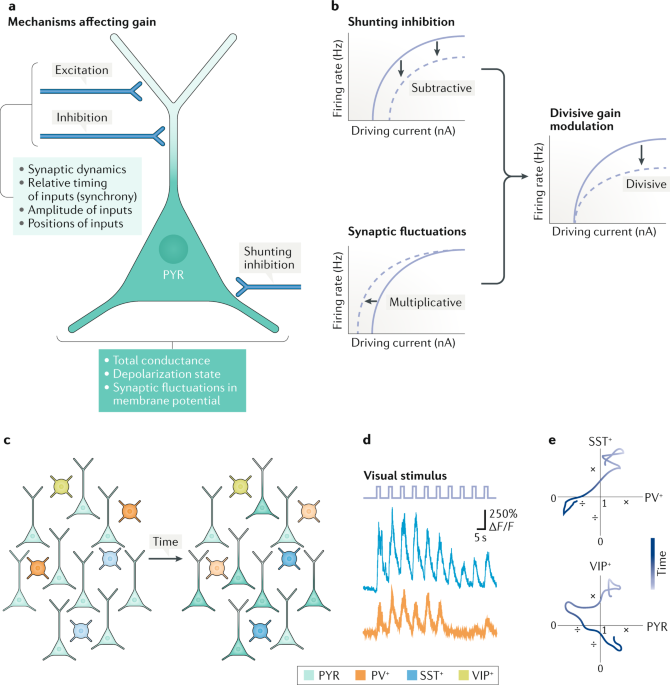
Play all audios:
ABSTRACT Cortical gain regulation allows neurons to respond adaptively to changing inputs. Neural gain is modulated by internal and external influences, including attentional and arousal
states, motor activity and neuromodulatory input. These influences converge to a common set of mechanisms for gain modulation, including GABAergic inhibition, synaptically driven
fluctuations in membrane potential, changes in cellular conductance and changes in other biophysical neural properties. Recent work has identified GABAergic interneurons as targets of
neuromodulatory input and mediators of state-dependent gain modulation. Here, we review the engagement and effects of gain modulation in the cortex. We highlight key recent findings that
link phenomenological observations of gain modulation to underlying cellular and circuit-level mechanisms. Finally, we place these cellular and circuit interactions in the larger context of
their impact on perception and cognition. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to
this journal Receive 12 print issues and online access $189.00 per year only $15.75 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS ENDOGENOUS ACTIVITY MODULATES STIMULUS AND CIRCUIT-SPECIFIC NEURAL TUNING AND PREDICTS PERCEPTUAL BEHAVIOR Article Open access 11 August 2020
FREQUENCY MODULATION OF CORTICAL RHYTHMICITY GOVERNS BEHAVIORAL VARIABILITY, EXCITABILITY AND SYNCHRONY OF NEURONS IN THE VISUAL CORTEX Article Open access 03 December 2022 OPPOSITE FORMS OF
ADAPTATION IN MOUSE VISUAL CORTEX ARE CONTROLLED BY DISTINCT INHIBITORY MICROCIRCUITS Article Open access 24 February 2022 REFERENCES * Reynolds, J. H. & Heeger, D. J. The normalization
model of attention. _Neuron_ 61, 168–185 (2009). Article CAS PubMed PubMed Central Google Scholar * Lee, S. H. et al. Activation of specific interneurons improves V1 feature
selectivity and visual perception. _Nature_ 488, 379–383 (2012). Article CAS PubMed PubMed Central Google Scholar * Ohshiro, T., Angelaki, D. E. & DeAngelis, G. C. A normalization
model of multisensory integration. _Nat. Neurosci._ 14, 775–782 (2011). Article CAS PubMed PubMed Central Google Scholar * Carandini, M. & Heeger, D. J. Summation and division by
neurons in primate visual cortex. _Science_ 264, 1333–1336 (1994). Article CAS PubMed Google Scholar * Carandini, M. & Heeger, D. J. Normalization as a canonical neural computation.
_Nat. Rev. Neurosci._ 13, 51–62 (2011). Article PubMed PubMed Central CAS Google Scholar * Salinas, E. & Thier, P. Gain modulation: a major computational principle of the central
nervous system. _Neuron_ 27, 15–21 (2000). Article CAS PubMed Google Scholar * Andersen, R. A., Snyder, L. H., Bradley, D. C. & Xing, J. Multimodal representation of space in the
posterior parietal cortex and its use in planning movements. _Annu. Rev. Neurosci._ 20, 303–330 (1997). Article CAS PubMed Google Scholar * Pouget, A. & Snyder, L. H. Computational
approaches to sensorimotor transformations. _Nat. Neurosci._ 3, 1192–1198 (2000). Article CAS PubMed Google Scholar * Salinas, E. & Sejnowski, T. J. Impact of correlated synaptic
input on output firing rate and variability in simple neuronal models. _J. Neurosci._ 20, 6193–6209 (2000). Article CAS PubMed PubMed Central Google Scholar * Finn, I. M., Priebe, N. J.
& Ferster, D. The emergence of contrast-invariant orientation tuning in simple cells of cat visual cortex. _Neuron_ 54, 137–152 (2007). Article CAS PubMed PubMed Central Google
Scholar * Sclar, G. & Freeman, R. D. Orientation selectivity in the cat’s striate cortex is invariant with stimulus contrast. _Exp. Brain Res._ 46, 457–461 (1982). Article CAS PubMed
Google Scholar * Skottun, B. C., Bradley, A., Sclar, G., Ohzawa, I. & Freeman, R. D. The effects of contrast on visual orientation and spatial frequency discrimination: a comparison
of single cells and behavior. _J. Neurophysiol._ 57, 773–786 (1987). Article CAS PubMed Google Scholar * Anderson, J. S., Lampl, I., Gillespie, D. C. & Ferster, D. The contribution
of noise to contrast invariance of orientation tuning in cat visual cortex. _Science_ 290, 1968–1972 (2000). Article CAS PubMed Google Scholar * McAdams, C. J. & Maunsell, J. H.
Effects of attention on the reliability of individual neurons in monkey visual cortex. _Neuron_ 23, 765–773 (1999). Article CAS PubMed Google Scholar * Somers, D. C., Nelson, S. B. &
Sur, M. An emergent model of orientation selectivity in cat visual cortical simple cells. _J. Neurosci._ 15, 5448–5465 (1995). Article CAS PubMed PubMed Central Google Scholar * Treue,
S. & Martinez Trujillo, J. C. Feature-based attention influences motion processing gain in macaque visual cortex. _Nature_ 399, 575–579 (1999). Article CAS PubMed Google Scholar *
Baccus, S. A. & Meister, M. Fast and slow contrast adaptation in retinal circuitry. _Neuron_ 36, 909–919 (2002). Article CAS PubMed Google Scholar * Ruff, D. A., Ni, A. M. &
Cohen, M. R. Cognition as a window into neuronal population space. _Annu. Rev. Neurosci._ 41, 77–97 (2018). Article CAS PubMed PubMed Central Google Scholar * Shadlen, M. N., Britten,
K. H., Newsome, W. T. & Movshon, J. A. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. _J. Neurosci._ 16, 1486–1510 (1996).
Article CAS PubMed PubMed Central Google Scholar * Barlow, H. B., Kaushal, T. P., Hawken, M. & Parker, A. J. Human contrast discrimination and the threshold of cortical neurons. _J.
Opt. Soc. Am. A_ 4, 2366–2371 (1987). Article CAS PubMed Google Scholar * Boynton, G. M., Demb, J. B., Glover, G. H. & Heeger, D. J. Neuronal basis of contrast discrimination. _Vis.
Res._ 39, 257–269 (1999). Article CAS PubMed Google Scholar * Clatworthy, P. L., Chirimuuta, M., Lauritzen, J. S. & Tolhurst, D. J. Coding of the contrasts in natural images by
populations of neurons in primary visual cortex (V1). _Vis. Res._ 43, 1983–2001 (2003). Article CAS PubMed Google Scholar * Parker, A. & Hawken, M. Capabilities of monkey cortical
cells in spatial-resolution tasks. _J. Opt. Soc. Am. A_ 2, 1101–1114 (1985). Article CAS PubMed Google Scholar * Watson, A. B. Gain, noise, and contrast sensitivity of linear visual
neurons. _Vis. Neurosci._ 4, 147–157 (1990). Article CAS PubMed Google Scholar * Eldar, E., Cohen, J. D. & Niv, Y. Amplified selectivity in cognitive processing implements the neural
gain model of norepinephrine function. _Behav. Brain Sci._ 39, e206 (2016). Article PubMed Google Scholar * Natan, R. G., Carruthers, I. M., Mwilambwe-Tshilobo, L. & Geffen, M. N.
Gain control in the auditory cortex evoked by changing temporal correlation of sounds. _Cereb. Cortex_ 27, 2385–2402 (2017). PubMed Google Scholar * Mineault, P. J., Tring, E.,
Trachtenberg, J. T. & Ringach, D. L. Enhanced spatial resolution during locomotion and heightened attention in mouse primary visual cortex. _J. Neurosci._ 36, 6382–6392 (2016). THIS
PAPER DEMONSTRATES THAT THE RELATIVE GAIN OF VISUAL RESPONSES BETWEEN QUIESCENCE AND LOCOMOTION IS HETEROGENEOUS ACROSS CELLS AND DEPENDS ON THE (SPATIAL-FREQUENCY) TUNING PROPERTIES OF THE
CELL. Article CAS PubMed PubMed Central Google Scholar * Reimer, J. et al. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. _Neuron_ 84, 355–362
(2014). Article CAS PubMed PubMed Central Google Scholar * Bennett, C., Arroyo, S. & Hestrin, S. Subthreshold mechanisms underlying state-dependent modulation of visual responses.
_Neuron_ 80, 350–357 (2013). Article CAS PubMed PubMed Central Google Scholar * Ohshiro, T., Angelaki, D. E. & DeAngelis, G. C. A neural signature of divisive normalization at the
level of multisensory integration in primate cortex. _Neuron_ 95, 399–411.e8 (2017). Article CAS PubMed PubMed Central Google Scholar * Ratan Murty, N. A. & Arun, S. P.
Multiplicative mixing of object identity and image attributes in single inferior temporal neurons. _Proc. Natl Acad. Sci. USA_ 115, E3276–E3285 (2018). Article PubMed CAS PubMed Central
Google Scholar * Niell, C. M. & Stryker, M. P. Modulation of visual responses by behavioral state in mouse visual cortex. _Neuron_ 65, 472–479 (2010). Article CAS PubMed PubMed
Central Google Scholar * Vinck, M., Batista-Brito, R., Knoblich, U. & Cardin, J. A. Arousal and locomotion make distinct contributions to cortical activity patterns and visual
encoding. _Neuron_ 86, 740–754 (2015). THIS PAPER SHOWS THAT AROUSAL AND LOCOMOTION DIFFERENTIALLY REGULATE NEURAL ACTIVITY AND SENSORY RESPONSE GAIN IN MOUSE V1. Article CAS PubMed
PubMed Central Google Scholar * Destexhe, A., Contreras, D. & Steriade, M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural
wake and sleep states. _J. Neurosci._ 19, 4595–4608 (1999). Article CAS PubMed PubMed Central Google Scholar * Livingstone, M. S. & Hubel, D. H. Effects of sleep and arousal on the
processing of visual information in the cat. _Nature_ 291, 554–561 (1981). Article CAS PubMed Google Scholar * Pakan, J. M. et al. Behavioral-state modulation of inhibition is
context-dependent and cell type specific in mouse visual cortex. _eLife_ 5, e14985 (2016). THIS PAPER SHOWS THAT LOCOMOTION-INDUCED GAIN MODULATION OF NEURONAL ACTIVITY IS CONTEXT DEPENDENT,
AND VARIES ACROSS LIGHT AND DARK CONDITIONS AND ACROSS DISTINCT CELL POPULATIONS. Article PubMed PubMed Central CAS Google Scholar * Dadarlat, M. C. & Stryker, M. P. Locomotion
enhances neural encoding of visual stimuli in mouse V1 _J. Neurosci._ 37, 3764–3775 (2017). THIS PAPER DEMONSTRATES THAT LOCOMOTION ENHANCES NEURAL ENCODING OF VISUAL STIMULI THROUGH
INCREASED FIRING RATES AND DECREASED NOISE CORRELATIONS ACROSS THE POPULATION. Article CAS PubMed PubMed Central Google Scholar * Schneider, D. M., Nelson, A. & Mooney, R. A
synaptic and circuit basis for corollary discharge in the auditory cortex. _Nature_ 513, 189–194 (2014). THIS ARTICLE SHOWS THAT LOCOMOTION DECREASES THE RESPONSE GAIN IN THE MOUSE PRIMARY
AUDITORY CORTEX VIA INHIBITORY INTERNEURON ACTIONS IN THE LOCAL CORTICAL CIRCUIT. Article CAS PubMed PubMed Central Google Scholar * Schneider, D. M., Sundararajan, J. & Mooney, R.
A cortical filter that learns to suppress the acoustic consequences of movement. _Nature_ 561, 391–395 (2018). Article CAS PubMed PubMed Central Google Scholar * Munoz, W., Tremblay,
R., Levenstein, D. & Rudy, B. Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. _Science_ 355, 954–959 (2017). THIS PAPER SHOWS THAT DISTINCT SST +
INTERNEURON POPULATIONS DEMONSTRATE LAMINA-DEPENDENT AND STATE-DEPENDENT DIFFERENCES IN GAIN MODULATION IN S1. Article CAS PubMed Google Scholar * Maunsell, J. H. R. Neuronal mechanisms
of visual attention. _Annu. Rev. Vis. Sci._ 1, 373–391 (2015). Article PubMed PubMed Central Google Scholar * McAdams, C. J. & Reid, R. C. Attention modulates the responses of
simple cells in monkey primary visual cortex. _J. Neurosci._ 25, 11023–11033 (2005). Article CAS PubMed PubMed Central Google Scholar * Reynolds, J. H., Pasternak, T. & Desimone, R.
Attention increases sensitivity of V4 neurons. _Neuron_ 26, 703–714 (2000). Article CAS PubMed Google Scholar * Connor, C. E., Gallant, J. L., Preddie, D. C. & Van Essen, D. C.
Responses in area V4 depend on the spatial relationship between stimulus and attention. _J. Neurophysiol._ 75, 1306–1308 (1996). Article CAS PubMed Google Scholar * Connor, C. E.,
Preddie, D. C., Gallant, J. L. & Van Essen, D. C. Spatial attention effects in macaque area V4. _J. Neurosci._ 17, 3201–3214 (1997). Article CAS PubMed PubMed Central Google Scholar
* Lee, J. & Maunsell, J. H. A normalization model of attentional modulation of single unit responses. _PLOS ONE_ 4, e4651 (2009). Article PubMed PubMed Central CAS Google Scholar
* Martinez-Trujillo, J. C. & Treue, S. Feature-based attention increases the selectivity of population responses in primate visual cortex. _Curr. Biol._ 14, 744–751 (2004). Article CAS
PubMed Google Scholar * Williford, T. & Maunsell, J. H. Effects of spatial attention on contrast response functions in macaque area V4. _J. Neurophysiol._ 96, 40–54 (2006). Article
PubMed Google Scholar * Reynolds, J. H., Chelazzi, L. & Desimone, R. Competitive mechanisms subserve attention in macaque areas V2 and V4. _J. Neurosci._ 19, 1736–1753 (1999). Article
CAS PubMed PubMed Central Google Scholar * Ecker, A. S., Denfield, G. H., Bethge, M. & Tolias, A. S. On the structure of neuronal population activity under fluctuations in
attentional state. _J. Neurosci._ 36, 1775–1789 (2016). Article CAS PubMed PubMed Central Google Scholar * Rabinowitz, N. C., Goris, R. L., Cohen, M. & Simoncelli, E. P. Attention
stabilizes the shared gain of V4 populations. _eLife_ 4, e08998 (2015). Article PubMed PubMed Central Google Scholar * Tiesinga, P. H. & Sejnowski, T. J. Rapid temporal modulation of
synchrony by competition in cortical interneuron networks. _Neural Comput._ 16, 251–275 (2004). Article CAS PubMed PubMed Central Google Scholar * Reynolds, J. H. & Chelazzi, L.
Attentional modulation of visual processing. _Annu. Rev. Neurosci._ 27, 611–647 (2004). Article CAS PubMed Google Scholar * Schoups, A., Vogels, R., Qian, N. & Orban, G. Practising
orientation identification improves orientation coding in V1 neurons. _Nature_ 412, 549–553 (2001). Article CAS PubMed Google Scholar * Jurjut, O., Georgieva, P., Busse, L. &
Katzner, S. Learning enhances sensory processing in mouse V1 before improving behavior. _J. Neurosci._ 37, 6460–6474 (2017). Article CAS PubMed PubMed Central Google Scholar * Kaneko,
M. & Stryker, M. P. Sensory experience during locomotion promotes recovery of function in adult visual cortex. _eLife_ 3, e02798 (2014). Article PubMed PubMed Central Google Scholar
* Chance, F. S., Abbott, L. F. & Reyes, A. D. Gain modulation from background synaptic input. _Neuron_ 35, 773–782 (2002). Article CAS PubMed Google Scholar * Ho, N. & Destexhe,
A. Synaptic background activity enhances the responsiveness of neocortical pyramidal neurons. _J. Neurophysiol._ 84, 1488–1496 (2000). Article CAS PubMed Google Scholar * Prescott, S. A.
& De Koninck, Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. _Proc. Natl Acad. Sci. USA_ 100, 2076–2081 (2003). Article CAS
PubMed PubMed Central Google Scholar * Shu, Y., Hasenstaub, A., Badoual, M., Bal, T. & McCormick, D. A. Barrages of synaptic activity control the gain and sensitivity of cortical
neurons. _J. Neurosci._ 23, 10388–10401 (2003). Article CAS PubMed PubMed Central Google Scholar * Cardin, J. A., Palmer, L. A. & Contreras, D. Cellular mechanisms underlying
stimulus-dependent gain modulation in primary visual cortex neurons in vivo. _Neuron_ 59, 150–160 (2008). Article CAS PubMed PubMed Central Google Scholar * Ly, C. & Doiron, B.
Divisive gain modulation with dynamic stimuli in integrate-and-fire neurons. _PLOS Comput. Biol._ 5, e1000365 (2009). Article PubMed PubMed Central CAS Google Scholar * Miller, K. D.
& Troyer, T. W. Neural noise can explain expansive, power-law nonlinearities in neural response functions. _J. Neurophysiol._ 87, 653–659 (2002). Article PubMed Google Scholar *
Hansel, D. & van Vreeswijk, C. How noise contributes to contrast invariance of orientation tuning in cat visual cortex. _J. Neurosci._ 22, 5118–5128 (2002). Article CAS PubMed PubMed
Central Google Scholar * Bulsara, A., Jacobs, E. W., Zhou, T., Moss, F. & Kiss, L. Stochastic resonance in a single neuron model: theory and analog simulation. _J. Theor. Biol._ 152,
531–555 (1991). Article CAS PubMed Google Scholar * Wiesenfeld, K. & Moss, F. Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. _Nature_ 373,
33–36 (1995). Article CAS PubMed Google Scholar * Khubieh, A., Ratte, S., Lankarany, M. & Prescott, S. A. Regulation of cortical dynamic range by background synaptic noise and
feedforward inhibition. _Cereb. Cortex_ 26, 3357–3369 (2016). Article PubMed PubMed Central Google Scholar * Atallah, B. V., Bruns, W., Carandini, M. & Scanziani, M.
Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. _Neuron_ 73, 159–170 (2012). Article CAS PubMed PubMed Central Google Scholar * Katzner, S.,
Busse, L. & Carandini, M. GABAA inhibition controls response gain in visual cortex. _J. Neurosci._ 31, 5931–5941 (2011). Article CAS PubMed PubMed Central Google Scholar * de la
Rocha, J., Doiron, B., Shea-Brown, E., Josic, K. & Reyes, A. Correlation between neural spike trains increases with firing rate. _Nature_ 448, 802–806 (2007). Article PubMed CAS
Google Scholar * Gentet, L. J., Avermann, M., Matyas, F., Staiger, J. F. & Petersen, C. C. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice.
_Neuron_ 65, 422–435 (2010). Article CAS PubMed Google Scholar * Pala, A. & Petersen, C. C. State-dependent cell-type-specific membrane potential dynamics and unitary synaptic inputs
in awake mice. _eLife_ 7, e35869 (2018). Article PubMed PubMed Central Google Scholar * Polack, P. O., Friedman, J. & Golshani, P. Cellular mechanisms of brain state-dependent gain
modulation in visual cortex. _Nat. Neurosci._ 16, 1331–1339 (2013). Article CAS PubMed PubMed Central Google Scholar * Poulet, J. F. & Petersen, C. C. Internal brain state regulates
membrane potential synchrony in barrel cortex of behaving mice. _Nature_ 454, 881–885 (2008). Article CAS PubMed Google Scholar * Chen, N., Sugihara, H. & Sur, M. An
acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. _Nat. Neurosci._ 18, 892–902 (2015). Article CAS PubMed PubMed Central Google Scholar * Carvalho, T.
P. & Buonomano, D. V. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input–output functions. _Neuron_ 61, 774–785 (2009). Article CAS
PubMed PubMed Central Google Scholar * Mitchell, S. J. & Silver, R. A. Shunting inhibition modulates neuronal gain during synaptic excitation. _Neuron_ 38, 433–445 (2003). Article
CAS PubMed Google Scholar * Murphy, B. K. & Miller, K. D. Multiplicative gain changes are induced by excitation or inhibition alone. _J. Neurosci._ 23, 10040–10051 (2003). Article
CAS PubMed PubMed Central Google Scholar * Abbott, L. F. & Chance, F. S. Drivers and modulators from push–pull and balanced synaptic input. _Prog. Brain Res._ 149, 147–155 (2005).
Article CAS PubMed Google Scholar * Ayaz, A. & Chance, F. S. Gain modulation of neuronal responses by subtractive and divisive mechanisms of inhibition. _J. Neurophysiol._ 101,
958–968 (2009). Article PubMed Google Scholar * Brozovic, M., Abbott, L. F. & Andersen, R. A. Mechanism of gain modulation at single neuron and network levels. _J. Comput. Neurosci._
25, 158–168 (2008). Article CAS PubMed Google Scholar * Vogels, T. P. & Abbott, L. F. Gating multiple signals through detailed balance of excitation and inhibition in spiking
networks. _Nat. Neurosci._ 12, 483–491 (2009). Article CAS PubMed PubMed Central Google Scholar * Fellous, J. M., Rudolph, M., Destexhe, A. & Sejnowski, T. J. Synaptic background
noise controls the input/output characteristics of single cells in an in vitro model of in vivo activity. _Neuroscience_ 122, 811–829 (2003). Article CAS PubMed Google Scholar * Holt, G.
R. & Koch, C. Shunting inhibition does not have a divisive effect on firing rates. _Neural Comput._ 9, 1001–1013 (1997). Article CAS PubMed Google Scholar * Litwin-Kumar, A.,
Oswald, A. M., Urban, N. N. & Doiron, B. Balanced synaptic input shapes the correlation between neural spike trains. _PLOS Comput. Biol._ 7, e1002305 (2011). Article CAS PubMed PubMed
Central Google Scholar * Rosenbaum, R. & Josic, K. Membrane potential and spike train statistics depend distinctly on input statistics. _Phys. Rev. E Stat. Nonlin. Soft Matter Phys._
84, 051902 (2011). Article PubMed CAS Google Scholar * Shea-Brown, E., Josic, K., de la Rocha, J. & Doiron, B. Correlation and synchrony transfer in integrate-and-fire neurons: basic
properties and consequences for coding. _Phys. Rev. Lett._ 100, 108102 (2008). Article PubMed CAS Google Scholar * Tchumatchenko, T. & Wolf, F. Representation of dynamical stimuli
in populations of threshold neurons. _PLOS Comput. Biol._ 7, e1002239 (2011). Article CAS PubMed PubMed Central Google Scholar * Arsiero, M., Luscher, H. R., Lundstrom, B. N. &
Giugliano, M. The impact of input fluctuations on the frequency–current relationships of layer 5 pyramidal neurons in the rat medial prefrontal cortex. _J. Neurosci._ 27, 3274–3284 (2007).
Article CAS PubMed PubMed Central Google Scholar * Higgs, M. H., Slee, S. J. & Spain, W. J. Diversity of gain modulation by noise in neocortical neurons: regulation by the slow
afterhyperpolarization conductance. _J. Neurosci._ 26, 8787–8799 (2006). Article CAS PubMed PubMed Central Google Scholar * Hong, S., Ratte, S., Prescott, S. A. & De Schutter, E.
Single neuron firing properties impact correlation-based population coding. _J. Neurosci._ 32, 1413–1428 (2012). Article CAS PubMed PubMed Central Google Scholar * Lundstrom, B. N.,
Famulare, M., Sorensen, L. B., Spain, W. J. & Fairhall, A. L. Sensitivity of firing rate to input fluctuations depends on time scale separation between fast and slow variables in single
neurons. _J. Comput. Neurosci._ 27, 277–290 (2009). Article PubMed Google Scholar * Rauch, A., La Camera, G., Luscher, H. R., Senn, W. & Fusi, S. Neocortical pyramidal cells respond
as integrate-and-fire neurons to in vivo-like input currents. _J. Neurophysiol._ 90, 1598–1612 (2003). Article PubMed Google Scholar * Larkum, M. E., Senn, W. & Luscher, H. R.
Top-down dendritic input increases the gain of layer 5 pyramidal neurons. _Cereb. Cortex_ 14, 1059–1070 (2004). Article PubMed Google Scholar * Mehaffey, W. H., Doiron, B., Maler, L.
& Turner, R. W. Deterministic multiplicative gain control with active dendrites. _J. Neurosci._ 25, 9968–9977 (2005). Article CAS PubMed PubMed Central Google Scholar * Jarvis, S.,
Nikolic, K. & Schultz, S. R. Neuronal gain modulability is determined by dendritic morphology: a computational optogenetic study. _PLOS Comput. Biol._ 14, e1006027 (2018). Article
PubMed PubMed Central CAS Google Scholar * Quiquempoix, M. et al. Layer 2/3 pyramidal neurons control the gain of cortical output. _Cell Rep._ 24, 2799–2807.e4 (2018). Article CAS
PubMed Google Scholar * Sato, T. K., Haider, B., Hausser, M. & Carandini, M. An excitatory basis for divisive normalization in visual cortex. _Nat. Neurosci._ 19, 568–570 (2016).
Article CAS PubMed PubMed Central Google Scholar * Haider, B. & McCormick, D. A. Rapid neocortical dynamics: cellular and network mechanisms. _Neuron_ 62, 171–189 (2009). Article
CAS PubMed PubMed Central Google Scholar * Silver, R. A. Neuronal arithmetic. _Nat. Rev. Neurosci._ 11, 474–489 (2010). Article CAS PubMed PubMed Central Google Scholar * Nelson,
S., Toth, L., Sheth, B. & Sur, M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. _Science_ 265, 774–777 (1994). Article CAS PubMed Google
Scholar * Atallah, B. V., Scanziani, M. & Carandini, M. Atallah et al. reply. _Nature_ 508, E3 (2014). Article CAS PubMed Google Scholar * El-Boustani, S., Wilson, N. R., Runyan, C.
A. & Sur, M. El-Boustani et al. reply. _Nature_ 508, E3–E4 (2014). Article CAS PubMed Google Scholar * Wilson, N. R., Runyan, C. A., Wang, F. L. & Sur, M. Division and
subtraction by distinct cortical inhibitory networks in vivo. _Nature_ 488, 343–348 (2012). Article CAS PubMed PubMed Central Google Scholar * Natan, R. G., Rao, W. & Geffen, M. N.
Cortical interneurons differentially shape frequency tuning following adaptation. _Cell Rep._ 21, 878–890 (2017). THIS PAPER SHOWS THAT DISTINCT CORTICAL INTERNEURON POPULATIONS DIFFERENTLY
MODULATE THE GAIN OF FREQUENCY-TUNED EXCITATORY RESPONSES DURING ADAPTATION. Article CAS PubMed PubMed Central Google Scholar * Phillips, E. A. & Hasenstaub, A. R. Asymmetric
effects of activating and inactivating cortical interneurons. _eLife_ 5, e18383 (2016). THIS PAPER SHOWS THAT OPTOGENETIC ACTIVATION OF GABAERGIC INTERNEURONS IN THE CORTEX DOES NOT FULLY
CAPTURE THE IMPACT OF INHIBITION ON EXCITATORY NEURON RESPONSE GAIN. Article PubMed PubMed Central CAS Google Scholar * Seybold, B. A., Phillips, E. A. K., Schreiner, C. E. &
Hasenstaub, A. R. Inhibitory actions unified by network integration. _Neuron_ 87, 1181–1192 (2015). Article CAS PubMed PubMed Central Google Scholar * Fishell, G. & Rudy, B.
Mechanisms of inhibition within the telencephalon: ‘‘where the wild things are’’. _Annu. Rev. Neurosci._ 34, 535–567 (2011). Article CAS PubMed PubMed Central Google Scholar * Markram,
H. et al. Interneurons of the neocortical inhibitory system. _Nat. Rev. Neurosci._ 5, 793–807 (2004). Article CAS PubMed Google Scholar * El-Boustani, S. & Sur, M. Response-dependent
dynamics of cell-specific inhibition in cortical networks in vivo. _Nat. Commun._ 5, 5689 (2014). Article CAS PubMed Google Scholar * Lee, A. M. et al. Identification of a brainstem
circuit regulating visual cortical state in parallel with locomotion. _Neuron_ 83, 455–466 (2014). Article CAS PubMed PubMed Central Google Scholar * Cardin, J. A. Inhibitory
interneurons regulate temporal precision and correlations in cortical circuits. _Trends Neurosci._ 41, 689–700 (2018). Article CAS PubMed PubMed Central Google Scholar * Cone, J. J.,
Scantlen, M. D., Histed, M. H. & Maunsell, J. H. R. Different inhibitory interneuron cell classes make distinct contributions to visual contrast perception. _eNeuro_
https://doi.org/10.1523/ENEURO.0337-18.2019 (2019). Article PubMed PubMed Central Google Scholar * Ayzenshtat, I., Karnani, M. M., Jackson, J. & Yuste, R. Cortical control of spatial
resolution by VIP+ interneurons. _J. Neurosci._ 36, 11498–11509 (2016). Article CAS PubMed PubMed Central Google Scholar * Hong, Y. K., Lacefield, C. O., Rodgers, C. C. & Bruno, R.
M. Sensation, movement and learning in the absence of barrel cortex. _Nature_ 561, 542–546 (2018). Article CAS PubMed PubMed Central Google Scholar * Otchy, T. M. et al. Acute
off-target effects of neural circuit manipulations. _Nature_ 528, 358–363 (2015). Article CAS PubMed Google Scholar * Wolff, S. B. & Olveczky, B. P. The promise and perils of causal
circuit manipulations. _Curr. Opin. Neurobiol._ 49, 84–94 (2018). Article CAS PubMed PubMed Central Google Scholar * Phillips, E. A. K., Schreiner, C. E. & Hasenstaub, A. R.
Cortical interneurons differentially regulate the effects of acoustic context. _Cell Rep._ 20, 771–778 (2017). Article CAS PubMed PubMed Central Google Scholar * Fu, Y. et al. A
cortical circuit for gain control by behavioral state. _Cell_ 156, 1139–1152 (2014). THIS PAPER PROVIDES EVIDENCE THAT VIP + INTERNEURONS ARE ACTIVATED BY LOCOMOTION AND MAY CONTRIBUTE TO
STATE-DEPENDENT VISUAL RESPONSE GAIN MODULATION IN MOUSE V1. Article CAS PubMed PubMed Central Google Scholar * Karnani, M. M. et al. Opening holes in the blanket of inhibition:
localized lateral disinhibition by VIP interneurons. _J. Neurosci._ 36, 3471–3480 (2016). Article CAS PubMed PubMed Central Google Scholar * Karnani, M. M. et al. Cooperative
subnetworks of molecularly similar interneurons in mouse neocortex. _Neuron_ 90, 86–100 (2016). Article CAS PubMed PubMed Central Google Scholar * Lee, S., Kruglikov, I., Huang, Z. J.,
Fishell, G. & Rudy, B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. _Nat. Neurosci._ 16, 1662–1670 (2013). Article CAS PubMed PubMed Central Google
Scholar * Pi, H. J. et al. Cortical interneurons that specialize in disinhibitory control. _Nature_ 503, 521–524 (2013). Article CAS PubMed PubMed Central Google Scholar *
Batista-Brito, R. et al. Developmental dysfunction of VIP interneurons impairs cortical circuits. _Neuron_ 95, 884–895.e9 (2017). Article CAS PubMed PubMed Central Google Scholar *
Dipoppa, M. et al. Vision and locomotion shape the interactions between neuron types in mouse visual cortex. _Neuron_ 98, 602–615.e8 (2018). Article CAS PubMed PubMed Central Google
Scholar * Ozeki, H., Finn, I. M., Schaffer, E. S., Miller, K. D. & Ferster, D. Inhibitory stabilization of the cortical network underlies visual surround suppression. _Neuron_ 62,
578–592 (2009). Article CAS PubMed PubMed Central Google Scholar * Tsodyks, M. V., Skaggs, W. E., Sejnowski, T. J. & McNaughton, B. L. Paradoxical effects of external modulation of
inhibitory interneurons. _J. Neurosci._ 17, 4382–4388 (1997). Article CAS PubMed PubMed Central Google Scholar * Kato, H. K., Asinof, S. K. & Isaacson, J. S. Network-level control
of frequency tuning in auditory cortex. _Neuron_ 95, 412–423.e4 (2017). Article CAS PubMed PubMed Central Google Scholar * Garcia Del Molino, L. C., Yang, G. R., Mejias, J. F. &
Wang, X. J. Paradoxical response reversal of top-down modulation in cortical circuits with three interneuron types. _eLife_ 6, e29742 (2017). Article PubMed PubMed Central Google Scholar
* Litwin-Kumar, A., Rosenbaum, R. & Doiron, B. Inhibitory stabilization and visual coding in cortical circuits with multiple interneuron subtypes. _J. Neurophysiol._ 115, 1399–1409
(2016). Article PubMed PubMed Central Google Scholar * Zhou, M. et al. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. _Nat. Neurosci._
17, 841–850 (2014). Article CAS PubMed PubMed Central Google Scholar * Boly, M. et al. Baseline brain activity fluctuations predict somatosensory perception in humans. _Proc. Natl
Acad. Sci. USA_ 104, 12187–12192 (2007). Article CAS PubMed PubMed Central Google Scholar * Fox, M. D., Snyder, A. Z., Vincent, J. L. & Raichle, M. E. Intrinsic fluctuations within
cortical systems account for intertrial variability in human behavior. _Neuron_ 56, 171–184 (2007). Article CAS PubMed Google Scholar * Hesselmann, G., Kell, C. A., Eger, E. &
Kleinschmidt, A. Spontaneous local variations in ongoing neural activity bias perceptual decisions. _Proc. Natl Acad. Sci. USA_ 105, 10984–10989 (2008). Article CAS PubMed PubMed Central
Google Scholar * Hesselmann, G., Kell, C. A. & Kleinschmidt, A. Ongoing activity fluctuations in hMT+ bias the perception of coherent visual motion. _J. Neurosci._ 28, 14481–14485
(2008). Article CAS PubMed PubMed Central Google Scholar * Palva, J. M. & Palva, S. Roles of multiscale brain activity fluctuations in shaping the variability and dynamics of
psychophysical performance. _Prog. Brain Res._ 193, 335–350 (2011). Article PubMed Google Scholar * Diamond, D. M., Campbell, A. M., Park, C. R., Halonen, J. & Zoladz, P. R. The
temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes–Dodson law.
_Neural. Plast._ 2007, 60803 (2007). Article PubMed PubMed Central Google Scholar * Yerkes, R. M. & Dodson, J. D. The relation of strength of stimulus to rapidity of habit-formation.
_J. Comp. Neurol. Psychol._ 18, 459–482 (1908). Article Google Scholar * He, B. J. Spontaneous and task-evoked brain activity negatively interact. _J. Neurosci._ 33, 4672–4682 (2013).
Article PubMed PubMed Central CAS Google Scholar * Bullock, T., Elliott, J. C., Serences, J. T. & Giesbrecht, B. Acute exercise modulates feature-selective responses in human
cortex. _J. Cognit. Neurosci._ 29, 605–618 (2017). Article Google Scholar * He, B. J. & Zempel, J. M. Average is optimal: an inverted-U relationship between trial-to-trial brain
activity and behavioral performance. _PLOS Comput. Biol._ 9, e1003348 (2013). Article PubMed PubMed Central CAS Google Scholar * Murphy, P. R., Vandekerckhove, J. & Nieuwenhuis, S.
Pupil-linked arousal determines variability in perceptual decision making. _PLOS Comput. Biol._ 10, e1003854 (2014). Article PubMed PubMed Central CAS Google Scholar * Aston-Jones, G.
& Cohen, J. D. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. _Annu. Rev. Neurosci._ 28, 403–450 (2005). Article CAS PubMed
Google Scholar * Murphy, P. R., O’Connell, R. G., O’Sullivan, M., Robertson, I. H. & Balsters, J. H. Pupil diameter covaries with BOLD activity in human locus coeruleus. _Hum. Brain
Mapp._ 35, 4140–4154 (2014). Article PubMed PubMed Central Google Scholar * Joshi, S., Li, Y., Kalwani, R. M. & Gold, J. I. Relationships between pupil diameter and neuronal activity
in the locus coeruleus, colliculi, and cingulate cortex. _Neuron_ 89, 221–234 (2016). Article CAS PubMed Google Scholar * Reimer, J. et al. Pupil fluctuations track rapid changes in
adrenergic and cholinergic activity in cortex. _Nat. Commun._ 7, 13289 (2016). Article CAS PubMed PubMed Central Google Scholar * Erisken, S. et al. Effects of locomotion extend
throughout the mouse early visual system. _Curr. Biol._ 24, 2899–2907 (2014). Article CAS PubMed Google Scholar * Tang, L. & Higley, M. J. Layer 5 circuits in V1 differentially
control visuomotor behavior. _bioRxiv_ https://doi.org/10.1101/540807 (2019). * Saleem, A. B., Ayaz, A., Jeffery, K. J., Harris, K. D. & Carandini, M. Integration of visual motion and
locomotion in mouse visual cortex. _Nat. Neurosci._ 16, 1864–1869 (2013). Article CAS PubMed PubMed Central Google Scholar * Neske, G. T., Nestvogel, D., Steffan, P. J. & McCormick,
D. A. Distinct waking states for strong evoked responses in primary visual cortex and optimal visual detection performance. _J. Neurosci_. 39, 10044–10059 (2019). Article PubMed PubMed
Central Google Scholar * Bullock, T., Cecotti, H. & Giesbrecht, B. Multiple stages of information processing are modulated during acute bouts of exercise. _Neuroscience_ 307, 138–150
(2015). Article CAS PubMed Google Scholar * Benjamin, A. V., Wailes-Newson, K., Ma-Wyatt, A., Baker, D. H. & Wade, A. R. The effect of locomotion on early visual contrast processing
in humans. _J. Neurosci._ 38, 3050–3059 (2018). Article CAS PubMed PubMed Central Google Scholar * McGinley, M. J., David, S. V. & McCormick, D. A. Cortical membrane potential
signature of optimal states for sensory signal detection. _Neuron_ 87, 179–192 (2015). Article CAS PubMed PubMed Central Google Scholar * Barson, D. et al. Simultaneous mesoscopic and
two-photon imaging of neuronal activity in cortical circuits. _Nat. Methods_ https://doi.org/10.1038/s41592-019-0625-2 (2019). Article PubMed CAS PubMed Central Google Scholar * Clancy,
K. B., Orsolic, I. & Mrsic-Flogel, T. D. Locomotion-dependent remapping of distributed cortical networks. _Nat. Neurosci._ 22, 778–786 (2019). Article CAS PubMed PubMed Central
Google Scholar * Shimaoka, D., Harris, K. D. & Carandini, M. Effects of arousal on mouse sensory cortex depend on modality. _Cell Rep._ 22, 3160–3167 (2018). Article CAS PubMed
PubMed Central Google Scholar * Musall, S., Kaufman, M. T., Juavinett, A. L., Gluf, S. & Churchland, A. K. Single-trial neural dynamics are dominated by richly varied movements. _Nat.
Neurosci._ 22, 1677–1686 (2019). THIS STUDY REPORTS THAT ANIMAL MOVEMENTS CAPTURE THE MAJORITY OF NEURAL VARIABILITY ACROSS THE CORTEX, AND THOSE THAT ARE TASK-ALIGNED ACCOUNT FOR FEATURES
COMMONLY ATTRIBUTED TO COGNITIVE TASK DEMANDS. Article CAS PubMed PubMed Central Google Scholar * Disney, A. A., Alasady, H. A. & Reynolds, J. H. Muscarinic acetylcholine receptors
are expressed by most parvalbumin-immunoreactive neurons in area MT of the macaque. _Brain Behav._ 4, 431–445 (2014). Article PubMed PubMed Central Google Scholar * Disney, A. A. &
Aoki, C. Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin-immunoreactive neurons. _J. Comp. Neurol._ 507, 1748–1762 (2008). Article PubMed
PubMed Central Google Scholar * Disney, A. A., Domakonda, K. V. & Aoki, C. Differential expression of muscarinic acetylcholine receptors across excitatory and inhibitory cells in
visual cortical areas V1 and V2 of the macaque monkey. _J. Comp. Neurol._ 499, 49–63 (2006). Article CAS PubMed Google Scholar * Disney, A. A. & Reynolds, J. H. Expression of m1-type
muscarinic acetylcholine receptors by parvalbumin-immunoreactive neurons in the primary visual cortex: a comparative study of rat, guinea pig, ferret, macaque, and human. _J. Comp. Neurol._
522, 986–1003 (2014). Article CAS PubMed PubMed Central Google Scholar * Letzkus, J. J. et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex.
_Nature_ 480, 331–335 (2011). Article CAS PubMed Google Scholar * Porter, J. T. et al. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. _J. Neurosci._
19, 5228–5235 (1999). Article CAS PubMed PubMed Central Google Scholar * Urban-Ciecko, J., Jouhanneau, J. S., Myal, S. E., Poulet, J. F. A. & Barth, A. L. Precisely timed nicotinic
activation drives SST inhibition in neocortical circuits. _Neuron_ 97, 611–625.e5 (2018). Article CAS PubMed PubMed Central Google Scholar * Disney, A. A., Aoki, C. & Hawken, M. J.
Gain modulation by nicotine in macaque V1. _Neuron_ 56, 701–713 (2007). Article CAS PubMed PubMed Central Google Scholar * Gil, Z., Connors, B. W. & Amitai, Y. Differential
regulation of neocortical synapses by neuromodulators and activity. _Neuron_ 19, 679–686 (1997). Article CAS PubMed Google Scholar * Hasselmo, M. E. & Bower, J. M. Cholinergic
suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. _J. Neurophysiol._ 67, 1222–1229 (1992). Article CAS PubMed Google Scholar * Kimura, F.
Cholinergic modulation of cortical function: a hypothetical role in shifting the dynamics in cortical network. _Neurosci. Res._ 38, 19–26 (2000). Article CAS PubMed Google Scholar *
Kimura, F., Fukuda, M. & Tsumoto, T. Acetylcholine suppresses the spread of excitation in the visual cortex revealed by optical recording: possible differential effect depending on the
source of input. _Eur. J. Neurosci._ 11, 3597–3609 (1999). Article CAS PubMed Google Scholar * Disney, A. A., Aoki, C. & Hawken, M. J. Cholinergic suppression of visual responses in
primate V1 is mediated by GABAergic inhibition. _J. Neurophysiol._ 108, 1907–1923 (2012). Article CAS PubMed PubMed Central Google Scholar * Soma, S., Shimegi, S., Osaki, H. & Sato,
H. Cholinergic modulation of response gain in the primary visual cortex of the macaque. _J. Neurophysiol._ 107, 283–291 (2012). Article CAS PubMed Google Scholar * Herrero, J. L.,
Gieselmann, M. A. & Thiele, A. Muscarinic and nicotinic contribution to contrast sensitivity of macaque area V1 neurons. _Front. Neural Circuits_ 11, 106 (2017). Article PubMed PubMed
Central Google Scholar * Askew, C., Intskirveli, I. & Metherate, R. Systemic nicotine increases gain and narrows receptive fields in A1 via integrated cortical and subcortical actions.
_eNeuro_ https://doi.org/10.1523/ENEURO.0192-17.2017 (2017). Article PubMed PubMed Central Google Scholar * Herrero, J. L. et al. Acetylcholine contributes through muscarinic receptors
to attentional modulation in V1. _Nature_ 454, 1110–1114 (2008). Article CAS PubMed PubMed Central Google Scholar * Pinto, L. et al. Fast modulation of visual perception by basal
forebrain cholinergic neurons. _Nat. Neurosci._ 16, 1857–1863 (2013). Article CAS PubMed PubMed Central Google Scholar * Stewart, A. E., Yan, Z., Surmeier, D. J. & Foehring, R. C.
Muscarine modulates Ca2+ channel currents in rat sensorimotor pyramidal cells via two distinct pathways. _J. Neurophysiol._ 81, 72–84 (1999). Article CAS PubMed Google Scholar *
Lorenzon, N. M. & Foehring, R. C. Relationship between repetitive firing and afterhyperpolarizations in human neocortical neurons. _J. Neurophysiol._ 67, 350–363 (1992). Article CAS
PubMed Google Scholar * McCormick, D. A. & Prince, D. A. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. _J. Physiol._ 375, 169–194 (1986). Article
CAS PubMed PubMed Central Google Scholar * Schwindt, P. C., Spain, W. J. & Crill, W. E. Influence of anomalous rectifier activation on afterhyperpolarizations of neurons from cat
sensorimotor cortex in vitro. _J. Neurophysiol._ 59, 468–481 (1988). Article CAS PubMed Google Scholar * Wang, Z. & McCormick, D. A. Control of firing mode of corticotectal and
corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. _J. Neurosci._ 13, 2199–2216 (1993). Article CAS PubMed PubMed Central Google Scholar *
Eggermann, E. & Feldmeyer, D. Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. _Proc. Natl Acad. Sci. USA_ 106, 11753–11758 (2009). Article CAS
PubMed PubMed Central Google Scholar * Gulledge, A. T., Park, S. B., Kawaguchi, Y. & Stuart, G. J. Heterogeneity of phasic cholinergic signaling in neocortical neurons. _J.
Neurophysiol._ 97, 2215–2229 (2007). Article CAS PubMed Google Scholar * Gulledge, A. T. & Stuart, G. J. Cholinergic inhibition of neocortical pyramidal neurons. _J. Neurosci._ 25,
10308–10320 (2005). Article CAS PubMed PubMed Central Google Scholar * Dasgupta, R., Seibt, F. & Beierlein, M. Synaptic release of acetylcholine rapidly suppresses cortical activity
by recruiting muscarinic receptors in layer 4. _J. Neurosci._ 38, 5338–5350 (2018). Article CAS PubMed PubMed Central Google Scholar * Higley, M. J., Soler-Llavina, G. J. &
Sabatini, B. L. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. _Nat. Neurosci._ 12, 1121–1128 (2009). Article CAS PubMed PubMed
Central Google Scholar * Giessel, A. J. & Sabatini, B. L. M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK
channels. _Neuron_ 68, 936–947 (2010). Article CAS PubMed PubMed Central Google Scholar * Foehring, R. C., Schwindt, P. C. & Crill, W. E. Norepinephrine selectively reduces slow
Ca2+- and Na+-mediated K+ currents in cat neocortical neurons. _J. Neurophysiol._ 61, 245–256 (1989). Article CAS PubMed Google Scholar * Madison, D. V. & Nicoll, R. A. Actions of
noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. _J. Physiol._ 372, 221–244 (1986). Article CAS PubMed PubMed Central Google Scholar *
Mueller, D., Porter, J. T. & Quirk, G. J. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. _J. Neurosci._ 28, 369–375
(2008). Article CAS PubMed PubMed Central Google Scholar * Dodt, H. U., Pawelzik, H. & Zieglgansberger, W. Actions of noradrenaline on neocortical neurons in vitro. _Brain Res._
545, 307–311 (1991). Article CAS PubMed Google Scholar * Mynlieff, M. & Dunwiddie, T. V. Noradrenergic depression of synaptic responses in hippocampus of rat: evidence for mediation
by α1-receptors. _Neuropharmacology_ 27, 391–398 (1988). Article CAS PubMed Google Scholar * Guan, D., Armstrong, W. E. & Foehring, R. C. Electrophysiological properties of
genetically identified subtypes of layer 5 neocortical pyramidal neurons: Ca2+ dependence and differential modulation by norepinephrine. _J. Neurophysiol._ 113, 2014–2032 (2015). Article
PubMed PubMed Central Google Scholar * Waterhouse, B. D., Mouradian, R., Sessler, F. M. & Lin, R. C. Differential modulatory effects of norepinephrine on synaptically driven responses
of layer V barrel field cortical neurons. _Brain Res._ 868, 39–47 (2000). Article CAS PubMed Google Scholar * Armstrong-James, M. & Fox, K. Effects of ionophoresed noradrenaline on
the spontaneous activity of neurones in rat primary somatosensory cortex. _J. Physiol._ 335, 427–447 (1983). Article CAS PubMed PubMed Central Google Scholar * Bassant, M. H., Ennouri,
K. & Lamour, Y. Effects of iontophoretically applied monoamines on somatosensory cortical neurons of unanesthetized rats. _Neuroscience_ 39, 431–439 (1990). Article CAS PubMed Google
Scholar * Foote, S. L., Freedman, R. & Oliver, A. P. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. _Brain Res._ 86, 229–242 (1975). Article CAS
PubMed Google Scholar * Waterhouse, B. D., Moises, H. C. & Woodward, D. J. Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative
neurotransmitters. _Exp. Neurol._ 69, 30–49 (1980). Article CAS PubMed Google Scholar * Waterhouse, B. D., Moises, H. C. & Woodward, D. J. Alpha-receptor-mediated facilitation of
somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. _Neuropharmacology_ 20, 907–920 (1981). Article CAS PubMed Google
Scholar * Ego-Stengel, V., Bringuier, V. & Shulz, D. E. Noradrenergic modulation of functional selectivity in the cat visual cortex: an in vivo extracellular and intracellular study.
_Neuroscience_ 111, 275–289 (2002). Article CAS PubMed Google Scholar * Seillier, L. et al. Serotonin decreases the gain of visual responses in awake macaque V1. _J. Neurosci._ 37,
11390–11405 (2017). Article CAS PubMed PubMed Central Google Scholar * Watakabe, A. et al. Enriched expression of serotonin 1B and 2A receptor genes in macaque visual cortex and their
bidirectional modulatory effects on neuronal responses. _Cereb. Cortex_ 19, 1915–1928 (2009). Article PubMed Google Scholar * Dugue, G. P. et al. Optogenetic recruitment of dorsal raphe
serotonergic neurons acutely decreases mechanosensory responsivity in behaving mice. _PLOS ONE_ 9, e105941 (2014). Article PubMed PubMed Central CAS Google Scholar * Davis, M.,
Strachan, D. I. & Kass, E. Excitatory and inhibitory effects of serotonin on sensorimotor reactivity measured with acoustic startle. _Science_ 209, 521–523 (1980). Article CAS PubMed
Google Scholar * Vijayraghavan, S., Wang, M., Birnbaum, S. G., Williams, G. V. & Arnsten, A. F. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory.
_Nat. Neurosci._ 10, 376–384 (2007). Article CAS PubMed Google Scholar * Williams, G. V. & Goldman-Rakic, P. S. Modulation of memory fields by dopamine D1 receptors in prefrontal
cortex. _Nature_ 376, 572–575 (1995). Article CAS PubMed Google Scholar * Noudoost, B. & Moore, T. Control of visual cortical signals by prefrontal dopamine. _Nature_ 474, 372–375
(2011). Article CAS PubMed PubMed Central Google Scholar * Lur, G. & Higley, M. J. Glutamate receptor modulation is restricted to synaptic microdomains. _Cell Rep._ 12, 326–334
(2015). Article CAS PubMed PubMed Central Google Scholar * Athilingam, J. C., Ben-Shalom, R., Keeshen, C. M., Sohal, V. S. & Bender, K. J. Serotonin enhances excitability and gamma
frequency temporal integration in mouse prefrontal fast-spiking interneurons. _eLife_ 6, e31991 (2017). Article PubMed PubMed Central Google Scholar * Kawaguchi, Y. & Shindou, T.
Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. _J. Neurosci._ 18, 6963–6976 (1998). Article CAS PubMed PubMed Central Google Scholar * Demb, J.
B. Multiple mechanisms for contrast adaptation in the retina. _Neuron_ 36, 781–783 (2002). Article CAS PubMed Google Scholar * Farley, B. J., Quirk, M. C., Doherty, J. J. &
Christian, E. P. Stimulus-specific adaptation in auditory cortex is an NMDA-independent process distinct from the sensory novelty encoded by the mismatch negativity. _J. Neurosci._ 30,
16475–16484 (2010). Article CAS PubMed PubMed Central Google Scholar * Fishman, Y. I. & Steinschneider, M. Searching for the mismatch negativity in primary auditory cortex of the
awake monkey: deviance detection or stimulus specific adaptation? _J. Neurosci._ 32, 15747–15758 (2012). Article CAS PubMed PubMed Central Google Scholar * Kohn, A. & Movshon, J. A.
Neuronal adaptation to visual motion in area MT of the macaque. _Neuron_ 39, 681–691 (2003). Article CAS PubMed Google Scholar * Szymanski, F. D., Garcia-Lazaro, J. A. & Schnupp, J.
W. Current source density profiles of stimulus-specific adaptation in rat auditory cortex. _J. Neurophysiol._ 102, 1483–1490 (2009). Article PubMed Google Scholar * Ulanovsky, N., Las,
L. & Nelken, I. Processing of low-probability sounds by cortical neurons. _Nat. Neurosci._ 6, 391–398 (2003). Article CAS PubMed Google Scholar * Dean, I., Robinson, B. L., Harper,
N. S. & McAlpine, D. Rapid neural adaptation to sound level statistics. _J. Neurosci._ 28, 6430–6438 (2008). Article CAS PubMed PubMed Central Google Scholar * Barlow, H. in
_Sensory Communication_ (MIT Press, 1961). * Niyogi, R. K. & Wong-Lin, K. Dynamic excitatory and inhibitory gain modulation can produce flexible, robust and optimal decision-making.
_PLOS Comput. Biol._ 9, e1003099 (2013). Article CAS PubMed PubMed Central Google Scholar * Busse, L., Wade, A. R. & Carandini, M. Representation of concurrent stimuli by population
activity in visual cortex. _Neuron_ 64, 931–942 (2009). Article CAS PubMed PubMed Central Google Scholar * Hahnloser, R. H., Douglas, R. J. & Hepp, K. Attentional recruitment of
inter-areal recurrent networks for selective gain control. _Neural Comput._ 14, 1669–1689 (2002). Article PubMed Google Scholar * Thiele, A. & Bellgrove, M. A. Neuromodulation of
attention. _Neuron_ 97, 769–785 (2018). Article CAS PubMed PubMed Central Google Scholar * Schwartz, O. & Simoncelli, E. P. Natural signal statistics and sensory gain control. _Nat.
Neurosci._ 4, 819–825 (2001). Article CAS PubMed Google Scholar * Willmore, B. D., Bulstrode, H. & Tolhurst, D. J. Contrast normalization contributes to a biologically-plausible
model of receptive-field development in primary visual cortex (V1). _Vis. Res._ 54, 49–60 (2012). Article PubMed Google Scholar * Ni, A. M., Ruff, D. A., Alberts, J. J., Symmonds, J.
& Cohen, M. R. Learning and attention reveal a general relationship between population activity and behavior. _Science_ 359, 463–465 (2018). Article CAS PubMed PubMed Central Google
Scholar * Lee, S., Park, J. & Smirnakis, S. M. Internal gain modulations, but not changes in stimulus contrast, preserve the neural code. _J. Neurosci._ 39, 1671–1687 (2019). CAS
PubMed PubMed Central Google Scholar * McGinley, M. J. et al. Waking state: rapid variations modulate neural and behavioral responses. _Neuron_ 87, 1143–1161 (2015). Article CAS PubMed
PubMed Central Google Scholar * Rose, D. & Blakemore, C. Effects of bicuculline on functions of inhibition in visual cortex. _Nature_ 249, 375–377 (1974). Article CAS PubMed
Google Scholar * Carandini, M. & Ferster, D. Membrane potential and firing rate in cat primary visual cortex. _J. Neurosci._ 20, 470–484 (2000). Article CAS PubMed PubMed Central
Google Scholar * Isaacson, J. S. & Scanziani, M. How inhibition shapes cortical activity. _Neuron_ 72, 231–243 (2011). Article CAS PubMed PubMed Central Google Scholar * Zhang, Y.
P. & Oertner, T. G. Optical induction of synaptic plasticity using a light-sensitive channel. _Nat. Methods_ 4, 139–141 (2007). Article CAS PubMed Google Scholar * Allen, B. D.,
Singer, A. C. & Boyden, E. S. Principles of designing interpretable optogenetic behavior experiments. _Learn. Mem._ 22, 232–238 (2015). Article PubMed PubMed Central Google Scholar *
Cottam, J. C., Smith, S. L. & Hausser, M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. _J. Neurosci._ 33, 19567–19578 (2013).
Article CAS PubMed PubMed Central Google Scholar * Pfeffer, C. K., Xue, M., He, M., Huang, Z. J. & Scanziani, M. Inhibition of inhibition in visual cortex: the logic of connections
between molecularly distinct interneurons. _Nat. Neurosci._ 16, 1068–1076 (2013). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was
supported by US National Institutes of Health (NIH) R01 MH102365, NIH R01 EY022951, NIH R01 MH113852, a Simons Foundation Autism Research Initiative (SFARI) Research Grant, a Smith Family
Award for Excellence in Biomedical Research, a Klingenstein Fellowship Award, an Alfred P. Sloan Fellowship, a US National Alliance for Research on Schizophrenia & Depression (NARSAD)
Young Investigator Award, a McKnight Fellowship and a grant from the Ludwig Family Foundation to J.A.C.; and a Brown-Coxe fellowship and a NARSAD Young Investigator Award to K.A.F. The
authors thank M. J. Higley and members of the Cardin and Higley laboratories for insightful discussions, and Q. Perrenoud for help with illustration. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Neuroscience, Yale University, New Haven, CT, USA Katie A. Ferguson & Jessica A. Cardin * Kavli Institute for Neuroscience, Yale University, New Haven, CT,
USA Jessica A. Cardin Authors * Katie A. Ferguson View author publications You can also search for this author inPubMed Google Scholar * Jessica A. Cardin View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS Both authors researched data for article, made substantial contributions to discussions of the content, wrote the manuscript
and reviewed or edited the manuscript before submission. CORRESPONDING AUTHOR Correspondence to Jessica A. Cardin. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Reviews Neuroscience_ thanks C. Angeloni, M. Geffen, J. Reynolds and the other, anonymous, reviewer(s) for their
contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. GLOSSARY
* Gain modulation A phenomenon whereby the gain or sensitivity of a neuron to inputs, such as visual stimuli, is altered without changing selectivity. * Input–output (I/O) relationship The
relationship between the inputs a neuron receives (such as synaptic inputs, direct currents or sensory stimulation) and the firing rate responses of that neuron. * Synaptic summation The
summation of synaptic inputs to a neuron either spatially (when nearby synapses are coactive on a dendritic branch) or temporally (when synaptic inputs occur within a short time window
mediated by the membrane time constant, τ). * Iceberg effect An effect whereby, if subthreshold responses to a stimulus are less selective than the neuron’s firing, a linear increase or
decrease in activity may alter the neuron’s selectivity by raising or lowering the tuning curve of the neuron across the threshold. * Monocular deprivation An experimental paradigm in which
an animal is deprived of vision from one eye during a critical developmental period. The mature binocular visual cortex then responds predominantly to inputs from the non-deprived eye. *
Stochastic resonance A phenomenon in which the addition of noise non-linearly enhances the information content of a signal, by boosting resonant frequencies over a sensor’s detection
threshold (such as a cell’s spike threshold). * Shunting inhibition A GABAergic synaptic input that minimally affects the membrane potential of a cell that is near the inhibitory synaptic
reversal potential, but that leads to a reduction of nearby excitatory postsynaptic potential amplitudes. * Pairwise correlations A normalized measure of covariation between pairs of neurons
that can give insight into their tuning similarity (signal correlations) or shared trial-to-trial variability (noise correlations). * Dendritic saturation A phenomenon in which an already
depolarized dendritic branch shows reduced excitatory responses to temporally correlated excitatory inputs due to reduced driving force. * Synaptic efficacy The influence that a presynaptic
input has on a postsynaptic cell’s probability of firing an action potential. * Adaptation A decrease in sensitivity to constant or repeated stimuli, leading to reduced stimulus-evoked
neural responses over time. * Forward suppression A rapid form of sensory adaptation whereby the response to a stimulus is reduced when preceded by a stimulus with similar features. *
Feedback inhibition A type of inhibition delivered through recurrent connections: that is, local inhibitory cells target the same population of excitatory cells that drive local inhibitory
activity. * Brain states Spatiotemporal patterns of neural-network activity across the brain that are dynamically regulated by behaviour, the environment and the internal state. * Pupil
diameter The diameter of the pupil of the eye. The diameter is tightly coupled to various emotional and cognitive factors, including global arousal and attention, even when controlling for
changes in luminance and depth accommodation. * Attractor dynamics Temporal patterns that evolve towards a stable state from a large range of starting conditions. Attractor network
characterization facilitates the identification of key network properties. * Winner-take-all mechanism A computational principle in which non-linearities in a recurrent neural network create
strong competition between neurons. Only neurons (or sets thereof) with the strongest responses remain active, providing a mechanism for input selection or segregation. * Dimensionality
reduction Reduction of the number of random variables of a system to a smaller set of principal variables to aid analysis. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Ferguson, K.A., Cardin, J.A. Mechanisms underlying gain modulation in the cortex. _Nat Rev Neurosci_ 21, 80–92 (2020). https://doi.org/10.1038/s41583-019-0253-y Download
citation * Accepted: 25 November 2019 * Published: 07 January 2020 * Issue Date: February 2020 * DOI: https://doi.org/10.1038/s41583-019-0253-y SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative