all three cases, we found a 4-fold increase in MIC for the strain expressing mutant variant of
the MacB domain as compared with the wild-type (the observed increase in MIC from 4 to 16 µM for J23119 is presented in Fig. 5a,b). RUMICIDINS HAVE A WIDE SPECTRUM OF ANTIBACTERIAL ACTIVITY
To estimate the therapeutic potential of rumicidins as candidate compounds for combating bacterial infections, we assessed their antimicrobial activity against a wide panel of strains. The
resulting MIC heatmap is shown in Fig. 6a. As expected, antibacterial activities of rumicidins, like those of known PrAMPs, decreased in most cases in the presence of salt, which might
inhibit the adsorption of the peptides to the bacterial surface. Besides, their activities against Gram-negative bacteria deficient in SbmA protein (_Pseudomonas aeruginosa_ and _Proteus
mirabilis_) were also less pronounced. Among all tested PrAMPs, rumicidin-3 from _A. americana_ was of particular practical interest, having a minimal median MIC of about 2 µM (Fig. 6a).
Notably, rumicidins also exhibited significant activities against Gram-positive bacteria, in particular micrococci, bacilli, and mycobacteria. All rumicidins have a similar activity against
_Mycobacterium phlei_ with MICs of 0.25-1 µM in the salt-free MHB and of 4-8 µM in the medium supplemented with 0.9% NaCl. We also accessed activities of the peptides against another
fast-growing mycobacterial strain _M. smegmatis_ mc(2)155 and found rumicidin-1 to be the most active variant with MICs of 0.125-0.5 µM depending on the test medium (Supplementary Table 5).
Activities of two other rumicidins were at least 4-fold lower. As PR-39 is the only described PrAMP having an activity against mycobacteria (MIC ~ 10 µM32), we used its analog PR-39[1-22] as
a reference peptide. Notably, similar MICs of 8–16 µM were determined when testing activities against _M. smegmatis_ (Supplementary Table 5). In total, these preliminary data make
rumicidin-1 a comparatively effective and promising antimycobacterial agent as well. Considering the presence of aromatic Trp and Phe residues in the structure of all rumicidins, we
investigated the ability of these peptides to damage _E. coli_ membranes. The effect of rumicidin-1 on the cytoplasmic membrane was quite modest (less than 20% of permeabilized cells) even
at a concentration of 64 µM, which is 16-fold higher than the MICs against _E. coli_ (Supplementary Fig. 13). The obtained results agree with the previous data on several cetacean
ribosome-targeting PrAMPs, which did not alter cytoplasmic membrane permeability at MICs12. Notably, we found no significant differences in the action of rumicidin-1 and Bac7[1-22] on both
the outer and inner membrane of _E. coli_ (Supplementary Fig. 14). Interestingly, both peptides at concentrations ≥ MICs were shown to be quite effective in damaging the outer membrane of
bacterial cells washed with sodium phosphate buffer, whereas the addition of salts at physiological concentrations, in particular, 0.9% NaCl and/or divalent cations (0.5 mM Ca2+ and 0.5 mM
Mg2+) minimized the activity. Perhaps, translocation of rumicidin-1 into the periplasmic space depends on electrostatic interaction with the outer membrane followed by porin-mediated uptake
(the _ompF_ gene knockout resulted in a 4-fold increase in MIC, Fig. 5a). CYTOTOXIC AND IMMUNOMODULATORY ACTIVITY OF RUMICIDIN-1 It is known that most PrAMPs have no pronounced toxicity
toward mammalian cells12. A quite effective inhibition of eukaryotic ribosome translation by the truncated analogs of Bac7 was shown5. At the same time, the absence of toxicity against HaCaT
and erythrocytes was demonstrated for the Bac7[1-35]12. Therefore, the antibacterial selectivity of the peptide is achieved due to a low efficiency of penetration into mammalian cells
rather than to a low affinity for 80S and mitochondrial ribosomes. In this study, we showed that rumicidins as well as the control peptide Bac7[1-22] lysed less than 2% of red blood cells
(RBCs) at the concentration of 128 µM (Supplementary Fig. 15). Very modest cytotoxic effects of the peptides against HaCaT (IC50 > 128 µM) and HEK293T (IC50 ~ 128 µM) cell lines were
observed as well (Fig. 6a). Membrane-active PrAMPs were shown to undergo significant conformational changes when transiting from bulk solution into membrane-mimicking environments12. In
contrast, circular dichroism (CD) spectra of rumicidin-1 were similar in water and zwitterionic dodecylphosphocholine (DPC) micelles (Supplementary Fig. 16), possibly suggesting the absence
of specific interactions with neutral mammalian membranes. Cathelicidins are known to have different immunomodulatory effects on various types of cells33. Given the absence of proline-rich
cathelicidins in humans and, therefore, the likelihood of unpredictable effects on the human immune system, next, we assessed the effect of rumicidin-1 at a potential therapeutic
concentration of 2 µM in blood stream on the expression profile of key cytokines and chemokines by immune and epithelial cells (SUPPLEMENTARY DISCUSSION, Supplementary Fig. 17, Supplementary
Table 6). The peptide was shown to display both pro- and anti-inflammatory action similar to human cathelicidin LL-37. Expectedly, the most significant effects on human cells were
specifically triggered by LL-37. However, we also found a number of pro-inflammatory effects unique to rumicidin-1. In particular, it is able to significantly induce production of the
chemotactic IL-8/CXCL8 by the epithelial Caco-2 cells as well as to inhibit the soluble interleukin-1 receptor antagonist (IL-1RA) production by different cell lines. Together, our data
indicate the lack of significant adverse immunomodulatory effects of rumicidin-1 towards human cells in vitro. RUMICIDINS SHOW AN EFFICACY IN THE MOUSE SEPTICEMIA MODEL Next, we examined an
efficacy of rumicidins in the mouse septicemia model (Fig. 6b). In comparison with membrane-active peptides, PrAMPs are known as low-toxic substances having maximum tolerated doses (MTD) of
above 50 mg/kg34,35. Here, we used two peptides – rumicidin-1 and -3 and applied each of them three times within the first day after infection. Each injection of rumicidin-1 was
administrated at the dosage of 10 mg per kg of body weight and of 5 mg/kg for rumicidin-3 as the most active variant. Intraperitoneal infection of BALB/c mice with _E. coli_ ATCC 25922 in
the presence of mucin resulted in the death of all 5 mice within two days if treated using the vehicle control (saline) and survival of all mice when treated with 10 mg/kg ciprofloxacin. We
demonstrated a therapeutic efficacy of 80% and 60% when applying the triple dose of rumicidin-1 and rumicidin-3, respectively, after one-week experiment. Our results are comparable to those
for Bac7PS - a lead candidate compound selected by high-throughput screening of Bac7 analogs35. These data suggest that rumicidins are promising natural compounds for developing
antimicrobials. DISCUSSION Many animal genomes contain several distinct AMP gene families. As a result, host organisms deploy them in synergistic mixtures that minimize the probability of
evolution of bacterial resistance in nature36, and PrAMPs are the key ingredients in these cocktails in artiodactyls and insects22,37. Here, we discovered and investigated a family of
ribosome-targeting PrAMPs named rumicidins. The high distribution of corresponding orthologous genes among ruminants suggest their appearance before the split of pronghorns and other
pecorans (all ruminants excluding Tragulidae) at the Oligocene/Miocene boundary38,39. Our results obtained both in vitro (Fig. 2b, c) and in vivo (Fig. 2d) suggested that rumicidins exerted
their antibacterial activity via inhibition of protein biosynthesis. In particular, they inhibit the first peptide bond formation and block the elongation stage of translation, similarly to
other known oncocin- and Bac7-like PrAMPs. Notably, complete translational inhibition was achieved at concentrations equal to or even lower than the MIC values against different _E. coli_
strains. We cannot fully exclude any secondary intracellular targets (e.g., chaperone DnaK3), but in total, the obtained data allowed us to consider the ribosome as the main molecular target
of rumicidins, which was also confirmed by cryo-EM data (Fig. 4). The structure of rumicidins consists of two functional parts: its central region enriched with PR repeats (which are
essential for a peptide binding within the NPET23) is crucial for translation inhibition, whereas the _N_-terminal residues are likely to be important for the uptake of the peptide and
provide low MICs against _E. coli_. Notably, these findings are in contrast to features of the known Bac7- and Bac5-like cathelicidins where the _N_-terminal residues are essential for
ribosome binding5,40,41. The density was observed for the _C_-terminal fragment [12-27] in the case of rumicidin-2 (Fig. 4c), rationalizing our SAR data (Fig. 3). This fragment appears to be
consistent with the sequence of the pharmacophore essential for the on-target activity: while the analog 11-29 is still able to impede protein synthesis and stop cell growth at a relatively
low concentrations ( ≥8 µM, Fig. 3, Supplementary Fig. 6a), a subsequent shortening of rumicidin-1 leads to a dramatic loss of activity and an increase in MICs. Superpositioning of
structures of rumicidin-2 and other PrAMPs bound to bacterial ribosomes provides insight into the consensus sequence (R/K)XX(R/Y)LPRPR of oncocin- and Bac7-like peptides (class I PrAMPs)
localized in the A-site cleft and the upper tunnel. According to the alignment shown in Fig. 2a, the key functional site Arg/Tyr-Leu changed to His-Arg in the case of rumicidins. The
incorporation of Arg-Leu instead of His-Arg at the key site as well as the point substitution [H14A] did not influence the activity of rumicidin-1. This is in sharp contrast with the short
PrAMPs like oncocin or Bac7[1-16], where amino acid residues in conservative sites were not tolerable even to a single substitution11,42. Next, we assessed a role of the conservative dyad
Trp23-Phe24 forming key stacking interactions in the constriction site. Surprisingly, the analog of rumicidin-1 with a single substitution [W23A] exhibits a similar activity against _E.
coli_ in the salt-free MHB and strongly inhibits protein biosynthesis in vitro. A further modification of the dyad by a double substitution [W23A, F24A] resulted to a slight activity
decrease. A 4-8-fold rise in MICs of both analogs in the test medium with an increased ionic strength is likely resulting from a weaker ability to interact/penetrate cell membranes (Fig. 3,
Supplementary Figs. 13,14). At the same time, the effectiveness of inhibition of protein biosynthesis is reduced significantly for the analog [W23A, F24A] as compared to the wild-type
peptide (Supplementary Fig. 6b), that points at a dual function of this hydrophobic fragment. A more pronounced decrease in both activities was found for the shortened analog 9-29 with a
similar [W23A, F24A] modification of the dyad. To destabilize the interaction of the dyad in the tunnel, the Pro22 residue was also substituted with alanine, however only a 2-fold increase
in MIC was detected. We can assume that extensive interactions with the 50S ribosomal subunit make rumicidins highly tolerable to amino acid substitutions (even at the key sites according to
our cryo-EM data), which opens the door to further rational design of new analogs. We did not find any mutations in ribosomal genes in the obtained rumicidin-resistant _E. coli_ strains,
which confirmed the high efficiency of multisite binding of these extended peptides in the NPET. Moreover, no increase in MIC was observed for _E. coli_ strains bearing single nucleotide
substitutions in the 23S rRNA, in particular, A2058G or U2584C, which caused resistance to macrolide antibiotics, or klebsazolicin and some oncocin-like insect PrAMPs, respectively3,27. On
the other hand, resistance to rumicidins is mediated by the proteins like SbmA involved in their uptake. Here, we also report an example of the inducted mutation that increased the
selectivity of MacAB-TolC efflux pump to rumicidins and other PrAMPs. The wild-type and obtained resistant strains showed similar growth rates (Supplementary Fig. 9b), therefore the
physiological costs of evolving resistance to rumicidin-1 in a rich medium appear to be minimal. However, the detailed mechanism conferring the mutant MacB[N470D] resistance and its
influence on the strain virulence is unclear and requires an additional study. Surprisingly, a strong cross-resistance of this mutant against ‘last resort’ antibiotic polymyxin B was
detected as well (Supplementary Table 7). A 64-fold rise in MIC was shown to be comparable with the effect of MCR-1-mediated colistin resistance reported previously43. While this
cross-effect is not extended to a key human cathelicidin LL-37 and many other cationic AMPs as well as to different conventional antibiotics (Supplementary Table 7), it should be carefully
studied before the potential use of PrAMPs as therapeutics. Together, these results show MacAB-TolC has a broader role in antimicrobial adaptation than previously thought and is not limited
to the efflux of polymyxins, bacitracin, and macrolides44. In total, the obtained data on biological activity indicate a significant antibacterial selectivity which is an important advantage
of PrAMPs over many other membrane-active cationic AMPs. Rumicidins have a reasonable activity against mycobacteria and a range of important Gram-negative bacteria that belong to so-called
“ESKAPE” pathogens45. Moreover, the efficacy of rumicidins was proved in the mouse lethal septicemia model induced by _E. coli_. We believe that the obtained detailed information about the
discovered original peptides interacting with the ribosomal tunnel would provide insights for the rational design of more potent translation inhibitors, in particular, chimeric peptides
carrying the structural elements of rumicidins and other known PrAMPs. METHODS IDENTIFICATION OF CATHL GENES IN CETARTIODACTYLA WGS DATABASE The TBLASTN program was used to identify
cathelicidin genes in the whole-genome sequencing (WGS, GenBank) database using conservative cathelin-like domain (CLD) fragment FTVKETVCPRTSPQPPEQCDFKE encoded by the nucleotide sequence
located in the second exon of the cattle procathelicidin-3 (Bac7 precursor, GenBank: NP_776426.1) as a query against all Cetartiodactyla WGS projects deposed in NCBI using the values of the
default parameters (matrix: BLOSUM62, gap costs: existence 11, extension 1). Then, the obtained hit DNA contigs ( ± 3000 bp relative to the query) were analyzed to identify exons within the
genomic sequence. The putative elastase processing sites in the fourth exon were predicted based on information about known Cetartiodactyla cathelicidins and also using the ExPASy peptide
cutter with neutrophil elastase (http://web.expasy.org/peptide_cutter/). Finally, putative mature cathelicidin sequences were manually (visually) inspected and additionally analyzed by
blasting against AMP databases to classify them. BACTERIAL STRAINS The following strains were utilized: _Escherichia coli_ BL21 (DE3) (Novagen), _E. coli_ BL21 (DE3) Star (Novagen), _E.
coli_ DH10B (Invitrogen), _E. coli_ ML-35p, _E. coli_ ATCC 25922, _Klebsiella pneumoniae_ ATCC 700603, _Pseudomonas aeruginosa_ ATCC 27853, _Staphylococcus aureus_ ATCC 6538 P, _S. aureus_
ATCC 29213, _Bacillus subtilis_ B-886, _Micrococcus luteus_ B-1314, _Mycobacterium phlei_ Ac-1291. The _Mycobacterium smegmatis_ strain mc(2)155 was kindly provided by Dr. Tatyana L.
Azhikina (M.M. Shemyakin & Yu.A. Ovchinnikov Institute of Bioorganic Chemistry, Russia). The strain _E. coli_ SQ110 (Δ_rrnA_, Δ_rrnB_, Δ_rrnC_, Δ_rrnD_, Δ_rrnG_, Δ_rrnH_) and its
modified variants _E. coli_ SQ110DTC (Δ_tolC_), and _E. coli_ SQ110LPTD were from20. The strain _E. coli_ BW25113 and its knockout variants Δ_sbmA_, Δ_mdtM_, Δ_ompF_, Δ_rpoS_, Δ_macA_,
Δ_macB_ were from the Keio collection46. The antibiotic-resistant strains _Enterobacter cloacae_ XDR CI 4172, _Acinetobacter baumannii_ XDR CI 2675, _Proteus mirabilis_ XDR CI 3423, and _E.
coli_ MDR CI 1057 (_E. coli_ 1057, SRCAMB collection № B-10910) were collected and provided by the I.M. Sechenov First Moscow State Medical University hospital. The detailed characteristics
of utilized clinical isolates are presented in ref. 22. EXPRESSION AND PURIFICATION OF THE ANTIMICROBIAL PEPTIDES Nucleotide sequences were designed based on _E. coli_ codon usage bias data.
At the first stage, the recombinant plasmids for the expression of _N_-terminal fragments of rumicidins (Rum-1[1-22], Rum-2[1-22], Rum-3[1-23]), Bac7[1-22], and PR-39[1-22] were
constructed. Briefly, the AMP-coding sequences were generated by the annealing of partially self-complementary oligonucleotides (Supplementary Table 8) followed by PCR and cloning in the
pET-based vector as described previously22. The expression plasmids encoding full-length rumicidins and rumicidin-1 modified analogs were then obtained using ligation independent cloning
procedure47 (Supplementary Table 8). The expression cassette was composed of the T7 promoter, the ribosome binding site, and the sequence encoding the chimeric protein that included His-tag,
the _E. coli_ thioredoxin A with the M37L substitution (TrxL), methionine residue, and a target peptide. _E. coli_ BL21 (DE3) cells were transformed with corresponding plasmids and grown up
from an initial OD600 of 0.01 for 24 h at 30 °C under stirring in a shaker at a speed of 220 rpm in ZYP-5052 auto-inducing medium based on lysogeny broth (LB) supplemented with 0.2%
lactose, 0.05% glucose, 0.5% glycerol, 1 mM MgSO4, 50 mM Na2HPO4, 50 mM KH2PO4, 25 mM (NH4)2SO4, 100 µg/mL of ampicillin, and trace metals according to Studier18. The cultured cells were
harvested by centrifugation and sonicated in the 100 mM phosphate buffer (pH 7.8) containing 20 mM imidazole and 6 M guanidine hydrochloride. The clarified lysate was loaded on a column
packed with Ni Sepharose (GE Healthcare). The recombinant protein was eluted with the buffer containing 0.5 M imidazole. The eluate was acidified (to pH 1.0) by the concentrated hydrochloric
acid, and the fusion protein was cleaved by a 100-fold molar excess of CNBr over the number of methionine residues at 25 °C for 18 h in the dark. The lyophilized products of the cleavage
reaction were dissolved in water and loaded on a semi-preparative Reprosil-pur C18-AQ column (10 × 250 mm2, 5-µm particle size, Dr. Maisch GmbH). RP-HPLC was performed with a linear gradient
of acetonitrile in water containing 0.1% TFA. The peaks were collected and analyzed by MALDI-TOF MS using Reflex III mass-spectrometer (Bruker Daltonics). Purity of the obtained recombinant
peptides was monitored using Tricine-SDS-PAGE in 16.5% gel containing 6 M urea48. The obtained fractions with corresponding molecular masses (Supplementary Table 2) were dried _in vacuo_
and dissolved in water. Cathelicidin LL-37, melittin, and VicBac (of >98% purity for all the peptides) were synthesized using a standard solid-phase method and were kindly provided by Dr.
Maxim N. Zhmak and Dr. Sergey V. Sychev. ANTIMICROBIAL ASSAY Minimum inhibitory concentrations (MIC) were determined by broth microdilution assay based on Clinical and Laboratory Standards
Institute (CLSI) guidelines using BSA in the growth medium to minimize AMP nonspecific adsorption49. Bacterial test cultures were grown in the Mueller-Hinton broth (MHB, Sigma) at 37 °C to
mid-log phase and then diluted with the 2× MH medium supplemented with 1.8% NaCl (or without salt) so that to reach a final cell concentration of 106 CFU/mL. 50 µL of the obtained bacterial
suspension were added to aliquots of 50 µL of the peptide solutions serially diluted with sterilized 0.1% bovine serum albumin (BSA) in 96-well flat-bottom polystyrene microplates (Eppendorf
#0030730011). The optical density (OD570) of each well was measured, and the lowest concentration of the tested compound that did not result in bacterial growth after incubation for 24 h at
37 °C and 950 rpm was determined as MIC. To verify MIC values, the respiratory activity of the bacteria was determined. Briefly, 5 µL of 0.5 mg/mL resazurin (Sigma) was added to the wells
after 24 h (or after 48 h for _M. smegmatis_) of incubation, and the plate was incubated for additional 3 h. In some cases, MICs were determined in the Lysogeny Broth (LB) or Middlebrook 7H9
medium. The reduction of resazurin to resorufin was recorded. In most cases, no significant divergence of MIC values was observed (within ±1 dilution step). The results were expressed as
the median values determined based on at least three independent experiments performed in triplicate. SELECTION OF RESISTANT BACTERIAL STRAINS Resistance induction experiments were performed
using the previously described method22. This approach allows for monitoring MIC values after each transfer. Briefly, on the first day, the overnight culture of the wild-type bacteria was
diluted with the 2× MHB supplemented with 1.8% NaCl (or without salt) to reach a final cell concentration of 106 CFU/mL. 50 µL of the obtained bacterial suspension were added to aliquots of
50 µL of the peptide solutions serially diluted with the sterilized 0.1% BSA in 96-well flat-bottom polystyrene microplates. After incubation for 20 ± 2 h at 37 °C and 950 rpm, MICs were
determined as described above. For each subsequent daily transfer, 2–4 μL of the inoculum taken from the first well containing a sub-inhibitory drug concentration was diluted with 2 mL of
the fresh 2× MHB supplemented with 1.8% NaCl (or without salt). Then, 50 µL of this suspension was sub-cultured into the next passage wells containing 50 µL aliquots of the peptide at
concentrations from 0.25× to 8–16× of the current MIC of each agent. Multiple repeated passages in the presence of antimicrobial agents were performed for each bacterial strain during the
experiment. Bacteria that grew at the highest concentrations of AMPs on the final day were passaged further 3 times on drug-free agar plates before determining the final MIC value. Control
serial passages in the absence of the agent were also performed, and the resulting cultures showed unchanged MICs against antibacterial agents. WHOLE-GENOME SEQUENCING OF BACTERIA To
identify potential mechanisms conferring resistance to rumicidin-1, we performed whole-genome sequencing of the corresponding strains followed by genomic DNA de novo assembly and variant
calling. The genome of wild-type _E. coli_ 1057 strain (SRCAMB collection № B-10910) was used as a reference28. 2 × 100 bp pair-end sequencing of the prepared genomic DNA was performed with
an Illumina NextSeq550 and MiSeq platforms (Illumina). Evaluation of read quality was performed using the FastQC software (v0.11.9)50, then reads were filtered, and adapters were cut with
TrimmomaticPE (v0.39)51. The SPAdes software (v3.13.0) was used to assemble genomes utilizing both filtered paired-end and unpaired reads52. Assembly quality was then evaluated with the
QUAST program (v5.0.2)53. Gene prediction and annotation of assembled contigs were made with the Prokka program (v1.14.6)54. Alignment of paired-end reads on reference genome was made using
the BWA-MEM (v0.7.17-r1188) algorithm55. To call actual variants, VarScan software (v2.4.0) was launched with a minimal reported variant frequency set to 0.956. To prove the obtained
whole-genome sequencing data, the analyzed genes (_sbmA_, _rpoS_, _macB_) were amplified by PCR using specific primers (Supplementary Table 8) and inserted into the pAL-2T vector (Evrogen).
The ligation products were transformed into the chemically competent _E. coli_ DH10B cells. The obtained plasmids were sequenced on both strands using the ABI PRISM 3100-Avant automatic
sequencer (Applied Biosystems). CONSTRUCTION OF COMPLEMENTATION PLASMIDS Three different plasmid vectors (pUC-based with the strong constitutive artificial promoter J2311931, the pQE30-based
vector with an IPTG/lactose-inducible hybrid T5lac promoter, and pBAD/myc-HisA with an arabinose-inducible promoter) were used for the preparation of the complementation plasmids
overexpressing MacB or MacB[N470D] in _E. coli_ BW25113 Δ_rpoS_. Two plasmids were obtained by ligase-independent cloning procedure47 using designed primer pairs (Supplementary Table 8).
Briefly, DNA parts were produced by PCR-amplification of vectors and target _macB_ gene from the wild-type _E. coli_ 1057 or rumicidin-1-resistant strain (R2) expressing the mutant MacB
variant. The DNA fragments purified by gel electrophoresis and having 22–23 bp overhangs were mixed with molar ratios of 2:1 (insert to linearized vector) and were subsequently transformed
into chemically competent _E. coli_ DH10B cells. pBAD-based complementation vectors were obtained by cloning the target genes with restriction enzymes NcoI and EcoRI followed by ligation and
transformation into _E. coli_ DH10B. Target plasmids (Supplementary Fig. 12) were isolated from individual clones and then analyzed by DNA sequencing. CELL-FREE PROTEIN EXPRESSION ASSAY To
investigate the effects of AMPs and antibiotics on the coupled transcription/translation process, the test compounds were added to a cell-free protein synthesis (CFPS) reaction mix with a
plasmid encoding enhanced green fluorescent protein (EGFP) variant (F64L, S65T, Q80R, F99S, M153T, and V163A) under control of the T7 promoter. The cell lysate was prepared using the _E.
coli_ BL21 Star (DE3) overexpressed T7 RNA polymerase as described previously22. The reaction mix consisted of the following components: 1.2 mM ATP, 0.8 mM UTP, 0.8 mM GTP, 0.8 mM CTP, 2 mM
of each of 20 proteinogenic amino acids, 1.5 mM spermidine, 1 mM putrescine dihydrochloride, 0.06647 mM calcium folinate, 170 ng/ml tRNA from the _E. coli_ MRE 600 strain, 0.33 mM NAD, 120
mM HEPES-KOH (pH 8.0), 10 mM ammonium glutamate, 175 mM potassium glutamate, 60 mM glucose, 15 mM magnesium glutamate, 2% PEG 8000, 25% _E. coli_ BL21 Star (DE3) cell lysate, 1 ng/ml plasmid
DNA. The reaction volume was 50 µL. The peptides were dissolved in PBS with the addition of 0.1% BSA. Erythromycin was used in the positive control reactions. The fluorescence of the sample
without inhibitor was set as the 100% value. The reaction proceeded for 2 h in 96-well v-bottom black polystyrene microplates (Eppendorf #0030601904) in a plate shaker (30 °C, 1000 rpm).
The fluorescence of the synthesized EGFP was measured with the microplate reader AF2200 (λEx = 488 nm, λEm = 510 nm; Eppendorf). The experimental data were obtained from at least two
independent experiments performed in triplicate. Non-linear regression curves were generated using GraphPad Prism v.8.0.1 (GraphPad Software Inc.). IN VITRO TRANSLATION INHIBITION ASSAY In
vitro translation reactions were carried out using a system of _E. coli_ S30 extracts for linear templates (Promega) in 5 µL with the addition of 100 ng of Fluc mRNA and 0.05 mM of
D-luciferin, preincubated for 5 min with 5 μM of the tested compounds. Chemiluminescence was measured every 30 s for 20 min at 37 °C using a VICTOR X5 Multilabel Plate Reader (Perkin Elmer,
USA). Two independent experiments were performed, and the curve pattern was the same. DETECTION OF THE TRANSLATION INHIBITORS WITH PDUALREP2 REPORTER For in vivo bioactivity test, the
reporter strain BW25113 Δ_tolC_-pDualrep2 was used as previously described57. Briefly, 10 µL of solutions of tested peptides (0.2 mM of full-length rumicidin isoforms; 0.5 mM of Rum-1[1-22]
and Rum-1[9-29]) were applied to an agar plate contained a lawn of the reporter strain. 5 mg/ml erythromycin (Ery, 1 µL) and 25 µg/ml levofloxacin (Lev, 1 µL) solutions were used as control
samples. After being incubated overnight at 37 °C, the plate was scanned by ChemiDoc (Bio-Rad) using “Cy3-blot” settings for RFP and “Cy5-blot” for the Katushka2S protein. At least, two
independent experiments were performed, and the observed effects were the same. TOE-PRINTING ANALYSIS Toe-print analysis was performed as previously described20 on two matrices: ErmCL and
RST1. Briefly, the PURExpress system (NEB) was used for transcription and subsequent translation, then a reverse transcription reaction was performed. The ErmCL template was generated by PCR
with two partially self-complementary oligonucleotides (Erm-F and Erm-R, Supplementary Table 8). GGTTATAATGAATTTTGCTTATTAAC oligonucleotide was used for reverse transcription. RST1 template
was prepared as previously described58 using PCR primers (T7fwd and NV1). Concentrations of tested compounds in toe-print were 0.5, 5, or 50 μM. 1% (v/v) DMSO water solution was used as a
negative control sample. Two independent experiments were performed using both matrices, and the observed effects were the same. PEPTIDE BOND FORMATION Initiation complexes and ternary
complexes were prepared as described previously21,59. Where necessary initiation complexes were supplemented with rumicidin-1 (1 μM) or amicoumacin A (30 μM) followed by additional
incubation for 5 min at 37 °C. To analyze formation of dipeptide fMet-Val, 0.2 μM 70S initiation complexes containing fMet-tRNAfMet in the P site were mixed with 0.375 μM ternary complexes
EF-Tu⋅GTP⋅[14C]Val-tRNAVal (444 dpm/pmol) and incubated for 2 min at 37 °C. Then samples were quenched with 1/10 volume of 5 M KOH and hydrolyzed for 30 min at 37 °C. Samples were
neutralized with 1/10 volume of glacial acetic acid and analyzed by reversed-phase HPLC on RP8 (Merck) with an acetonitrile gradient in 0.1% trifluoroacetic acid. Percentage of synthesized
dipeptide was determined by incorporation of the radioactive label as a ratio of peptide formed to the amount of the 70S ribosomes in the reaction mixture, as described earlier60.
Experiments were done in three replicates. ASSESSMENT OF BACTERIAL MEMBRANE PERMEABILIZATION To examine the ability of the peptides to affect the barrier function of outer and inner
membranes of Gram-negative bacteria, we slightly modified the previously described procedure22 with the use of the _E. coli_ ML-35p strain constitutively expressing cytoplasmic
β-galactosidase but lacking lactose permease, and also containing β-lactamase in the periplasmic space. The state of the _E. coli_ ML-35p outer and cytoplasmic membranes was assessed based
on their permeability to chromogenic markers nitrocefin (Calbiochem) and _o_-nitrophenyl-β-D-galactopyranoside (ONPG, AppliChem) which are the β-lactamase and β-galactosidase substrates,
respectively. The cells were incubated in the trypticase soy broth (TSB) for 16 h at 37 °C, washed three times with phosphate-buffered saline (PBS, pH 7.4) to remove residual growth media,
adjusted to the concentration of 2.5 × 108 CFU/mL, and stored on ice until used. Experiments were performed in 10 mM sodium phosphate buffer (NaPB, pH 7.4) with or without the addition of
0.9% NaCl and/or a mixture of divalent cations (0.5 mM CaCl2 and 0.5 mM MgSO4). The final concentration of _E. coli_ ML-35p cells was of 2.5 × 107 CFU/mL. The concentrations of ONPG and
nitrocefin were of 2.5 mM and 20 µM, respectively. Peptide samples were placed in the wells of a 96-well flat-bottom polystyrene microplate, and optical density of the solution enhanced due
to an appearance of the hydrolyzed nitrocefin or ONPG and was measured at 492 and 405 nm, respectively, using a microplate reader AF2200. The final volume in each well was 200 µL. Assays
were performed at 37 °C under stirring at 500 rpm. Control experiments were performed under the same conditions without adding a peptide. Two independent experiments were performed, and the
curve pattern was the same. HEMOLYSIS AND CYTOTOXICITY ASSAY Hemolytic activity of the peptides was tested against the fresh suspension of human red blood cells (hRBC) using the hemoglobin
release assay as described previously61. Two experiments were performed with the hRBC from blood samples collected from healthy donors by certified medical personnel upon informed written
consent. All procedures were approved by the Ethics Committee of the Institute of Experimental Medicine (protocol 1/20 of 2/27/2020) and comply with the ethical principles of the Declaration
of Helsinki. The quantitative data were represented as average means with standard deviations. The colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye
reduction assay was used to determine cytotoxicity of the peptides against human keratinocyte cell line (HaCaT) and transformed human embryonic kidney cells (HEK293T). Both cell lines were
purchased from American Type Culture Collection (ATCC). 104 cells per well in Dulbecco’s modified Eagle’s medium (DMEM/F12) supplemented with 10% fetal bovine serum (FBS, Invitrogen) were
placed into 96-well plates and then cultured in the CO2-incubator (5% CO2, 37 °C). After the media were removed, the peptides were dissolved in 100 µL of the same medium and added to cell
cultures at different final concentrations. 20 h later, 20 μL of MTT (5 mg/mL, Sigma) was added to each well, and the plates were incubated for 4 h at 37 °C. Then, the media were discarded
and 100 µL DMSO-isopropanol mixture at a ratio of 1:1 (v/v) was added to each well to dissolve the crystallized formazan. The absorbance at 570 nm was measured by a microplate reader AF2200
(Eppendorf). An optical density in the wells containing cells cultured without the peptides was assumed to represent 100% cell viability. Two independent experiments were performed for each
peptide. CYTOKINE RESPONSE TO CATHELICIDINS ON HUMAN CELLS IN VITRO The acute monocytic leukemia THP-1 cell line (ATCC TIB-202) was cultured in the complete RPMI 1640 medium (Invitrogen)
containing 10% FBS, 1× antibiotic-antimycotic solution (Invitrogen), and 0.05 mM β-mercaptoethanol, in the CO2-incubator (5% CO2, 37 °C). THP-1 cells were differentiated into proinflammatory
macrophages (MΦ1) according to the previously reported protocol62. Primary peripheral blood mononuclear cells (PBMC) collected from a healthy donor were purchased from ATCC (PCS-800-011),
thawed, and seeded into a 96-well plate one day prior to the experiment at a density of 2 × 105 cells/well. Two different cell subpopulations (monocytes and T-/B-/NK-lymphocytes) were
isolated from PBMC based on their adherence ability. Macrophages MΦ1 were washed out and seeded into 96-well plates at a density of 105 cells/well one day prior to the experiment. The next
day, medium in each well was replaced by a fresh complete RPMI 1640 medium with or without 2 μM of a tested peptide (rumicidin-1 or LL-37). Cell cultures were kept in a CO2-incubator (5%
CO2, 37 °C) for 24 h. Culture supernatants were collected 24 h later and stored at −70 °C degrees less than one week prior to the assessment of analytes. 26 analytes were measured at a
protein level by multiplex xMAP technology using the MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Immunology Panel kit (HCYTOMAG-60K-27, Merck): eotaxin-1/CCL11, TGFα, GM-CSF, IFNα2,
IFNγ, IL-10, IL-12p40, IL-12p70, IL-15, sCD40L, IL-17A, IL-1RA, IL-1α, IL-9, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8/CXCL8, IP-10/CXCL10, RANTES/CCL5, TNFα, TNFβ. Multiplex-based
assay read-out was performed using the MAGPIX system (Merck) with the xPONENT 4.2 software (Merck) in accordance with the manufacturer’s instruction with overnight incubation of the samples
with primary antibodies. The final analysis was carried out with the MILLIPLEX Analyst v5.1 software (Merck). Measurements were performed twice for each sample. The release of the analytes
in control and experimental samples were compared by unpaired two-sample _t_-test using GraphPad Prism v.8.0.1. The _p_ values ≤ 0.05 were considered significant. SYSTEMIC SEPTICEMIA
INFECTION MOUSE MODEL All animal studies were performed at the State Research Center for Applied Microbiology & Biotechnology (SRCAMB) in Obolensk (Russia), approved by the Institutional
Bioethics Committee of the SRCAMB (Animal Research Protocol #VP-2022/1) and performed according to the Russian Federal rules and Directive 2010/63/EU of the European Parliament and of the
Council. Experiments were performed with female eight- to ten-week-old BALB/c mice (“Andreevka” laboratory animal nursery FMBA, Russia). The animals were housed in groups of 5 in
polycarbonate cages, and the following housing conditions were used: 12/12 h dark/light cycle, ambient temperature of 20 ÷ 22 °C, 50% humidity. Food and water were provided _ad libitum_. In
vivo efficacy of rumicidins was tested in the mouse septicemia model induced by _E. coli_ ATCC 25922. Mice were infected with 0.5 mL of bacterial suspension (1 × 106 CFU per animal) in
saline supplemented with 2.5% mucin (w/v) via intraperitoneal (IP) injection. A total of 4 groups with 5 animals were used. The first group received ciprofloxacin (as a positive control)
administered IP once (1 h post-infection) at a dose of 10 mg/kg. The second group received saline (as a vehicle control) and administered IP once (1 h post-infection). Two other groups
received rumicidin-1 or rumicidin-3 each administered IP three times (1, 4, and 8 h post-infection) at a dose of 10 mg/kg or of 5 mg/kg, respectively. Survival was monitored for 7 days.
After that survived animals were euthanized by CO2 asphyxiation. The spleen was aseptically removed, homogenized, serially diluted and placed on Endo agar for CFU identification. CRYO-EM
SAMPLE PREPARATION AND DATA COLLECTION Initiation complex (70S/mRNA/fMet-tRNAfMet) was prepared as described previously63,64. Purified complex (0.3 µM) was incubated with rumicidin-2 (10 µM)
and spermine (0.6 mM) at 0 °C for 5 min before application onto EM grids. Quantifoil R1.2/1.3 grids with an additional 2 nm amorphous carbon film were glow-discharged for 10 sec at 15 mA
using PELCO easiGlow. A total of 3.0 μl of the sample was applied onto the grids and plunge-frozen in liquid ethane using Vitrobot Mark IV (Thermo Fisher) at 10 °C and 100% humidity. Grids
were transferred to Krios Cryo-TEM (Thermo-Fisher Scientific) equipped with a Cs-corrector, and a Falcon II electron detector. Data were collected at nominal magnification of ×75,000 and a
pixel size of 0.863 Å/pixel, with a defocus range from 0.5 to 1.8 μm at a total dose of 80 e-/Å2 per exposure distributed across 32 frames. CRYO-EM DATA PROCESSING Dataset consisting of 3528
movies was imported into Relion v3.1.365 for preprocessing. Motion correction was performed using an implementation of the MC2 algorithm66, followed by defocus and astigmatism estimation in
CTFFIND67 using the sum of PS generated during motion correction. Next, crYOLO 1.7.668 was used for particle picking using a re-trained model, resulting in 501000 particles which were
extracted at 3.452 Å pixel size which were used for a 3D refinement, followed by 3D classification with the local search of 20°, angular sampling of 1.8° and an offset range/step derived
from the corresponding values determined during 3D refinement. 371858 particles were re-extracted with a 1.08 Å pixel size and used for another 3D refinement combined with the local masked
refinement of the 50S subunit, resulting in a 2.53 Å cryo-EM map. This map was used for consecutive CTF refinement, including defocus, anisotropic magnification and high-order aberrations
followed by another run of 3D refinement. Next, a 2.25 Å map was used for polishing and extracting of particles with the original pixel size. Finally, particles were imported into cryoSPARC
3.369. 3D classification and Heterogeneous refinement were performed to characterize ribosome conformations. Peptide conformations were found to be identical across different classes. All
particles were combined for the focused refinement, aberration values were re-estimated and Local refinement with a mask covering 50S subunit70 was performed, resulting in the 1.95 Å cryo-EM
map. Resolution was estimated in cryoSPARC using default parameters. MODEL BUILDING Both unsharpened and sharpened map (B-factor of −41 Å2 was applied) from cryoSPARC were used for modeling
of rumicidin-2 in Coot v9.671. For an initial model of the 50S subunit the structure of the _E. coli_ 50S ribosomal subunit was used (PDB: 8B0X)72. Model was manually curated in Coot,
followed by refinement in Coot and Servalcat73. Water molecules were added in Coot and validated using the sharpened map which was resampled to a pixel size of 0.3 Å.Visualization and
analysis of the structures were performed using Chimera v1.1574 and ChimeraX v1.375. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting
Summary linked to this article. DATA AVAILABILITY Coordinates and charge density map for the _E. coli_ 50S ribosomal subunit in complex with rumicidin-2 were deposited in the RCSB Protein
Data Bank and EMDB with the accession codes 9D89 and EMD-46632, respectively. Data on bacterial genome sequencing were deposited in NCBI SRA database under accession code PRJNA1168027. The
data that support the findings of this study are provided as Supplementary Data 1 and Supplementary Information files. Additional data that support the findings of this study are available
on request from the corresponding authors. Source data are provided with this paper. REFERENCES * Czaplewski, L. et al. Alternatives to antibiotics—a pipeline portfolio review. _Lancet
Infect. Dis._ 16, 239–251 (2016). Article CAS Google Scholar * Gagnon, M. G. et al. Structures of proline-rich peptides bound to the ribosome reveal a common mechanism of protein
synthesis inhibition. _Nucleic Acids Res._ 44, 2439–2450 (2016). Article ADS CAS Google Scholar * Graf, M. & Wilson, D. N. _Intracellular Antimicrobial Peptides Targeting the Protein
Synthesis Machinery_. in _Antimicrobial Peptides_ (ed. Matsuzaki, K.) 1117, 73–89 (Springer Singapore, Singapore, 2019). * Mookherjee, N., Anderson, M. A., Haagsman, H. P. & Davidson,
D. J. Antimicrobial host defence peptides: functions and clinical potential. _Nat. Rev. Drug Discov._ 19, 311–332 (2020). Article CAS Google Scholar * Seefeldt, A. C. et al. Structure of
the mammalian antimicrobial peptide Bac7(1–16) bound within the exit tunnel of a bacterial ribosome. _Nucleic Acids Res._ 44, 2429–2438 (2016). Article CAS Google Scholar * Florin, T. et
al. An antimicrobial peptide that inhibits translation by trapping release factors on the ribosome. _Nat. Struct. Mol. Biol._ 24, 752–757 (2017). Article CAS Google Scholar * Mangano, K.
et al. Inhibition of translation termination by the antimicrobial peptide Drosocin. _Nat. Chem. Biol_. https://doi.org/10.1038/s41589-023-01300-x (2023). * Ge, R.-L. et al. Draft genome
sequence of the Tibetan antelope. _Nat. Commun._ 4, 1858 (2013). Article ADS Google Scholar * Chen, L. et al. Large-scale ruminant genome sequencing provides insights into their evolution
and distinct traits. _Science_ 364, eaav6202 (2019). Article ADS CAS Google Scholar * Tomasinsig, L. & Zanetti, M. The cathelicidins - structure, function and evolution. _Curr.
Protein Pept. Sci._ 6, 23–34 (2005). Article CAS Google Scholar * Mardirossian, M. et al. Peptide inhibitors of bacterial protein synthesis with broad spectrum and Sbma-independent
Bactericidal Activity Against Clinical Pathogens. _J. Med. Chem._ 63, 9590–9602 (2020). Article CAS Google Scholar * Sola, R. et al. Characterization of cetacean proline-rich
antimicrobial peptides displaying activity against ESKAPE pathogens. _Int. J. Mol. Sci._ 21, 7367 (2020). Article CAS PubMed Central Google Scholar * The Bovine Genome Sequencing and
Analysis Consortium. et al. The genome sequence of taurine cattle: a window to ruminant biology and evolution. _Science_ 324, 522–528 (2009). Article ADS PubMed Central Google Scholar *
Whelehan, C. J. et al. Characterisation and expression profile of the bovine cathelicidin gene repertoire in mammary tissue. _BMC Genomics_ 15, 128 (2014). Article PubMed Central Google
Scholar * Zhang et al. Genomic identification and expression analysis of the cathelicidin gene family of the forest musk deer. _Animals_ 9, 481 (2019). Article PubMed Central Google
Scholar * Veldhuizen, E. J. A. et al. Antimicrobial and immunomodulatory activities of PR-39 derived peptides. _PLoS ONE_ 9, e95939 (2014). Article ADS PubMed Central Google Scholar *
Benincasa, M. et al. Antimicrobial activity of Bac7 fragments against drug-resistant clinical isolates. _Peptides_ 25, 2055–2061 (2004). Article CAS Google Scholar * Studier, F. W.
Protein production by auto-induction in high density shaking cultures. _Protein Expr. Purif._ 41, 207–234 (2005). Article CAS Google Scholar * Vázquez-Laslop, N. et al. Role of antibiotic
ligand in nascent peptide-dependent ribosome stalling. _Proc. Natl Acad. Sci._ 108, 10496–10501 (2011). Article ADS Google Scholar * Orelle, C. et al. Tools for characterizing bacterial
protein synthesis inhibitors. _Antimicrob. Agents Chemother._ 57, 5994–6004 (2013). Article CAS PubMed Central Google Scholar * Maksimova, E. M. et al. Multifaceted mechanism of
amicoumacin A inhibition of bacterial translation. _Front. Microbiol._ 12, 618857 (2021). Article PubMed Central Google Scholar * Panteleev, P. V. et al. Combined antibacterial effects of
goat cathelicidins with different mechanisms of action. _Front. Microbiol._ 9, 2983 (2018). Article PubMed Central Google Scholar * Loveland, A. B. et al. Ribosome inhibition by
C9ORF72-ALS/FTD-associated poly-PR and poly-GR proteins revealed by cryo-EM. _Nat. Commun._ 13, 2776 (2022). Article ADS CAS PubMed Central Google Scholar * Seefeldt, A. C. et al. The
proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. _Nat. Struct. Mol. Biol._ 22, 470–475 (2015). Article CAS Google
Scholar * Komp Lindgren, P., Karlsson, A. & Hughes, D. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract
infections. _Antimicrob. Agents Chemother._ 47, 3222–3232 (2003). Article PubMed Central Google Scholar * Ghilarov, D. et al. Molecular mechanism of SbmA, a promiscuous transporter
exploited by antimicrobial peptides. _Sci. Adv._ 7, eabj5363 (2021). Article ADS CAS PubMed Central Google Scholar * Metelev, M. et al. Klebsazolicin inhibits 70S ribosome by
obstructing the peptide exit tunnel. _Nat. Chem. Biol._ 13, 1129–1136 (2017). Article CAS PubMed Central Google Scholar * Panteleev, P. V. et al. A novel proline-rich cathelicidin from
the alpaca vicugna _pacos_ with potency to combat antibiotic-resistant bacteria: mechanism of action and the functional role of the C-terminal region. _Membranes_ 12, 515 (2022). Article
CAS PubMed Central Google Scholar * Schellhorn, H. E. Function, evolution, and composition of the RpoS regulon in escherichia coli. _Front. Microbiol._ 11, 560099 (2020). Article PubMed
Central Google Scholar * Fitzpatrick, A. W. P. et al. Structure of the MacAB–TolC ABC-type tripartite multidrug efflux pump. _Nat. Microbiol._ 2, 17070 (2017). Article CAS Google Scholar
* Yan, Q. & Fong, S. S. Study of in vitro transcriptional binding effects and noise using constitutive promoters combined with UP element sequences in Escherichia coli. _J. Biol. Eng._
11, 33 (2017). Article Google Scholar * Linde, C. M. A., Hoffner, S. E., Refai, E. & Andersson, M. In vitro activity of PR-39, a proline-arginine-rich peptide, against susceptible and
multi-drug-resistant Mycobacterium tuberculosis. _J. Antimicrob. Chemother._ 47, 575–580 (2001). Article CAS Google Scholar * Scheenstra, M. R., van Harten, R. M., Veldhuizen, E. J. A.,
Haagsman, H. P. & Coorens, M. Cathelicidins Modulate TLR-Activation and Inflammation. _Front. Immunol._ 11, 1137 (2020). Article CAS Google Scholar * Benincasa, M. et al. The
proline-rich peptide Bac7(1-35) reduces mortality from Salmonella typhimurium in a mouse model of infection. _BMC Microbiol._ 10, 178 (2010). Article Google Scholar * Koch, P. et al.
Optimization of the antimicrobial peptide Bac7 by deep mutational scanning. _BMC Biol._ 20, 114 (2022). Article CAS Google Scholar * Lazzaro, B. P., Zasloff, M. & Rolff, J.
Antimicrobial peptides: application informed by evolution. _Science_ 368, eaau5480 (2020). Article CAS Google Scholar * Chernysh, S., Gordya, N. & Suborova, T. Insect antimicrobial
peptide complexes prevent resistance development in bacteria. _PLOS ONE_ 10, e0130788 (2015). Article Google Scholar * Wang, Y. et al. Genetic basis of ruminant headgear and rapid antler
regeneration. _Science_ 364, eaav6335 (2019). Article ADS CAS Google Scholar * Hassanin, A. et al. Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as
revealed by a comprehensive analysis of mitochondrial genomes. _C. R. Biol._ 335, 32–50 (2012). Article Google Scholar * Mardirossian, M. et al. The dolphin proline-rich antimicrobial
peptide Tur1A inhibits protein synthesis by targeting the bacterial ribosome. _Cell Chem. Biol._ 25, 530–539.e7 (2018). Article CAS Google Scholar * Mardirossian, M., Sola, R., Degasperi,
M. & Scocchi, M. Search for shorter portions of the proline‐rich antimicrobial peptide fragment Bac5(1–25) that retain antimicrobial activity by blocking protein synthesis.
_ChemMedChem_ 14, 343–348 (2019). Article CAS Google Scholar * Lai, P.-K., Geldart, K., Ritter, S., Kaznessis, Y. N. & Hackel, B. J. Systematic mutagenesis of oncocin reveals enhanced
activity and insights into the mechanisms of antimicrobial activity. _Mol. Syst. Des. Eng._ 3, 930–941 (2018). Article CAS Google Scholar * Liu, Y.-Y. et al. Emergence of
plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. _Lancet Infect. Dis._ 16, 161–168 (2016). Article
Google Scholar * Crow, A., Greene, N. P., Kaplan, E. & Koronakis, V. Structure and mechanotransmission mechanism of the MacB ABC transporter superfamily. _Proc. Natl Acad. Sci._ 114,
12572–12577 (2017). Article ADS CAS Google Scholar * Boucher, H. W. et al. Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. _Clin. Infect. Dis._
48, 1–12 (2009). Article Google Scholar * Baba, T. et al. Construction of _Escherichia coli_ K‐12 in‐frame, single‐gene knockout mutants: the Keio collection. _Mol. Syst. Biol_. 2,
2006.0008 (2006). * Beyer, H. M. et al. AQUA cloning: a versatile and simple enzyme-free cloning approach. _PLOS ONE_ 10, e0137652 (2015). Article Google Scholar * Schägger, H.
Tricine–SDS-PAGE. _Nat. Protoc._ 1, 16–22 (2006). Article Google Scholar * Wiegand, I., Hilpert, K. & Hancock, R. E. W. Agar and broth dilution methods to determine the minimal
inhibitory concentration (MIC) of antimicrobial substances. _Nat. Protoc._ 3, 163–175 (2008). Article CAS Google Scholar * de Sena Brandine, G. & Smith, A. D. Falco: high-speed FastQC
emulation for quality control of sequencing data. _F1000Research_ 8, 1874 (2021). Article PubMed Central Google Scholar * Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a
flexible trimmer for Illumina sequence data. _Bioinformatics_ 30, 2114–2120 (2014). Article CAS PubMed Central Google Scholar * Bankevich, A. et al. SPAdes: a new genome assembly
algorithm and its applications to single-cell sequencing. _J. Comput. Biol._ 19, 455–477 (2012). Article MathSciNet CAS PubMed Central Google Scholar * Gurevich, A., Saveliev, V.,
Vyahhi, N. & Tesler, G. QUAST: quality assessment tool for genome assemblies. _Bioinformatics_ 29, 1072–1075 (2013). Article CAS PubMed Central Google Scholar * Seemann, T. Prokka:
rapid prokaryotic genome annotation. _Bioinformatics_ 30, 2068–2069 (2014). Article CAS Google Scholar * Li, H. & Durbin, R. Fast and accurate short read alignment with
Burrows-Wheeler transform. _Bioinformatics_ 25, 1754–1760 (2009). Article CAS PubMed Central Google Scholar * Koboldt, D. C. et al. VarScan 2: Somatic mutation and copy number alteration
discovery in cancer by exome sequencing. _Genome Res._ 22, 568–576 (2012). Article CAS PubMed Central Google Scholar * Osterman, I. A. et al. Sorting out antibiotics’ mechanisms of
action: a double fluorescent protein reporter for high-throughput screening of ribosome and DNA biosynthesis inhibitors. _Antimicrob. Agents Chemother._ 60, 7481–7489 (2016). Article CAS
PubMed Central Google Scholar * Orelle, C. et al. Identifying the targets of aminoacyl-tRNA synthetase inhibitors by primer extension inhibition. _Nucleic Acids Res._ 41, e144 (2013).
Article CAS PubMed Central Google Scholar * Paleskava, A. et al. Differential contribution of protein factors and 70S ribosome to elongation. _Int. J. Mol. Sci._ 22, 9614 (2021). Article
CAS PubMed Central Google Scholar * Rodnina, M. V. & Wintermeyer, W. GTP consumption of elongation factor Tu during translation of heteropolymeric mRNAs. _Proc. Natl Acad. Sci._ 92,
1945–1949 (1995). Article ADS CAS PubMed Central Google Scholar * Panteleev, P. V., Bolosov, I. A. & Ovchinnikova, T. V. Bioengineering and functional characterization of arenicin
shortened analogs with enhanced antibacterial activity and cell selectivity: bioengineering of arenicin shortened analogs with enhanced selectivity. _J. Pept. Sci._ 22, 82–91 (2016). Article
CAS Google Scholar * Genin, M., Clement, F., Fattaccioli, A., Raes, M. & Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer
cells to etoposide. _BMC Cancer_ 15, 577 (2015). Article PubMed Central Google Scholar * Pichkur, E. B. et al. Insights into the improved macrolide inhibitory activity from the
high-resolution cryo-EM structure of dirithromycin bound to the _E. coli_ 70S ribosome. _RNA_ 26, 715–723 (2020). Article CAS PubMed Central Google Scholar * Konevega, A. L. et al.
Spontaneous reverse movement of mRNA-bound tRNA through the ribosome. _Nat. Struct. Mol. Biol._ 14, 318–324 (2007). Article CAS Google Scholar * Zivanov, J. et al. New tools for automated
high-resolution cryo-EM structure determination in RELION-3. _eLife_ 7, e42166 (2018). Article PubMed Central Google Scholar * Zheng, S. Q. et al. MotionCor2: anisotropic correction of
beam-induced motion for improved cryo-electron microscopy. _Nat. Methods_ 14, 331–332 (2017). Article CAS PubMed Central Google Scholar * Rohou, A. & Grigorieff, N. CTFFIND4: Fast
and accurate defocus estimation from electron micrographs. _J. Struct. Biol._ 192, 216–221 (2015). Article PubMed Central Google Scholar * Wagner, T. et al. SPHIRE-crYOLO is a fast and
accurate fully automated particle picker for cryo-EM. _Commun. Biol._ 2, 218 (2019). Article PubMed Central Google Scholar * Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker,
M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. _Nat. Methods_ 14, 290–296 (2017). Article CAS Google Scholar * Punjani, A., Zhang, H. & Fleet, D.
J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. _Nat. Methods_ 17, 1214–1221 (2020). Article CAS Google Scholar * Emsley, P., Lohkamp,
B., Scott, W. G. & Cowtan, K. Features and development of _Coot_. _Acta Crystallogr. D. Biol. Crystallogr._ 66, 486–501 (2010). Article ADS CAS Google Scholar * Fromm, S. A. et al.
The translating bacterial ribosome at 1.55 Å resolution generated by cryo-EM imaging services. _Nat. Commun._ 14, 1095 (2023). Article ADS CAS Google Scholar * Yamashita, K., Palmer, C.
M., Burnley, T. & Murshudov, G. N. Cryo-EM single-particle structure refinement and map calculation using _Servalcat_. _Acta Crystallogr. Sect. Struct. Biol._ 77, 1282–1291 (2021).
Article ADS CAS Google Scholar * Pettersen, E. F. et al. UCSF Chimera?a visualization system for exploratory research and analysis. _J. Comput. Chem._ 25, 1605–1612 (2004). Article CAS
Google Scholar * Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. _Protein Sci._ 30, 70–82 (2021). Article CAS Google Scholar
* Polikanov, Y. S., Aleksashin, N. A., Beckert, B. & Wilson, D. N. The mechanisms of action of ribosome-targeting peptide antibiotics. _Front. Mol. Biosci._ 5, 48 (2018). Article Google
Scholar Download references ACKNOWLEDGEMENTS We thank Yury S. Polikanov (University of Illinois at Chicago, USA) for valuable advice regarding some of the experiments as well as for
careful reading of the manuscript and critical feedback. This work was supported by the Russian Science Foundation: grant No. 21-74-00100 (identification, expression, purification, and
antimicrobial activity testing of rumicidins; analysis of rumicidin-resistant strains); grant No. 22-14-00380 (cytotoxicity and immunomodulatory activity testing); grant No. 21-64-00006
(detection of the translation inhibitors and toe-printing analysis); grant No. 22-14-00278 (cryo-EM and dipeptide formation experiments). AUTHOR INFORMATION Author notes * These authors
contributed equally: Pavel V. Panteleev, Eugene B. Pichkur. AUTHORS AND AFFILIATIONS * M.M. Shemyakin & Yu.A. Ovchinnikov Institute of Bioorganic Chemistry, the Russian Academy of
Sciences, Moscow, Russia Pavel V. Panteleev, Roman N. Kruglikov, Ilia A. Bolosov, Ivan V. Bogdanov, Victoria N. Safronova, Sergey V. Balandin, Alexey A. Bogdanov, Olga A. Dontsova &
Tatiana V. Ovchinnikova * Petersburg Nuclear Physics Institute named by B.P. Konstantinov of NRC “Kurchatov Institute”, Gatchina, Russia Eugene B. Pichkur, Alena Paleskava, Olga V.
Shulenina, Alexander G. Myasnikov & Andrey L. Konevega * Lomonosov Moscow State University, Moscow, Russia Valeriya I. Marina, Ilya A. Osterman, Petr V. Sergiev, Alexey A. Bogdanov, Olga
A. Dontsova & Tatiana V. Ovchinnikova * State Research Center for Applied Microbiology & Biotechnology (SRCAMB), Obolensk, Russia Tatiana I. Kombarova, Olga V. Korobova &
Alexander I. Borzilov * Institute of Experimental Medicine, Saint Petersburg, Russia Olga V. Shamova * Center of Life Sciences, Skolkovo Institute of Science and Technology, Skolkovo, Russia
Ilya A. Osterman, Petr V. Sergiev & Olga A. Dontsova * Department of Biotechnology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia Tatiana V.
Ovchinnikova Authors * Pavel V. Panteleev View author publications You can also search for this author inPubMed Google Scholar * Eugene B. Pichkur View author publications You can also
search for this author inPubMed Google Scholar * Roman N. Kruglikov View author publications You can also search for this author inPubMed Google Scholar * Alena Paleskava View author
publications You can also search for this author inPubMed Google Scholar * Olga V. Shulenina View author publications You can also search for this author inPubMed Google Scholar * Ilia A.
Bolosov View author publications You can also search for this author inPubMed Google Scholar * Ivan V. Bogdanov View author publications You can also search for this author inPubMed Google
Scholar * Victoria N. Safronova View author publications You can also search for this author inPubMed Google Scholar * Sergey V. Balandin View author publications You can also search for
this author inPubMed Google Scholar * Valeriya I. Marina View author publications You can also search for this author inPubMed Google Scholar * Tatiana I. Kombarova View author publications
You can also search for this author inPubMed Google Scholar * Olga V. Korobova View author publications You can also search for this author inPubMed Google Scholar * Olga V. Shamova View
author publications You can also search for this author inPubMed Google Scholar * Alexander G. Myasnikov View author publications You can also search for this author inPubMed Google Scholar
* Alexander I. Borzilov View author publications You can also search for this author inPubMed Google Scholar * Ilya A. Osterman View author publications You can also search for this author
inPubMed Google Scholar * Petr V. Sergiev View author publications You can also search for this author inPubMed Google Scholar * Alexey A. Bogdanov View author publications You can also
search for this author inPubMed Google Scholar * Olga A. Dontsova View author publications You can also search for this author inPubMed Google Scholar * Andrey L. Konevega View author
publications You can also search for this author inPubMed Google Scholar * Tatiana V. Ovchinnikova View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS P.V.P. and T.V.O. contributed to the conception of the work. P.V.P. and R.N.K. performed genome mining, and analyzed genome and transcriptome data; P.V.P. and R.N.K. assembled
plasmid constructs; P.V.P., R.N.K., I.A.B., and V.N.S. obtained recombinant peptides; E.B.P., A.P., O.V.Shu, A.G.M. and A.L.K. performed cryo-EM studies and analyzed results; A.P., O.V.Shu,
and A.L.K. performed and analyzed results of dipeptide formation experiments; P.V.P., R.N.K., I.A.B. and V.N.S. selected and analyzed resistant strains; P.V.P., R.N.K., I.V.B., I.A.B.,
V.N.S., S.V.B., V.I.M., I.A.O. and P.V.S. evaluated biological activities of rumicidins and performed biochemical assays; T.I.K., O.V.K., and A.I.B. performed in vivo studies. All authors
analyzed data; P.V.P., O.V.Sha, A.L.K., A.A.B., O.A.D. and T.V.O. interpreted the results. P.V.P, E.B.P., A.P., A.L.K., and T.V.O. wrote the manuscript. T.V.O. critically revised the
manuscript, approved and prepared it for publication. All authors have read and agreed to the published version of the manuscript. CORRESPONDING AUTHORS Correspondence to Andrey L. Konevega
or Tatiana V. Ovchinnikova. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Andrei
Korostelev and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL
SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 REPORTING SUMMARY SOURCE DATA TRANSPARENT PEER REVIEW FILE SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in
the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended
use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Panteleev, P.V., Pichkur, E.B., Kruglikov, R.N. _et al._ Rumicidins are a family of
mammalian host-defense peptides plugging the 70S ribosome exit tunnel. _Nat Commun_ 15, 8925 (2024). https://doi.org/10.1038/s41467-024-53309-y Download citation * Received: 25 January 2024
* Accepted: 08 October 2024 * Published: 16 October 2024 * DOI: https://doi.org/10.1038/s41467-024-53309-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative