Play all audios:
ABSTRACT Although larger species of animals typically live longer than smaller species, the relationship of body size to longevity within a species is generally opposite. The longevity
advantage of smaller individuals can be considerable and is best documented in laboratory mice and in domestic dogs. Importantly, it appears to apply broadly, including humans. It is not
known whether theses associations represent causal links between various developmental and physiological mechanisms affecting growth and/or aging. However, variations in growth hormone (GH)
signaling are likely involved because GH is a key stimulator of somatic growth, and apparently also exerts various “pro-aging” effects. Mechanisms linking GH, somatic growth, adult body
size, aging, and lifespan likely involve target of rapamycin (TOR), particularly one of its signaling complexes, mTORC1, as well as various adjustments in mitochondrial function, energy
metabolism, thermogenesis, inflammation, and insulin signaling. Somatic growth, aging, and longevity are also influenced by a variety of hormonal and nutritional signals, and much work will
be needed to answer the question of why smaller individuals may be likely to live longer. SIMILAR CONTENT BEING VIEWED BY OTHERS GENE-BY-ENVIRONMENT MODULATION OF LIFESPAN AND WEIGHT GAIN IN
THE MURINE BXD FAMILY Article 22 September 2021 POTENTIAL DOWNSIDES OF CALORIE RESTRICTION Article 17 April 2025 ON THE CAUSAL CONNECTION IN LIFESPAN CORRELATIONS AND THE POSSIBLE EXISTENCE
OF A ‘NUMBER OF LIFE’ AT MOLECULAR LEVEL Article Open access 30 December 2024 INTRODUCTION There is considerable evidence that developmental events, including pre-natal and post-natal
growth can have a profound impact on adult phenotypes and risk of chronic disease. In this context, it is interesting to consider to what extent somatic growth and adult body size can
influence the trajectory of aging and life expectancy. Pioneering studies of Samaras and his colleagues provided numerous examples of negative association of human height and lifespan.1,2,3
Other investigators emphasized a positive, rather than a negative, association of human stature with various health outcomes, including longevity.4,5 However, some of the reported
associations are controversial.6 Importantly, studies from several laboratories reported reduced old age mortality and exceptional longevity in individuals with shorter stature and reduced
somatotropic signaling.7,8,9,10 Evidence from genetic studies supports the negative association of somatotropic signaling and height with human longevity.11,12 A recent study revealed that
the total 24-hour secretion of GH was lower in middle-aged offspring of long-lived families than in their partners.13 Deciphering relationships between growth and longevity in human
populations is difficult because both can be powerfully influenced by environmental factors including nutrition, numerous public health measures, access to medical interventions and
lifestyle factors such as smoking, alcohol, and drug use. In this article, we will briefly review evidence for the links between somatic growth, aging, and longevity in experimental animals
and discuss the most likely underlying mechanisms. “LONGEVITY GENES” AND SOMATIC GROWTH The very exciting and largely unexpected discoveries of mutations that significantly extend longevity
in a worm (_Caenorhabditis elegans_) or in a fly (_Drosophila melanogaster_)14,15,16,17 are an important part of the present understanding of the genetic control of aging. The striking
effects of mutations of a single gene on longevity of heterothermic invertebrates led to the question of whether identifiable “longevity genes” also influence aging and lifespan in mammals,
including our own species. Studies conducted during the last 25 years in laboratory stocks of house mice (_Mus musculus_) demonstrated that longevity of these animals can be extended by a
natural loss-of-function mutation or targeted deletion of a single gene.18,19,20,21,22,23,24,25,26,27 With very few exceptions, the genetic modifications which produced a significant and
reproducible extension of longevity in both females and males disrupted the so called “somatotropic axis,” that is, biosynthesis or action of the pituitary growth hormone (GH) and/or the
insulin-like growth factor I (IGF-1), an important mediator of GH actions. Since in mammals the somatotropic axis is a key regulator of postnatal growth, these long-lived mutants exhibit
major reductions in growth rate and in adult body size. It should be emphasized that the remarkably extended longevity of GH-deficient and GH-resistant mice is associated with multiple signs
of delayed aging and with the extension of “healthspan,” the period of life free of functional deficits and chronic disease.19,20,21,28 This includes maintenance of normal cognitive
function (learning and memory) into advanced age.29,30 The rate of aging of these long-lived mutants was initially reported to be either reduced or unaltered, with life extension being due
to a delay, rather than a slowing, of age-related mortality.31 However, a more recent analysis using what we believe is a more pertinent methodology suggests that the rate of aging of
long-lived, GH-deficient and GH-resistant mice is initially slower than in genetically normal (control) animals and accelerates only later in their life, after most of the control animals
have died.32 Extended longevity and phenotypic characteristics of animals with deletion of genes acting downstream from the IGF-1 receptor such as IRS-1, IRS-2, or Akt25,26,27 and the
well-documented anti-aging effects of calorie restriction, provide additional examples of the negative association of somatotropic signaling and growth with healthy aging and lifespan.
GROWTH VS. LONGEVITY IN TRANSGENIC AND IN GENETICALLY NORMAL ANIMALS The reciprocal relationship of longevity to growth rate and adult body size discovered in various dwarf mutants also
applies to animals in which somatic growth and adult body size are experimentally enhanced. Transgenic mice, chronically expressing heterologous (in most cases bovine or human) GH in liver
and other organs under control of metallothionein I or phosphoenolpyruvate carboxykinase promoters, grow faster than normal animals and achieve a greater adult body size, often exhibiting a
very striking giant phenotype.33,34 These animals have much shorter lives than their normal siblings and exhibit multiple characteristics of early (premature) aging.35 Experimental animals
with extreme phenotypes, such as encountered in genetic dwarfs and giant transgenics, are useful for identifying the underlying mechanisms and previously unsuspected physiological
relationships. However, it is of obvious importance to determine whether the associations and causal relationships described in these animals apply to organisms that have not been
genetically altered and to genetic and phenotypic variations within the normal range. In fact, the negative association of longevity with adult body weight has been demonstrated in
comparisons of normal (“wild type”) animals form different stocks, inbred strains and selected lines in studies going back to the ‘60 s35,36,37 and, more recently, in comparisons of
individual animals from a normal, genetically heterogeneous population.38 Importantly, the negative association of body size and longevity extends to other mammalian species, with
differences in longevity between different breeds of domestic dogs, and between individual dogs differing in size providing the most striking example.39,40 It is interesting, but currently
difficult to explain, why these relationships within species are opposite to the fairly consistent trend for large species of mammals and birds living longer than smaller species. One could
speculate that because smaller species are more vulnerable to predation and other environmental hazards, they have developed life-course strategies for early reproduction and high fecundity.
This, in turn, may divert available resources away from repair and maintenance and thus lead to a shorter lifespan. The surprising tendency of circulating IGF-1 levels to be lower, rather
than higher, in large species41 may also prove to be a contributing factor to their longevity. SEARCH FOR MECHANISMS LINKING SOMATIC GROWTH WITH AGING AND LONGEVITY The (very consistent)
negative association of adult body size and longevity in laboratory mice and other mammalian species brings up the questions of causality and underlying mechanisms. Available data can be
interpreted as evidence that normal growth involves some intrinsic “costs” in terms of aging and longevity. Thus, faster or longer growth, and the consequent attainment of greater body size,
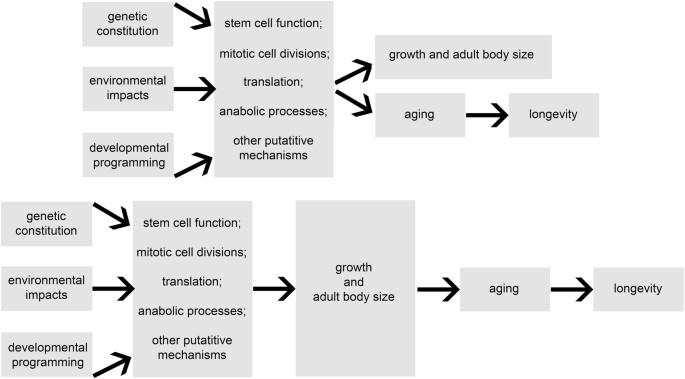
somehow predispose the organism to earlier and/or faster aging and a shorter lifespan. The underlying mechanisms could be envisaged to influence growth with a secondary impact on aging and
lifespan, or to independently influence both growth and aging, possibly via different signaling pathways or cellular processes (Fig. 1). In either case, identifying the mechanisms involved
is an important goal of our research and work in other laboratories. Comparing gene expression and phenotypic characteristics of long-lived mutants which have various defects in the
somatotropic axis with the same characteristics of age-matched and sex-matched normal animals from the same strain identified a number of suspected mechanisms of extended longevity.19,21
Defining the role of these mechanisms in the extension of healthspan and lifespan in the examined mutants is complicated by the fact that many of the differences between mutant and control
(“wild type”) animals could represent either mechanisms or markers of delayed and/or slower aging. For example, long-lived Ames dwarf and growth hormone receptor knockout (GHRKO) mice are
more insulin sensitive than their normal siblings42 but insulin sensitivity generally declines during aging.43,44 Thus, this difference could simply confirm the fact that at the same
chronological age, the long-lived dwarf mice are biologically younger. This difficult issue was addressed in one of our studies by comparing gene expression data in long-lived mutants to two
groups of normal animals that were either of the same chronological age or a comparable “biological age,” that is their age represented a similar percent of their life expectancy.45 In this
particular study, differences in hepatic expression of the examined genes in GHRKO vs. normal mice were shown to be due to the differences in genotype rather than in biological age.45
However, we are not aware of a similar analysis being performed in other long-lived mutants or in studies examining other candidate mechanisms of aging. Detailed discussion of all the
mechanisms that appear to be involved in linking reduced somatotropic signaling to slower aging and extended longevity is outside the scope of this review. Interested readers are referred to
several recent review articles.19,20,21 Mechanisms most likely related to the negative association of somatic growth and longevity and to the opposite effects of GH signaling on these
processes are briefly discussed below. MECHANISTIC TARGET OF RAPAMYCIN (MTOR) The ability of GH to directly or indirectly activate the mTOR signaling pathway provides one of the most likely
explanations of how this hormone can exert positive effects on somatic growth and negative effects on the lifespan. Mechanistic TOR (mTOR) pathway integrates somatotropic, nutritional and
stress signals and plays a role in the control of autophagy and cell senescence.46 While activation of mTOR signaling prevents cell death, promotes protein synthesis, growth and cell
divisions, it apparently also accelerates aging. Blagosklonny and his colleagues suggested that aging can be viewed as “a continuation of developmental growth driven by genetic pathways such
as mTOR”.47 These investigators also proposed that gender differences in longevity of mice may be related to greater mTOR activity in males48 and that conversion of reversible cell cycle
arrest to senescence (termed “geroconversion”) represents one of the mechanisms by which mTOR promotes aging.49 The excess “developmental” growth driven by mTOR presumably contributes to the
accumulation of unfolded proteins, endoplasmic reticulum (ER) stress, and reactive oxygen species (ROS) production, and leads to inhibition of autophagy and promotion of cell senescence.
Pharmacological or genetic suppression of mTOR signaling pathways results in extended longevity in organisms ranging from yeast to mice50,51,52 mTOR exerts its function via two complexes:
mTORC1 and mTORC2. There is increasing evidence that the “anti-aging” effect of mTOR suppression is due to inhibition of mTORC1 signaling. For example, long-lived mice with disrupted
somatotropic axis have diminished TORC1 signaling, while TORC2 activity may be increased53,54,55 mTORC2 appears to act to prevent, rather than promote aging, and consequently, its inhibition
can reduce longevity. The evidence for anti-aging effects of mTORC2 signaling includes demonstration that genetic deletion of Rictor, a mediator of TORC2 signaling, reduces longevity in
male mice.56 Interestingly, body weight and accumulation of white adipose tissue (WAT) in these animals were increased, suggesting that mTORC2, in contrast to mTORC1, may normally act as a
negative regulator of growth.57 Further support of the role of TORC-2 in the control of aging, adipose-specific deletion of Rictor leads to increases in body size, weight of non-adipose
organs and levels of IGF-1, IGFBP3, and insulin with a concomitant decrease of adiponectin, i.e., produces phenotypic characteristics generally associated with accelerated aging.57 ENERGY
METABOLISM AND THERMOGENESIS Another mechanism which may be contributing to the longevity benefits of small body size involves adjustments in mitochondrial function and energy metabolism in
response to greater heat loss and increased demand for thermogenesis. Smaller individuals have a greater body surface to body mass ratio and, thus, lose heat faster. Therefore, they have to
increase thermogenesis to maintain body temperature. This physiological response is of particular significance in laboratory mice whose thermoneutral temperature is ~30 °C (86 °F) and, thus,
housing at a “standard” animal room temperature (~22 °C, or 72 °F) represents a chronic mild cold stress.58 –60 The long-lived diminutive GHRKO and hypopituitary dwarf mice, in fact,
exhibit increases in the mass and activity of brown adipose tissue (BAT), the key site of non-shivering thermogenesis, along with evidence for thermogenic activation, the so-called
“browning” of WAT (Ref. 61, Darcy and Bartke, in press). Increased energy demand for maintaining a normal [or slight reduced,62 body temperature in these animals is reflected in increased
consumption of oxygen per gram of body weight63 and is accompanied by a marked reduction in respiratory quotient (RQ, equivalent to respiratory exchange ratio).63 Reduced RQ implies
increased reliance on fatty acids as a metabolic fuel which is generally considered a marker of improved mitochondrial efficiency leading to generation of smaller amounts of noxious
ROS.64,65,66,67 In fact, reduced RQ in long-lived dwarf mice is associated with increased expression of genes involved in β-oxidation of free fatty acids (Sun L, Darcy J, and Bartke A,
unpublished) and with reduced ROS generation.68 These alterations, together with improved anti-oxidant defenses18,69 probably account for less oxidative damage to DNA and other
macromolecules in these long-lived animals.69,70,71 Although it remains to be conclusively proven which of these associations are causally related to aging, we believe that improvements in
mitochondrial function in response to increased demand for thermogenesis represent one of the mechanisms linking reductions in somatotropic signaling, growth and adult body size to slower
(and/or delayed) aging and extended longevity.72,73 INDIRECT LINKS BETWEEN SOMATIC GROWTH AND LONGEVITY In addition to the mechanisms discussed above, the negative association of body size
and longevity within a species likely reflects several indirect, but important, mechanisms. These include pleiotropic actions of GH which affect both body size and aging, acting through
different, but likely physiologically related mechanisms. Growth hormone is a key regulator of hepatic IGF-1 expression, circulating IGF-1 levels, growth and adult body size, but it also
appears to promote aging by a variety of actions such as inducing insulin resistance35,42 promoting cell senescence74 and chronic low grade inflammation,75,76 as well as favoring
differentiation and depletion of at least one type of stem cell.77 Thus, the spectrum of known biological actions of GH could explain why growth and adult body size would tend to be
negatively associated with longevity.21,78 Another indirect link between body size and longevity concerns alterations in disease risk. Growth hormone and IGF-1, as well as stature, have been
related to cancer incidence on the basis of numerous in vitro, in vivo and epidemiological studies.79,80,81 Severe GH resistance in the syndrome of Laron dwarfism produces a remarkable
degree of protection from cancer.82 Neoplastic disease may have little if any influence on aging, but it has an obvious and major impact on longevity. This is particularly striking in
laboratory mice in which cancer is an important and, in many strains, by far the most common cause of death. The fact that GH induces insulin resistance, an important element of the
metabolic syndrome, prediabetes and diabetes, should also be mentioned in this context. The risk of diabetes is increased in association with abnormally elevated GH levels in acromegaly83
and reduced in the GH-resistant individuals with Laron syndrome.84 Impacts of nutrition on growth, body composition, disease risk and aging are outside of the scope of this brief article,
but can provide yet another indirect mechanism for the observed associations. Thus, both pre-natal and post-natal overnutrition can stimulate growth, as well as obesity and also increase
risk of various chronic diseases in adulthood.85,86 Rapid growth in response to food availability after a period of nutrient shortage, the so-called “catch-up growth”, may be particularly
important in this context.87,88,89 Increased risk of chronic disease in adult life can obviously reduce longevity and it also may represent a symptom of accelerated aging.90,91 Results of
recent and ongoing studies in our laboratory indicate that treatment of hypopituitary Ames dwarf mice with GH injections during a relatively brief period (6 weeks) during development
increases adult body size and significantly reduces the remarkably long lifespan of these animals.92,93 Importance of early (peripubertal) GH actions in the control of longevity is
indirectly supported by the recent report that disruption of the GH receptor gene in adult mice produces a relatively modest increase in lifespan only in females.94 It should also be
mentioned that the endocrine basis of the complex relationships between growth-related processes and aging is not limited to the somatotropic axis. Ames and Snell dwarfs, which represent
some of the extremes of mouse longevity, are both GH- and thyroid stimulating hormone-deficient and, thus, profoundly hypothyroid.18,19 Chronic thyroxine treatment can shorten longevity of
Snell dwarf mice,95 as well as normal rats 96 while subclinical hypothyroidism was associated with exceptional longevity in women.97,98 However, treatment of dwarf mice with thyroxine
limited to the peripubertal period did not alter their longevity.93,99 The relationships between the activity of the hypothalamic-pituitary-adrenal axis and aging are complex and poorly
understood.100 However, chronic elevation of glucocorticoid levels can promote age-related pathology, and is suspected of contributing to accelerated aging of GH transgenic mice.101 Much
more work will be needed to determine whether the association of reduced body size with extended longevity is truly causal, and to identify the mechanisms underlying this association.
CONCLUSIONS Negative association of longevity with GH signaling, somatic growth and adult body size discovered in GH-deficient and GH-resistant mutant mice applies also to genetically normal
individuals and to other mammalian species. Stimulation of mTORC1 singling by the somatotropic axis could explain this association but many other mechanisms appear to be involved.
REFERENCES * Samaras, T. T. & Storms, L. H. Impact of height and weight on life span. _Bull. World Health Organ._ 70, 259–267 (1992). PubMed PubMed Central CAS Google Scholar *
Samaras, T. T. & Elrick, H. Height, body size and longevity. _Acta Med. Okayama_ 53, 149–169 (1999). PubMed CAS Google Scholar * Samaras, T. T. _Human body size and the laws of
scaling: physiological, performance, growth, longevity and ecological ramifications_. (Nova Science Publishers, Inc., 1st edn, 2007), pp. 381. * Paajanen, T. A., Oksala, N. K., Kuukasjarvi,
P. & Karhunen, P. J. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. _Eur. Heart J._ 31, 1802–1809 (2010). Article
PubMed Google Scholar * Nelson, C. P. et al. Genetically determined height and coronary artery disease. _N. Engl. J. Med._ 372, 1608–1618 (2015). Article PubMed PubMed Central CAS
Google Scholar * Nuesch, E. et al. Adult height, coronary heart disease and stroke: a multi-locus Mendelian randomization meta-analysis. _Int. J. Epidemiol._ 45, 1927–1937 (2015). Article
PubMed Central Google Scholar * van Heemst, D. et al. Reduced insulin/IGF-1 signalling and human longevity. _Aging Cell_ 4, 79–85 (2005). Article PubMed CAS Google Scholar * Suh, Y. et
al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. _Proc. Natl Acad. Sci. USA_ 105, 3438–3442 (2008). Article PubMed Google Scholar * He, Q. et
al. Shorter men live longer: association of height with longevity and FOXO3 genotype in American men of Japanese ancestry. _PLoS One_ 9, e94385 (2014). Article PubMed PubMed Central CAS
Google Scholar * Milman, S. et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. _Aging Cell_ 13, 769–771 (2014). Article PubMed PubMed
Central CAS Google Scholar * Teumer, A. et al. Genomewide meta-analysis identifies loci associated with IGF-I and IGFBP-3 levels with impact on age-related traits. _Aging Cell_ 15,
811–824 (2016). Article PubMed PubMed Central CAS Google Scholar * De Luca, M., Crocco, P., De Rango, F., Passarino, G. & Rose, G. Association of the Laminin, Alpha 5 (LAMA5)
rs4925386 with height and longevity in an elderly population from Southern Italy. _Mech. Ageing Dev._ 155, 55–59 (2016). Article PubMed CAS Google Scholar * van der Spoel, E. et al.
Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity. _Aging Cell_ 15, 1126–1131 (2016). Article CAS PubMed Google Scholar * Johnson,
T. E., Conley, W. L. & Keller, M. L. Long-lived lines of _Caenorhabditis elegans_ can be used to establish predictive biomarkers of aging. _Exp. Gerontol._ 23, 281–295 (1988). Article
PubMed CAS Google Scholar * Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A _C. elegans_ mutant that lives twice as long as wild type. _Nature_ 366, 461–464 (1993).
Article PubMed CAS Google Scholar * Tatar, M., Bartke, A. & Antebi, A. The endocrine regulation of aging by insulin-like signals. _Science_ 299, 1346–1351 (2003). Article PubMed
CAS Google Scholar * Clancy, D. J. et al. Extension of life-span by loss of CHICO, a _Drosophila_ insulin receptor substrate protein. _Science_ 292, 104–106 (2001). Article PubMed CAS
Google Scholar * Brown-Borg, H. M. The somatotropic axis and longevity in mice. _Am. J. Physiol. Endocrinol. Metab._ 309, E503–E510 (2015). Article PubMed PubMed Central CAS Google
Scholar * Bartke, A. Single-gene mutations and healthy ageing in mammals. _Philos. Trans. R. Soc. Lond. B. Biol. Sci._ 366, 28–34 (2011). Article PubMed PubMed Central CAS Google
Scholar * Junnila, R. K., List, E. O., Berryman, D. E., Murrey, J. W. & Kopchick, J. J. The GH/IGF-1 axis in ageing and longevity. _Nat. Rev. Endocrinol._ 9, 366–376 (2013). Article
PubMed PubMed Central CAS Google Scholar * Bartke, A., Sun, L. Y. & Longo, V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. _Physiol.
Rev._ 93, 571–598 (2013). Article PubMed PubMed Central CAS Google Scholar * Flurkey, K., Papaconstantinou, J., Miller, R. A. & Harrison, D. E. Lifespan extension and delayed immune
and collagen aging in mutant mice with defects in growth hormone production. _Proc. Natl Acad. Sci. USA_ 98, 6736–6741 (2001). Article PubMed CAS Google Scholar * Coschigano, K. T. et
al. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span.
_Endocrinology_ 144, 3799–3810 (2003). Article PubMed CAS Google Scholar * Conover, C. A. PAPP-A: a new anti-aging target? _Aging Cell_ 9, 942–946 (2010). Article PubMed PubMed Central
CAS Google Scholar * Selman, C., Partridge, L. & Withers, D. J. Replication of extended lifespan phenotype in mice with deletion of insulin receptor substrate 1. _PLoS One_ 6, e16144
(2011). Article PubMed PubMed Central CAS Google Scholar * Taguchi, A. & White, M. F. Insulin-like signaling, nutrient homeostasis, and life span. _Annu. Rev. Physiol._ 70, 191–212
(2008). Article PubMed CAS Google Scholar * Nojima, A. et al. Haploinsufficiency of akt1 prolongs the lifespan of mice. _PLoS One_ 8, e69178 (2013). Article PubMed PubMed Central CAS
Google Scholar * Ikeno, Y. et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. _J. Gerontol. A. Biol.
Sci. Med. Sci._ 64, 522–529 (2009). Article PubMed CAS Google Scholar * Kinney, B. A., Meliska, C. J., Steger, R. W. & Bartke, A. Evidence that Ames dwarf mice age differently from
their normal siblings in behavioral and learning and memory parameters. _Horm. Behav._ 39, 277–284 (2001). Article PubMed CAS Google Scholar * Kinney, B. A., Coschigano, K. T., Kopchick,
J. J., Steger, R. W. & Bartke, A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. _Physiol. Behav._ 72, 653–660
(2001). Article PubMed CAS Google Scholar * de Magalhaes, J. P., Cabral, J. A. & Magalhaes, D. The influence of genes on the aging process of mice: a statistical assessment of the
genetics of aging. _Genetics_ 169, 265–274 (2005). Article PubMed PubMed Central CAS Google Scholar * Koopman, J. J. et al. Measuring aging rates of mice subjected to caloric
restriction and genetic disruption of growth hormone signaling. _Aging_ 8, 539–546 (2016). Article PubMed PubMed Central CAS Google Scholar * Palmiter, R. D. et al. Dramatic growth of
mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. _Nature_ 300, 611–615 (1982). Article PubMed PubMed Central CAS Google Scholar * McGrane, M.
M. et al. Metabolic effects of developmental, tissue-, and cell-specific expression of a chimeric phosphoenolpyruvate carboxykinase (GTP)/bovine growth hormone gene in transgenic mice. _J.
Biol. Chem._ 265, 22371–22379 (1990). PubMed CAS Google Scholar * Bartke, A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. _Neuroendocrinology_ 78, 210–216
(2003). Article PubMed CAS Google Scholar * Roberts, R. C. The lifetime growth and reproduction of selected strains of mice. _Heredity_ 16, 369–381 (1961). Article Google Scholar *
Eklund, J. & Bradford, G. E. Longeveity and lifetime body weight in mice selected for rapid growth. _Nature_ 265, 48–49 (1977). Article PubMed CAS Google Scholar * Miller, R. A.,
Harper, J. M., Galecki, A. & Burke, D. T. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. _Aging Cell_ 1, 22–29 (2002). Article PubMed
CAS Google Scholar * Patronek, G. J., Waters, D. J. & Glickman, L. T. Comparative longevity of pet dogs and humans: implications for gerontology research. _J. Gerontol. A. Biol. Sci.
Med. Sci._ 52, B171–B178 (1997). Article PubMed CAS Google Scholar * Greer, K. A., Canterberry, S. C. & Murphy, K. E. Statistical analysis regarding the effects of height and weight
on life span of the domestic dog. _Res. Vet. Sci._ 82, 208–214 (2007). Article PubMed Google Scholar * Stuart, J. A. & Page, M. M. Plasma IGF-1 is negatively correlated with body mass
in a comparison of 36 mammalian species. _Mech. Ageing Dev._ 131, 591–598 (2010). Article PubMed CAS Google Scholar * Masternak, M. M., Panici, J. A., Bonkowski, M. S., Hughes, L. F.
& Bartke, A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. _J. Gerontol. A. Biol. Sci. Med. Sci._ 64, 516–521 (2009). Article PubMed CAS Google Scholar
* DeFronzo, R. A. Glucose intolerance and aging. _Diabetes Care_ 4, 493–501 (1981). Article PubMed CAS Google Scholar * Xu, C. et al. Selective overexpression of human SIRT1 in adipose
tissue enhances energy homeostasis and prevents the deterioration of insulin sensitivity with ageing in mice. _Am. J. Transl. Res._ 5, 412–426 (2013). PubMed PubMed Central CAS Google
Scholar * Panici, J. A. et al. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans?
_J. Gerontol. A. Biol. Sci. Med. Sci._ 64, 1126–1133 (2009). Article PubMed CAS Google Scholar * Lees, H., Walters, H. & Cox, L. S. Animal and human models to understand ageing.
_Maturitas_ 93, 18–27 (2016). Article PubMed Google Scholar * Blagosklonny, M. V. Aging is not programmed: genetic pseudo-program is a shadow of developmental growth. _Cell Cycle_ 12,
3736–3742 (2013). Article PubMed PubMed Central CAS Google Scholar * Leontieva, O. V., Paszkiewicz, G. M. & Blagosklonny, M. V. Mechanistic or mammalian target of rapamycin (mTOR)
may determine robustness in young male mice at the cost of accelerated aging. _Aging_ 4, 899–916 (2012). Article PubMed PubMed Central CAS Google Scholar * Blagosklonny, M. V.
Geroconversion: irreversible step to cellular senescence. _Cell Cycle_ 13, 3628–3635 (2014). Article PubMed PubMed Central CAS Google Scholar * Harrison, D. E. et al. Rapamycin fed late
in life extends lifespan in genetically heterogeneous mice. _Nature_ 460, 392–395 (2009). Article PubMed PubMed Central CAS Google Scholar * Johnson, S. C., Rabinovitch, P. S. &
Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. _Nature_ 493, 338–345 (2013). Article PubMed PubMed Central CAS Google Scholar * McCormick, M. A., Tsai, S. Y.
& Kennedy, B. K. TOR and ageing: a complex pathway for a complex process. _Philos. Trans. R. Soc. Lond. B. Biol. Sci._ 366, 17–27 (2011). Article PubMed PubMed Central CAS Google
Scholar * Sharp, Z. D. & Bartke, A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory
signaling pathways in Ames dwarf mice. _J. Gerontol. A. Biol. Sci. Med. Sci._ 60, 293–300 (2005). Article PubMed Google Scholar * Dominick, G. et al. Regulation of mTOR activity in Snell
dwarf and GH receptor gene-disrupted mice. _Endocrinology_ 156, 565–575 (2015). Article PubMed CAS Google Scholar * Lamming, D. W. et al. Rapamycin-induced insulin resistance is mediated
by mTORC2 loss and uncoupled from longevity. _Science_ 335, 1638–1643 (2012). Article PubMed PubMed Central CAS Google Scholar * Lamming, D. W. et al. Depletion of Rictor, an essential
protein component of mTORC2, decreases male lifespan. _Aging Cell_ 13, 911–917 (2014). Article PubMed PubMed Central CAS Google Scholar * Cybulski, N., Polak, P., Auwerx, J., Ruegg, M.
A. & Hall, M. N. mTOR complex 2 in adipose tissue negatively controls whole-body growth. _Proc. Natl Acad. Sci. USA_ 106, 9902–9907 (2009). Article PubMed Google Scholar * Gordon, C.
J. Thermal physiology of laboratory mice: definining thermoneutrality. _J. Therm. Biol._ 37, 654–685 (2012). Article Google Scholar * Karp, C. L. Unstressing intemperate models: how cold
stress undermines mouse modeling. _J. Exp. Med._ 209, 1069–1074 (2012). Article PubMed PubMed Central CAS Google Scholar * Maloney, S. K., Fuller, A., Mitchell, D., Gordon, C. &
Overton, J. M. Translating animal model research: does it matter that our rodents are cold? _Physiol._ 29, 413–420 (2014). Article CAS Google Scholar * Li, Y., Knapp, J. R. &
Kopchick, J. J. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. _Exp. Biol. Med._ 228,
207–215 (2003). Article CAS Google Scholar * Hunter, W. S., Croson, W. B., Bartke, A., Gentry, M. V. & Meliska, C. J. Low body temperature in long-lived Ames dwarf mice at rest and
during stress. _Physiol. Behav._ 67, 433–437 (1999). Article PubMed CAS Google Scholar * Westbrook, R., Bonkowski, M. S., Arum, O., Strader, A. D. & Bartke, A. Metabolic alterations
due to caloric restriction and every other day feeding in normal and growth hormone receptor knockout mice. _J. Gerontol. A. Biol. Sci. Med. Sci._ 69, 25–33 (2014). Article PubMed CAS
Google Scholar * Anderson, R. M. & Weindruch, R. Metabolic reprogramming, caloric restriction and aging. _Trends Endocrinol. Metab._ 21, 134–141 (2010). Article PubMed CAS Google
Scholar * Perdomo, G. et al. Increased beta-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite
intramyocellular lipid accumulation. _J. Biol. Chem._ 279, 27177–27186 (2004). Article PubMed CAS Google Scholar * Gonzalez-Covarrubias, V. et al. Lipidomics of familial longevity.
_Aging Cell_ 12, 426–434 (2013). Article PubMed PubMed Central CAS Google Scholar * Orellana-Gavalda, J. M. et al. Molecular therapy for obesity and diabetes based on a long-term
increase in hepatic fatty-acid oxidation. _Hepatology_ 53, 821–832 (2011). Article PubMed CAS Google Scholar * Brown-Borg, H. M., Johnson, W. T. & Rakoczy, S. G. Expression of
oxidative phosphorylation components in mitochondria of long-living Ames dwarf mice. _Age_ 34, 43–57 (2012). Article PubMed CAS Google Scholar * Brown-Borg, H. M., Bode, A. M. &
Bartke, A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. _Endocrine_ 11, 41–48 (1999). Article PubMed CAS Google Scholar *
Brown-Borg, H. M. & Rakoczy, S. G. Catalase expression in delayed and premature aging mouse models. _Exp. Gerontol._ 35, 199–212 (2000). Article PubMed CAS Google Scholar * Sanz, A.,
Bartke, A. & Barja, G. Long-lived Ames dwarf mice: oxidative damage to mitochondrial DNA in heart and brain. _J. Am. Aging Assoc._ 25, 119–122 (2002). PubMed PubMed Central CAS
Google Scholar * Brown-Borg, H. M. & Bartke, A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. _J. Gerontol. A. Biol. Sci. Med. Sci._ 67, 652–660 (2012). Article
PubMed CAS Google Scholar * Westbrook R. Ph.D. _The effects of altered growth hormone signaling on murine metabolism_. Dissertation, Southern Illinois University (2012). * Stout, M. B.
et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. _Aging_ 6, 575–586 (2014). Article PubMed PubMed Central Google
Scholar * Bartke, A. Healthspan and longevity can be extended by suppression of growth hormone signaling. _Mamm. Genome_ 27, 289–299 (2016). Article PubMed PubMed Central CAS Google
Scholar * Sadagurski, M. et al. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. _Aging Cell_ 14, 1045–1054 (2015). Article PubMed PubMed Central
CAS Google Scholar * Ratajczak, J. et al. Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice--novel view on Igf-1, stem cells and aging.
_Leukemia_ 25, 729–733 (2011). Article PubMed PubMed Central CAS Google Scholar * Bartke, A., List, E. O. & Kopchick, J. J. The somatotropic axis and aging: Benefits of endocrine
defects. _Growth Horm. Igf. Res._ 27, 41–45 (2016). Article PubMed PubMed Central CAS Google Scholar * Green, J. et al. Height and cancer incidence in the Million Women Study:
prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. _Lancet Oncol._ 12, 785–794 (2011). Article PubMed PubMed Central Google Scholar * Batty, G.
D. et al. Adult height and cancer mortality in Asia: the Asia Pacific Cohort Studies Collaboration. _Ann. Oncol._ 21, 646–654 (2010). Article PubMed CAS Google Scholar * C. Emerging
Risk Factors. Adult height and the risk of cause-specific death and vascular morbidity in 1 million people: individual participant meta-analysis. _Int. J. Epidemiol._ 41, 1419–1433 (2012).
Article Google Scholar * Laron, Z. The GH-IGF1 axis and longevity. The paradigm of IGF1 deficiency. _Hormones-Athens_ 7, 24–27 (2008). Article PubMed Google Scholar * Mercado, M. et al.
Successful mortality reduction and control of comorbidities in patients with acromegaly followed at a highly specialized multidisciplinary clinic. _J. Clin. Endocrinol. Metab._ 99,
4438–4446 (2014). Article PubMed CAS Google Scholar * Guevara-Aguirre, J. et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer,
and diabetes in humans. _Sci. Transl. Med_. 3, 70ra13 (2011). Article PubMed PubMed Central CAS Google Scholar * Ozanne, S. E., Fernandez-Twinn, D. & Hales, C. N. Fetal growth and
adult diseases. _Semin. Perinatol._ 28, 81–87 (2004). Article PubMed Google Scholar * Blackmore, H. L. & Ozanne, S. E. Maternal diet-induced obesity and offspring cardiovascular
health. _J. Dev. Orig. Health Dis._ 4, 338–347 (2013). Article PubMed CAS Google Scholar * Hales, C. N. & Ozanne, S. E. The dangerous road of catch-up growth. _J. Physiol._ 547, 5–10
(2003). Article PubMed CAS Google Scholar * Eriksson, J. G. et al. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. _BMJ_ 318, 427–431 (1999).
Article PubMed PubMed Central CAS Google Scholar * Cettour-Rose, P. et al. Redistribution of glucose from skeletal muscle to adipose tissue during catch-up fat: a link between catch-up
growth and later metabolic syndrome. _Diabetes_ 54, 751–756 (2005). Article PubMed CAS Google Scholar * Kirkland, J. L. The biology of senescence: potential for prevention of disease.
_Clin. Geriatr. Med._ 18, 383–405 (2002). Article PubMed Google Scholar * Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. _Cell_ 159, 709–713 (2014). Article PubMed
PubMed Central CAS Google Scholar * Bartke, A. Early life events can shape aging and longevity. _Curr. Aging Sci._ 8, 11–13 (2015). Article PubMed CAS Google Scholar * Panici, J. A.
et al. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. _FASEB J._ 24, 5073–5079 (2010). Article PubMed PubMed
Central CAS Google Scholar * Junnila, R. K. et al. Disruption of the growth hormone receptor gene in adult mice increases maximal lifespan in females. _Endocrinology_ 157, 4502–4513
(2016). Article PubMed CAS Google Scholar * Vergara, M., Smith-Wheelock, M., Harper, J. M., Sigler, R. & Miller, R. A. Hormone-treated snell dwarf mice regain fertility but remain
long lived and disease resistant. _J. Gerontol. A. Biol. Sci. Med. Sci._ 59, 1244–1250 (2004). Article PubMed PubMed Central Google Scholar * Ooka, H. & Shinkai, T. Effects of
chronic hyperthyroidism on the lifespan of the rat. _Mech. Ageing Dev._ 33, 275–282 (1986). Article PubMed CAS Google Scholar * Rozing, M. P. et al. Familial longevity is associated with
decreased thyroid function. _J. Clin. Endocrinol. Metab._ 95, 4979–4984 (2010). Article PubMed CAS Google Scholar * Bowers, J. et al. Thyroid hormone signaling and homeostasis during
aging. _Endocr. Rev._ 34, 556–589 (2013). Article PubMed CAS Google Scholar * Darcy, J. et al. Original Research: metabolic alterations from early life thyroxine replacement therapy in
male Ames dwarf mice are transient. _Exp. Biol. Med._ 241, 1764–1771 (2016). Article CAS Google Scholar * Gupta, D. & Morley, J. E. Hypothalamic-pituitary-adrenal (HPA) axis and
aging. _Compr. Physiol._ 4, 1495–1510 (2014). Article PubMed Google Scholar * Cecim, M., Alvarez-Sanz, M., Van de Kar, L., Milton, S. & Bartke, A. Increased plasma corticosterone
levels in bovine growth hormone (bGH) transgenic mice: effects of ACTH, GH and IGF-I on in vitro adrenal corticosterone production. _Transgenic Res._ 5, 187–192 (1996). Article PubMed CAS
Google Scholar Download references ACKNOWLEDGEMENTS Helpful discussions with Dr. Yimin (Julia) Fang and contributions of past and present members of our laboratory are gratefully
acknowledged. Sincere apologies are extended to those whose work relevant to the topic of this article was not cited or discussed due to inadvertent omissions. Our work and the writing of
this article were supported by NIH R01AG019899 and R21AG051869. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Internal Medicine, Southern Illinois University School of
Medicine, 801N. Rutledge, P.O. Box 19628, Springfield, IL, 62794-9628, USA Andrzej Bartke Authors * Andrzej Bartke View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS A.B. searched the literature, reviewed unpublished data, and wrote the article. CORRESPONDING AUTHOR Correspondence to Andrzej Bartke. ETHICS DECLARATIONS
COMPETING INTERESTS The author declares that they have no competing financial interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bartke, A. Somatic growth, aging, and longevity. _npj Aging Mech Dis_ 3, 14 (2017).
https://doi.org/10.1038/s41514-017-0014-y Download citation * Received: 25 October 2016 * Revised: 06 September 2017 * Accepted: 13 September 2017 * Published: 29 September 2017 * DOI:
https://doi.org/10.1038/s41514-017-0014-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative