Play all audios:
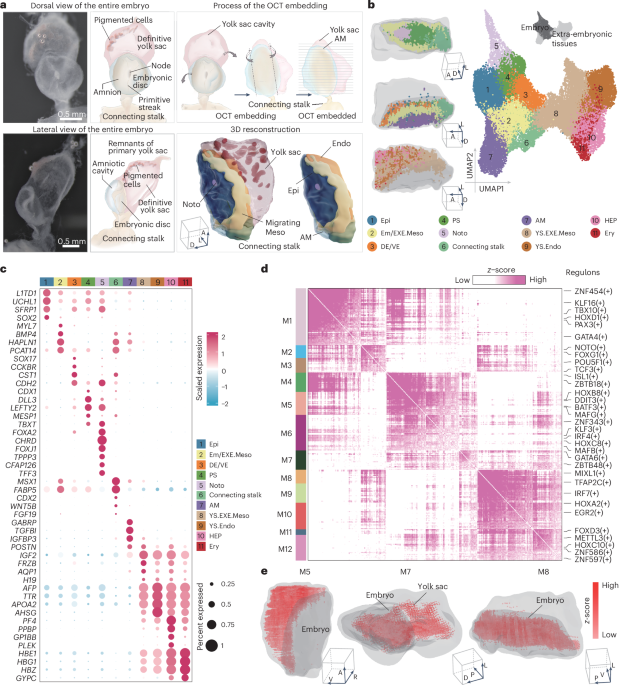
ABSTRACT Gastrulation marks a pivotal stage in mammalian embryonic development, establishing the three germ layers and body axis through lineage diversification and morphogenetic movements.
However, studying human gastrulating embryos is challenging due to limited access to early tissues. Here we show the use of spatial transcriptomics to analyse a fully intact Carnegie stage 7
human embryo at single-cell resolution, along with immunofluorescence validations in a second embryo. Employing 82 serial cryosections and Stereo-seq technology, we reconstructed a
three-dimensional model of the embryo. Our findings reveal early specification of distinct mesoderm subtypes and the presence of the anterior visceral endoderm. Notably, primordial germ
cells were located in the connecting stalk, and haematopoietic stem cell-independent haematopoiesis was observed in the yolk sac. This study advances our understanding of human gastrulation
and provides a valuable dataset for future research in early human development. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS SINGLE-CELL TRANSCRIPTOMIC CHARACTERIZATION OF A GASTRULATING HUMAN EMBRYO Article 17 November
2021 A SINGLE-CELL TRANSCRIPTOME ATLAS PROFILES EARLY ORGANOGENESIS IN HUMAN EMBRYOS Article 16 March 2023 SPATIOTEMPORAL TRANSCRIPTOMIC MAPS OF WHOLE MOUSE EMBRYOS AT THE ONSET OF
ORGANOGENESIS Article Open access 06 July 2023 DATA AVAILABILITY Sequencing data that support the findings of this study have been deposited in the Genome Sequence Archive63 in National
Genomics Data Center, China National Center for Bioinformation64, under accession code HRA006197. Previously published human CS7 embryo data that were re-analysed here are available under
accession code E-MTAB-9388. Previously published cynomolgus monkey embryo data that were re-analysed here are available under accession code GSE193007. Previously published marmoset CS5–CS7
embryo data that were re-analysed here are available under accession code E-MTAB-9367. Previously published mouse E5.25–E6.5 embryo data that were re-analysed here are available under
accession code CRA008972. Previously published human CS8 embryo data that were re-analysed here are available under accession code HRA005567. Previously published human primordial germ
cell-like cell data that were re-analysed here are available under accession code GSE205611. Previously published PGC data that were re-analysed here are available under accession code
GSE223036. Previously published human primordial germ cell-like cell data that were re-analysed here are available under accession code GSE231812. Human reference genome (hg38) and gene
annotations (Ensembl Genome Browser GRCh38.107) were used for alignment and gene count matrix generation. The CellchatDB.human was obtained through https://github.com/sqjin/CellChat. Data
supporting the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper. REFERENCES * Bergmann, S. et al. Spatial
profiling of early primate gastrulation in utero. _Nature_ 609, 136–143 (2022). Article CAS PubMed PubMed Central Google Scholar * Zhai, J. et al. Primate gastrulation and early
organogenesis at single-cell resolution. _Nature_ 612, 732–738 (2022). Article CAS PubMed PubMed Central Google Scholar * Tyser, R. C. V. et al. Single-cell transcriptomic
characterization of a gastrulating human embryo. _Nature_ 600, 285–289 (2021). Article CAS PubMed PubMed Central Google Scholar * Xiao, Z. et al. 3D reconstruction of a gastrulating
human embryo. _Cell_ 187, 2855–2874 e19 (2024). Article CAS PubMed Google Scholar * de Bakker, B. S. et al. An interactive three-dimensional digital atlas and quantitative database of
human development. _Science_ 354, aag0053 (2016). Article PubMed Google Scholar * Wang, M. et al. High-resolution 3D spatiotemporal transcriptomic maps of developing Drosophila embryos
and larvae. _Dev. Cell_ 57, 1271–1283 e1274 (2022). Article CAS PubMed Google Scholar * Chen, A. et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA
nanoball-patterned arrays. _Cell_ 185, 1777–1792 e1721 (2022). Article CAS PubMed Google Scholar * Van de Sande, B. et al. A scalable SCENIC workflow for single-cell gene regulatory
network analysis. _Nat. Protoc._ 15, 2247–2276 (2020). Article PubMed Google Scholar * DeTomaso, D. & Yosef, N. Hotspot identifies informative gene modules across modalities of
single-cell genomics. _Cell Syst._ 12, 446–456 e449 (2021). Article CAS PubMed Google Scholar * Liu, G. et al. Spatial transcriptomic profiling to identify mesoderm progenitors with
precision genomic screening and functional confirmation. _Cell Prolif._ 55, e13298 (2022). Article CAS PubMed PubMed Central Google Scholar * Tani, S., Chung, U. I., Ohba, S. &
Hojo, H. Understanding paraxial mesoderm development and sclerotome specification for skeletal repair. _Exp. Mol. Med._ 52, 1166–1177 (2020). Article CAS PubMed PubMed Central Google
Scholar * Newton, A. H., Williams, S. M., Major, A. T. & Smith, C. A. Cell lineage specification and signalling pathway use during development of the lateral plate mesoderm and forelimb
mesenchyme. _Development_ 149, dev200702 (2022). Article PubMed Google Scholar * Sambasivan, R. & Steventon, B. Neuromesodermal progenitors: a basis for robust axial patterning in
development and evolution. _Front. Cell Dev. Biol._ 8, 607516 (2020). Article PubMed Google Scholar * Yang, R. et al. Amnion signals are essential for mesoderm formation in primates.
_Nat. Commun._ 12, 5126 (2021). Article CAS PubMed PubMed Central Google Scholar * Pham, T. X. A. et al. Modeling human extraembryonic mesoderm cells using naive pluripotent stem cells.
_Cell Stem Cell_ 29, 1346–1365 e1310 (2022). Article CAS PubMed PubMed Central Google Scholar * Beddington, R. S. & Robertson, E. J. Axis development and early asymmetry in
mammals. _Cell_ 96, 195–209 (1999). Article CAS PubMed Google Scholar * Rivera-Perez, J. A. & Magnuson, T. Primitive streak formation in mice is preceded by localized activation of
Brachyury and Wnt3. _Dev. Biol._ 288, 363–371 (2005). Article CAS PubMed Google Scholar * Madabhushi, M. & Lacy, E. Anterior visceral endoderm directs ventral morphogenesis and
placement of head and heart via BMP2 expression. _Dev. Cell_ 21, 907–919 (2011). Article CAS PubMed PubMed Central Google Scholar * Soares, M. L., Torres-Padilla, M. E. &
Zernicka-Goetz, M. Bone morphogenetic protein 4 signaling regulates development of the anterior visceral endoderm in the mouse embryo. _Dev. Growth Differ._ 50, 615–621 (2008). Article CAS
PubMed PubMed Central Google Scholar * Sun, X., Meyers, E. N., Lewandoski, M. & Martin, G. R. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse
embryo. _Genes Dev._ 13, 1834–1846 (1999). Article CAS PubMed PubMed Central Google Scholar * Li, C., Li, Y. P., Fu, X. Y. & Deng, C. X. Anterior visceral endoderm SMAD4 signaling
specifies anterior embryonic patterning and head induction in mice. _Int J. Biol. Sci._ 6, 569–583 (2010). Article CAS PubMed PubMed Central Google Scholar * Andersson, O., Bertolino,
P. & Ibanez, C. F. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. _Dev. Biol._ 311, 500–511 (2007). Article CAS PubMed
Google Scholar * Kimura, C. et al. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. _Dev. Biol._ 225, 304–321 (2000). Article CAS PubMed Google
Scholar * Kinder, S. J. et al. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. _Development_ 128, 3623–3634 (2001).
Article CAS PubMed Google Scholar * Zhu, Q. et al. Decoding anterior-posterior axis emergence among mouse, monkey, and human embryos. _Dev. Cell_ 58, 63–79 e64 (2023). Article CAS
PubMed Google Scholar * Finley, K. R., Tennessen, J. & Shawlot, W. The mouse secreted frizzled-related protein 5 gene is expressed in the anterior visceral endoderm and foregut
endoderm during early post-implantation development. _Gene Expr. Patterns_ 3, 681–684 (2003). Article CAS PubMed Google Scholar * Kimura-Yoshida, C. et al. Canonical Wnt signaling and
its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. _Dev. Cell_ 9, 639–650 (2005). Article CAS PubMed Google Scholar *
Matsuda, K. & Kondoh, H. Dkk1-dependent inhibition of Wnt signaling activates Hesx1 expression through its 5’ enhancer and directs forebrain precursor development. _Genes Cells_ 19,
374–385 (2014). Article CAS PubMed Google Scholar * Kemp, C. R. et al. Expression of Frizzled5, Frizzled7, and Frizzled10 during early mouse development and interactions with canonical
Wnt signaling. _Dev. Dyn._ 236, 2011–2019 (2007). Article CAS PubMed Google Scholar * Richardson, B. E. & Lehmann, R. Mechanisms guiding primordial germ cell migration: strategies
from different organisms. _Nat. Rev. Mol. Cell Biol._ 11, 37–49 (2010). Article CAS PubMed PubMed Central Google Scholar * Guo, F. et al. The transcriptome and DNA methylome landscapes
of human primordial germ cells. _Cell_ 161, 1437–1452 (2015). Article CAS PubMed Google Scholar * Garcia-Alonso, L. et al. Single-cell roadmap of human gonadal development. _Nature_ 607,
540–547 (2022). Article CAS PubMed PubMed Central Google Scholar * Ai, Z. et al. Dissecting peri-implantation development using cultured human embryos and embryo-like assembloids.
_Cell Res_ 33, 661–678 (2023). Article PubMed PubMed Central Google Scholar * Tang, W. W. C. et al. Sequential enhancer state remodelling defines human germline competence and
specification. _Nat. Cell Biol._ 24, 448–460 (2022). Article CAS PubMed PubMed Central Google Scholar * La Manno, G. et al. RNA velocity of single cells. _Nature_ 560, 494–498 (2018).
Article PubMed PubMed Central Google Scholar * Yu, L. et al. Derivation of intermediate pluripotent stem cells amenable to primordial germ cell specification. _Cell Stem Cell_ 28,
550–567 e512 (2021). Article CAS PubMed Google Scholar * Irie, N. et al. DMRT1 regulates human germline commitment. _Nat. Cell Biol._ 25, 1439–1452 (2023). Article CAS PubMed PubMed
Central Google Scholar * Zheng, Y. et al. Single-cell analysis of embryoids reveals lineage diversification roadmaps of early human development. _Cell Stem Cell_ 29, 1402–1419 e1408
(2022). Article CAS PubMed PubMed Central Google Scholar * Harrison, S. E., Sozen, B., Christodoulou, N., Kyprianou, C. & Zernicka-Goetz, M. Assembly of embryonic and extraembryonic
stem cells to mimic embryogenesis in vitro. _Science_ 356, eaal1810 (2017). Article PubMed Google Scholar * Zhang, J. et al. OTX2 restricts entry to the mouse germline. _Nature_ 562,
595–599 (2018). Article CAS PubMed PubMed Central Google Scholar * Yu, S. et al. BMP4 drives primed to naive transition through PGC-like state. _Nat. Commun._ 13, 2756 (2022). Article
CAS PubMed PubMed Central Google Scholar * Zheng, Y. et al. Controlled modelling of human epiblast and amnion development using stem cells. _Nature_ 573, 421–425 (2019). Article CAS
PubMed PubMed Central Google Scholar * Murase, Y. et al. In vitro reconstitution of epigenetic reprogramming in the human germ line. _Nature_ 631, 170–178 (2024). Article CAS PubMed
PubMed Central Google Scholar * Esfahani, S. N. et al. Derivation of human primordial germ cell-like cells in an embryonic-like culture. _Nat. Commun._ 15, 167 (2024). Article CAS PubMed
PubMed Central Google Scholar * Vijayakumar, S. et al. Monolayer platform to generate and purify primordial germ-like cells in vitro provides insights into human germline specification.
_Nat. Commun._ 14, 5690 (2023). Article CAS PubMed PubMed Central Google Scholar * Bian, Z. et al. Deciphering human macrophage development at single-cell resolution. _Nature_ 582,
571–576 (2020). Article CAS PubMed Google Scholar * Dzierzak, E. & Bigas, A. Blood development: hematopoietic stem cell dependence and independence. _Cell Stem Cell_ 22, 639–651
(2018). Article CAS PubMed Google Scholar * Wang, H. et al. Decoding human megakaryocyte development. _Cell Stem Cell_ 28, 535–549 e538 (2021). Article CAS PubMed Google Scholar *
Stuart, T. et al. Comprehensive integration of single-cell data. _Cell_ 177, 1888–1902 e1821 (2019). Article CAS PubMed PubMed Central Google Scholar * Wolf, F. A., Angerer, P. &
Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. _Genome Biol._ 19, 15 (2018). Article PubMed PubMed Central Google Scholar * Palla, G. et al. Squidpy: a
scalable framework for spatial omics analysis. _Nat. Methods_ 19, 171–178 (2022). Article CAS PubMed PubMed Central Google Scholar * Kumar, N., Mishra, B., Athar, M. & Mukhtar, S.
Correction to: inference of gene regulatory network from single-cell transcriptomic data using pySCENIC. _Methods Mol. Biol._ 2328, C1 (2021). Article CAS PubMed Google Scholar * Gu, Z.,
Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. _Bioinformatics_ 32, 2847–2849 (2016). Article CAS PubMed Google Scholar
* Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. _Nat. Commun._ 12, 1088 (2021). Article CAS PubMed PubMed Central Google Scholar * Jin, S., Plikus,
M. V. & Nie, Q. CellChat for systematic analysis of cell-cell communication from single-cell transcriptomics. _Nat. Protoc._ 20, 180–219 (2025). Article CAS PubMed Google Scholar *
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. _Nat. Biotechnol._ 38, 1408–1414 (2020).
Article CAS PubMed Google Scholar * Wolf, F. A. et al. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. _Genome
Biol._ 20, 59 (2019). Article PubMed PubMed Central Google Scholar * Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. _Nat. Methods_ 14, 979–982 (2017).
Article CAS PubMed PubMed Central Google Scholar * Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. _Nature_ 566, 496–502 (2019). Article CAS
PubMed PubMed Central Google Scholar * Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. _Innovation_ 2, 100141 (2021). CAS PubMed PubMed
Central Google Scholar * Zeira, R., Land, M., Strzalkowski, A. & Raphael, B. J. Alignment and integration of spatial transcriptomics data. _Nat. Methods_ 19, 567–575 (2022). Article
CAS PubMed PubMed Central Google Scholar * Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor
package biomaRt. _Nat. Protoc._ 4, 1184–1191 (2009). Article CAS PubMed PubMed Central Google Scholar * Chen, T. et al. The genome sequence archive family: toward explosive data growth
and diverse data types. _Genomics Proteom. Bioinform._ 19, 578–583 (2021). Article Google Scholar * CNCB-NGDC Members and Partners. Database resources of the National Genomics Data Center,
China National Center for Bioinformation in 2022. _Nucleic Acids Res._ 50, D27–D38 (2022). Article Google Scholar Download references ACKNOWLEDGEMENTS We thank Q. Zhou for his invaluable
support and guidance. This work was supported by the National Key Research and Development Program of China (2022YFA1104100 and 2022YFC2702600 to J.G. and 2022YFA1103100 and 2022YFA1104300
to L.Y.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0820000 to J.G. and L.Y.), the start-up funding support from the Institute of Zoology and Chinese
Academy of Sciences to J.G. and L.Y., Initiative Scientific Research program supported by the Institute of Zoology, Chinese Academy of Sciences (202310Z0102 to J.G.), the Chinese
Universities Scientific Fund to Y.W., the Biological Breeding-National Science and Technology Major Project 2023ZD0407504 to Y.W., The National Natural Science Foundation of China (32400663
to Y.W., 2022CX11015 and 82371685 to Z.X., and 82101764 to N.H.) and the Beijing Nova Program to Z.X. We acknowledge assistance with the access of analytic instruments from the Translational
Medicine Center at The First Affiliated Hospital of Zhengzhou University. We thank all the members from Guo Laboratory, Wei Laboratory, Xiao Laboratory and Yu Laboratory for their fruitful
discussions. AUTHOR INFORMATION Author notes * These authors contributed equally: Lina Cui, Sirui Lin, Xiaolong Yang, Xinwei Xie, Xiaoyan Wang, Nannan He. AUTHORS AND AFFILIATIONS * Key
Laboratory of Organ Regeneration and Reconstruction, State Key Laboratory of Stem Cell and Reproductive Biology, Institute of Zoology, University of Chinese Academy of Sciences, Chinese
Academy of Sciences, Beijing, China Lina Cui, Xiaoyan Wang, Xiaojian Lu, Xiaodi Yan, Yifei Guo, Bailing Zhang, Ran Li, Hefan Miao, Leqian Yu & Jingtao Guo * Frontiers Science Center for
Molecular Design Breeding (MOE), China Agricultural University, Beijing, China Sirui Lin, Xin Zhang & Yulei Wei * State Key Laboratory of Animal Biotech Breeding, College of Biological
Sciences, China Agricultural University, Beijing, China Sirui Lin, Xin Zhang & Yulei Wei * School of Life Science, Beijing Institute of Technology, Beijing, China Xiaolong Yang, Xinwei
Xie, Jingyu Yang, Runzhao Zhang & Zhenyu Xiao * Beijing Institute for Stem Cell and Regenerative Medicine, Beijing, China Xiaoyan Wang, Leqian Yu & Jingtao Guo * Department of
Gynecology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China Nannan He & Mei Ji * Institute for Stem Cell and Regeneration, Chinese Academy of Sciences, Beijing,
China Leqian Yu & Jingtao Guo Authors * Lina Cui View author publications You can also search for this author inPubMed Google Scholar * Sirui Lin View author publications You can also
search for this author inPubMed Google Scholar * Xiaolong Yang View author publications You can also search for this author inPubMed Google Scholar * Xinwei Xie View author publications You
can also search for this author inPubMed Google Scholar * Xiaoyan Wang View author publications You can also search for this author inPubMed Google Scholar * Nannan He View author
publications You can also search for this author inPubMed Google Scholar * Jingyu Yang View author publications You can also search for this author inPubMed Google Scholar * Xin Zhang View
author publications You can also search for this author inPubMed Google Scholar * Xiaojian Lu View author publications You can also search for this author inPubMed Google Scholar * Xiaodi
Yan View author publications You can also search for this author inPubMed Google Scholar * Yifei Guo View author publications You can also search for this author inPubMed Google Scholar *
Bailing Zhang View author publications You can also search for this author inPubMed Google Scholar * Ran Li View author publications You can also search for this author inPubMed Google
Scholar * Hefan Miao View author publications You can also search for this author inPubMed Google Scholar * Mei Ji View author publications You can also search for this author inPubMed
Google Scholar * Runzhao Zhang View author publications You can also search for this author inPubMed Google Scholar * Leqian Yu View author publications You can also search for this author
inPubMed Google Scholar * Zhenyu Xiao View author publications You can also search for this author inPubMed Google Scholar * Yulei Wei View author publications You can also search for this
author inPubMed Google Scholar * Jingtao Guo View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.G. and L.Y. conceptualized the idea. J.G.,
Y.W., Z.X. and L.Y. designed, interpreted and supervised the experiments. N.H. performed the human embryo sample collection. Z.X. and Y.W. performed Stereo-seq library construction and
sequencing. L.C., S.L., X. Yang, X.W., X.X., J.Y., X.Z., X.L., X. Yan, Y.G., R.L., R.Z., H.M. and B.Z. performed bioinformatics analyses. L.C. and X.W. constructed the online website. N.H.
performed immunostaining and confocal photography with assistance from M.J. J.G., Y.W., Z.X. and L.Y. performed paper writing, review and editing with input and feedback from all authors.
CORRESPONDING AUTHORS Correspondence to Leqian Yu, Zhenyu Xiao, Yulei Wei or Jingtao Guo. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER
REVIEW INFORMATION _Nature Cell Biology_ thanks the anonymous reviewers for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 QUALITY CONTROL AND CLUSTERS ANNOTATION. A.
Immunofluorescent staining results showing the localization of AFP, GABBR2, and DAPI in the human CS7 embryo. Biologically independent experiments were repeated three times with similar
results. B. Histogram graph of UMIs/genes/percent.mito per slice. The dashed lines represent the threshold value of quality control. C. Bar graph showing the number of filtered spots in each
slice. D. UMAP plots as in Fig. 1b, showing the log expression of _UCHL1, TBXT, NOTO, LEFTY2, BMP4, GABRP, AFP, IGF2_ and _HBG1_. E. Bar plot of cell cycle phase by analysing the expression
levels of S- and M-phase genes. F. Spatial visualization of clusters with coloured spots in slice 1 (S1), slice 5 (S5), slice 10 (S10), slice 15 (S15), slice 20 (S20), slice 25 (S25), slice
30 (S30), slice 35 (S35), slice 40 (S40), slice 45 (S45), slice 50 (S50), slice 55 (S55), slice 60 (S60), slice 65 (S65), slice 70 (S70), slice 75 (S75), slice 80 (S80). Source data
EXTENDED DATA FIG. 2 SPATIAL CHARACTERISTICS IN HUMAN CS7 EMBRYO. A. Bar plot showing the fractions of clusters in each slice. B. Scatter-plot showing the spatial distribution of the
clusters including Epi, Em/EXE.Meso, DE/VE, PS, Noto, Connecting stalk, AM, YS.EXE.Meso, YS.Endo, HEP, Ery. C. Scatter-plot showing the normalized expression of _CHRD, CER1, SOX17, BMP4_,
_NANOS3, AFP_, _CDX1_, _IGF2_, _SOX2, TBXT, CDX2, SHH and GABRP_ in human CS7 reconstructed 3D model. Source data EXTENDED DATA FIG. 3 SPATIAL CHARACTERISTICS IN HUMAN CS7 EMBRYO. A. Bubble
plot showing the regulon specificity score (RSS) of significant top 8 regulons by SCENIC analysis in clusters shown in Fig. 1b. Significant, p < 0.05. The hypergeometric P values were
adjusted for multiple testing using the Benjamini–Hochberg method. B. Spatial visualization of functional regulon modules based on hotspot analysis in CS7 embryo. Colours represents module
scores in each spot. C. Heatmap plot showing the dynamic patterns of regulon modules along anterior-posterior axes. D. Heatmap plot showing the dynamic patterns of regulon modules across
cell types. E. Bar plot of the enriched significantly pathways in each regulon module. The hypergeometric P values were adjusted for multiple testing using the Benjamini–Hochberg method.
Source data EXTENDED DATA FIG. 4 SPATIAL DISTRIBUTION OF EMBRYONIC CLUSTERS. A. Bar graph of the number of spots shown in Fig. 2a in per slice. B. UMAP plots showing the log-normalized
expression of _MT1G, SOX2, CDX1, TBXT, CHRD, LEFTY2, MSX1, CCKBR, CYB5A, TFAP2A, RGS4_, and _MYL7_. C. Spatial plots showing the embryonic clusters in each slice. The spots coloured in grey
indicate extra-embryonic tissues. Source data EXTENDED DATA FIG. 5 COMPARISON AND TRAJECTORY ANALYSIS IN HUMAN CS7 EMBRYO. A. UMAP plot overlaid with PAGA graph showing the developmental
trajectory in human CS7 embryonic clusters. B. The scatter-plots showing the expression levels of the changed genes along the velocity pseudotime in lateral plate mesoderm (LP.Meso), axial
mesoderm (Axial.Meso) paraxial mesoderm (Para.Meso) and extra-embryonic mesoderm progenitor (EXE.Meso.Prog) lineages. C. UMAP plot showing the cell trajectory in Gast-derived cells along the
pseudotime by Monocle3 analysis. D. Heatmap of the percentage cells of embryonic subclusters from Stereo-seq mapped to human cell types at CS7, which suggests our Gast closely resembled
their Primitive Steak, our LP.Meso and Axial.Meso closely resembled their Nascent Mesoderm, our Para.Meso closely resembled Emergent Mesoderm, and our EXE.Meso.Prog closely resembled
Advanced Mesoderm. E. UMAP visualization of the integrated datasets based on 2000 HVGs of human CS7 embryonic clusters from Stereo-seq and Smart-seq. F. UMAP plot showing the cell trajectory
of embryonic subclusters from Stereo-seq and Smart-seq at CS7 along the pseudotime by Monocle3 analysis. G. Ridge plot showing the cell distribution along the pseudotime by Monocle3
analysis. Source data EXTENDED DATA FIG. 6 COMPARISON ANALYSIS WITH THE PUBLISHED DATASET. A. Heatmaps, and bar plots with enrichment pathways showing the expression patterns of the genes
that change along the pseudotime trajectory in our data and Tyser data by Monocle3 analysis. The hypergeometric P values were adjusted for multiple testing using the Benjamini–Hochberg
method. B. Heatmap showing the scaled expression pattern of genes in human CS7 gastrula. C. Bar plot showing the average expression of _CDH2_, _TBXT_, _NOTO_, _MIXL1_, _TBX6_, _NTS_ and
_FGF4_ in human CS7 embryonic clusters from Stereo-seq (blue) and Smart-seq (sky blue) datasets. Source data EXTENDED DATA FIG. 7 COMPARISON ANALYSIS OF AVE FROM HUMAN, MONKEY AND MOUSE. A.
Heatmap plot showing the expression of top 20 markers in endoderm cells. B. Left: PCA plot of visceral endoderm subclusters in mouse E5.25-E6.5 stages. Right: PCA plot of visceral endoderm
subclusters in monkey CS8 gastrula. ExVE, embryonic visceral endoderm; EmVE, embryonic visceral endoderm; AVE, anterior visceral endoderm; VE, visceral endoderm. C. PCA plot of the visceral
endoderm (VE) cells from human CS7 and monkey CS8 stage. Left: colours indicate clusters; shape indicates monkey. Right: colours indicate cell clusters of monkeys. D. PCA plot of the
visceral endoderm (VE) cells from human CS7 and mouse E5.25-E6.5 stages. Left: colours indicate clusters; shape indicate mouse. Right: colours indicate cell clusters of mice. E. Bar plot
showing the percentage of cells based on cell cycle-related genes in endodermal subclusters. F. Heatmap showing the percentage cells of endoderm cells allocated to human cells at CS7, monkey
cells at CS8, and mouse cells at E5.25-E6.5. DE, definitive endoderm; VE, visceral endoderm; AVE, anterior visceral endoderm; EmVE, embryonic visceral endoderm. Source data EXTENDED DATA
FIG. 8 SPATIAL ANALYSIS OF KEY SIGNALLING PATHWAYS IN AVE. A. Bar plots showing the communication counts of source cells (left) and target cells (right) by ligand–receptor analysis. B.
Bubble plots showing the communications between anterior visceral endoderm (AVE) and other clusters in human CS7 embryo. Red indicates target/receptor; Blue indicates source/ligand. The p
values obtained from the permutation test were adjusted for multiple testing using the Benjamini–Hochberg method. C. Bubble plots showing the communications between anterior visceral
endoderm (AVE) or definitive endoderm (DE2) (left) and other clusters in human CS7 embryo. Red indicates target/receptor; Blue indicates source/ligand. The p values obtained from the
permutation test were adjusted for multiple testing using the Benjamini–Hochberg method. Source data EXTENDED DATA FIG. 9 MOLECULAR CHARACTERIZATION OF AVE AND PGCS. A. Heatmaps showing the
expression of genes in BMP, NOTCH, FGF, TGFb, VEGF, and WNT pathways. Blue/red/green represent ligands/receptors/inhibitors. B. Heatmap of top 20 markers of epiblast-derived cells and
Connecting stalk. Gast, gastrulating cells; AM.Ecto, amniotic ectoderm; PGCs, primordial germ cells; Epi(P), posterior epiblast. C. PAGA graph showing the two lineage cells by
partition-based graph abstraction analysis. D. Left: 3D scatter-plot showing the spatial location of primordial germ cells (PGCs), posterior epiblast (Epi(P)), amniotic ectoderm (AM.Ecto)
and amnion (AM). Right: 3D Scatter-plot showing the spatial latent time of primordial germ cells (PGCs), posterior epiblast (Epi(P)), amniotic ectoderm (AM.Ecto) and amnion (AM) by RNA
velocity analysis. E. Deconvolution of the Monocle2 pseudotime plot according to clusters. AM.Ecto, amniotic ectoderm; PGC, primordial germ cells; Epi(P), posterior epiblast; AM, amnion. F.
Ridge plots showing the distribution of primordial germ cells (PGCs), posterior epiblast (Epi(P)), amniotic ectoderm (AM.Ecto) and amnion (AM) along the pseudotime in CS7 by Monocle2
analysis. G. Bubble plot of ligand–receptor interactions among PGCs, Gast, connecting stalk, and AM.Ecto. Colours indicate communication probability, size indicate significance. The p values
obtained from the permutation test were adjusted for multiple testing using the Benjamini–Hochberg method. Source data EXTENDED DATA FIG. 10 MOLECULAR CHARACTERIZATION OF SUBTYPES OF
HEMATOPOIETIC PRECURSORS. A. Bubble plot showing the expression of markers in hematopoietic progenitors. Mesoderm.Prog, mesoderm progenitor; Primitive.HSPCs, primitive hematopoietic
stem/progenitor cells; Definitive.HSPCs, definitive hematopoietic stem/progenitor cells; HEC, hemogenic endothelial cells; MP, myeloid progenitors; MEP, megakaryocyte/erythroid progenitors;
YS.Endo.Mega, yolk sac endoderm megakaryocyte; YS.Endo.MP, yolk sac endoderm myeloid progenitors; YS.Endo.Ery, yolk sac endoderm erythroblast. B. 3D scatter-plot showing the spatial
distribution of hematopoietic precursors in human 3D reconstructed model. C. Pearson correlations among clusters. The numbers indicate Pearson correlations. D. UMAP plot overlayed with RNA
velocity showing the trajectory among hematopoietic precursors. E. Immunofluorescent staining results showing the localization of CD45(PTPRC), TFRC(CD71), CD14, AFP and RUNX1 in the human
CS7 embryo. Biologically independent experiments were repeated three times with similar results. F. Immunofluorescent staining results showing the localization of DAPI, AFP and CDH1 in the
human CS7 embryo. Biologically independent experiments were repeated three times with similar results. G. Enrichment pathways in yolk sac endoderm myeloid progenitors (YS.Endo.MP). The size
of the dots represents the counts of genes. The hypergeometric P values were adjusted for multiple testing using the Benjamini–Hochberg method. H. The chord diagram showing the cell-cell
communication networks in myeloid progenitors (MP) and hemogenic endothelial cells (HEC). The thickness of the line represents the weight of ligand–receptor pairs. The p values obtained from
the permutation test were adjusted for multiple testing using the Benjamini–Hochberg method. I. Bubble plot showing the significant cell-cell communications between MP and other
hematopoietic precursors in human CS7. J. Enrichment pathways in yolk sac endoderm megakaryocyte (YS.Endo.Mega). The size of the dots represents the counts of genes. The hypergeometric P
values were adjusted for multiple testing using the Benjamini–Hochberg method. Source data SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Fig. 1. REPORTING SUMMARY
SUPPLEMENTARY TABLES 1–8 Supplementary Table 1. Top 20 marker genes in each cluster in human CS7 embryo. Supplementary Table 2. Abbreviations in this study. Supplementary Table 3. The
representative markers in mesodermal subtypes in the embryonic clusters. Supplementary Table 4. The top 20 markers in subclusters in this study. Supplementary Table 5. The results of
ligand–receptor pairs analysis among embryo clusters in this study. Supplementary Table 6. The results of ligand–receptor pairs analysis among epiblast-derived cells in this study.
Supplementary Table 7. Markers in haematopoietic progenitors. Supplementary Table 8. Top 20 marker genes in haematopoietic progenitors. SUPPLEMENTARY VIDEO 1 Video of the process of
constructing the 3D model of the human CS7 embryo. SOURCE DATA SOURCE DATA FIG. 1 Statistical source data. SOURCE DATA FIG. 2 Statistical source data. SOURCE DATA FIG. 3 Statistical source
data. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG. 5 Statistical source data. SOURCE DATA EXTENDED DATA FIG.1 Statistical source data. SOURCE DATA EXTENDED DATA FIG.2
Statistical source data. SOURCE DATA EXTENDED DATA FIG.3 Statistical source data. SOURCE DATA EXTENDED DATA FIG.4 Statistical source data. SOURCE DATA EXTENDED DATA FIG.5 Statistical source
data. SOURCE DATA EXTENDED DATA FIG.6 Statistical source data. SOURCE DATA EXTENDED DATA FIG.7 Statistical source data. SOURCE DATA EXTENDED DATA FIG.8 Statistical source data. SOURCE DATA
EXTENDED DATA FIG.9 Statistical source data. SOURCE DATA EXTENDED DATA FIG.10 Statistical source data. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other
partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this
article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cui, L., Lin, S., Yang, X. _et al._
Spatial transcriptomic characterization of a Carnegie stage 7 human embryo. _Nat Cell Biol_ 27, 360–369 (2025). https://doi.org/10.1038/s41556-024-01597-3 Download citation * Received: 23
August 2024 * Accepted: 12 December 2024 * Published: 10 January 2025 * Issue Date: February 2025 * DOI: https://doi.org/10.1038/s41556-024-01597-3 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative