Play all audios:
ABSTRACT The psychedelic alkaloid ibogaine has anti-addictive properties in both humans and animals1. Unlike most medications for the treatment of substance use disorders, anecdotal reports
suggest that ibogaine has the potential to treat addiction to various substances, including opiates, alcohol and psychostimulants. The effects of ibogaine—like those of other psychedelic
compounds—are long-lasting2, which has been attributed to its ability to modify addiction-related neural circuitry through the activation of neurotrophic factor signalling3,4. However,
several safety concerns have hindered the clinical development of ibogaine, including its toxicity, hallucinogenic potential and tendency to induce cardiac arrhythmias. Here we apply the
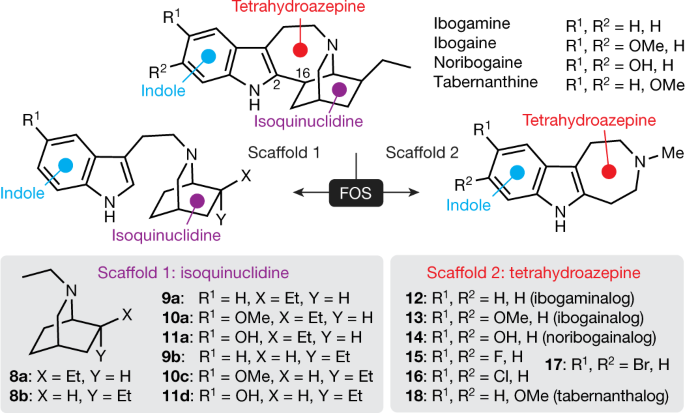
principles of function-oriented synthesis to identify the key structural elements of the potential therapeutic pharmacophore of ibogaine, and we use this information to engineer
tabernanthalog—a water-soluble, non-hallucinogenic, non-toxic analogue of ibogaine that can be prepared in a single step. In rodents, tabernanthalog was found to promote structural neural
plasticity, reduce alcohol- and heroin-seeking behaviour, and produce antidepressant-like effects. This work demonstrates that, through careful chemical design, it is possible to modify a
psychedelic compound to produce a safer, non-hallucinogenic variant that has therapeutic potential. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access
subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article
* Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn
about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EFFICIENT AND MODULAR SYNTHESIS OF IBOGAINE AND RELATED ALKALOIDS Article
06 February 2025 (2-AMINOPROPYL)BENZO[Β]THIOPHENES (APBTS) ARE NOVEL MONOAMINE TRANSPORTER LIGANDS THAT LACK STIMULANT EFFECTS BUT DISPLAY PSYCHEDELIC-LIKE ACTIVITY IN MICE Article 08
November 2021 PSYCHEDELICS: PRECLINICAL INSIGHTS PROVIDE DIRECTIONS FOR FUTURE RESEARCH Article 17 March 2023 DATA AVAILABILITY Data are available at
https://doi.org/10.6084/m9.figshare.11634795. Source data are provided with this paper. CODE AVAILABILITY Custom-written data analysis codes are available upon request from the corresponding
author. REFERENCES * Wasko, M. J., Witt-Enderby, P. A. & Surratt, C. K. DARK classics in chemical neuroscience: ibogaine. _ACS Chem. Neurosci_. 9, 2475–2483 (2018). Article CAS PubMed
Google Scholar * Noller, G. E., Frampton, C. M. & Yazar-Klosinski, B. Ibogaine treatment outcomes for opioid dependence from a twelve-month follow-up observational study. _Am. J. Drug
Alcohol Abuse_ 44, 37–46 (2018). Article PubMed Google Scholar * He, D. Y. et al. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug
ibogaine against alcohol consumption. _J. Neurosci_. 25, 619–628 (2005). Article CAS PubMed PubMed Central Google Scholar * Marton, S. et al. Ibogaine administration modifies GDNF and
BDNF expression in brain regions involved in mesocorticolimbic and nigral dopaminergic circuits. _Front. Pharmacol_. 10, 193 (2019). Article CAS PubMed PubMed Central Google Scholar *
Jenks, C. W. Extraction studies of _Tabernanthe iboga_ and _Voacanga africana_. _Nat. Prod. Lett_. 16, 71–76 (2002). Article CAS PubMed Google Scholar * Iyer, R. N., Favela, D., Zhang,
G. & Olson, D. E. The iboga enigma: the chemistry and neuropharmacology of iboga alkaloids and related analogs. _Nat. Prod. Rep_. https://doi.org/10.1039/D0NP00033G (2020). * Hough, L.
B., Pearl, S. M. & Glick, S. D. Tissue distribution of ibogaine after intraperitoneal and subcutaneous administration. _Life Sci_. 58, PL119–PL122 (1996). Article CAS PubMed Google
Scholar * Koenig, X., Kovar, M., Boehm, S., Sandtner, W. & Hilber, K. Anti-addiction drug ibogaine inhibits hERG channels: a cardiac arrhythmia risk. _Addict. Biol_. 19, 237–239 (2014).
Article CAS PubMed Google Scholar * Thurner, P. et al. Mechanism of hERG channel block by the psychoactive indole alkaloid ibogaine. _J. Pharmacol. Exp. Ther_. 348, 346–358 (2014).
Article PubMed Google Scholar * Alper, K. R., Stajić, M. & Gill, J. R. Fatalities temporally associated with the ingestion of ibogaine. _J. Forensic Sci_. 57, 398–412 (2012). Article
CAS PubMed Google Scholar * Koenig, X. & Hilber, K. The anti-addiction drug ibogaine and the heart: a delicate relation. _Molecules_ 20, 2208–2228 (2015). Article PubMed PubMed
Central CAS Google Scholar * Baumann, M. H., Pablo, J. P., Ali, S. F., Rothman, R. B. & Mash, D. C. Noribogaine (12-hydroxyibogamine): a biologically active metabolite of the
antiaddictive drug ibogaine. _Ann. NY Acad. Sci_. 914, 354–368 (2000). Article ADS CAS PubMed Google Scholar * Olson, D. E. Psychoplastogens: a promising class of plasticity-promoting
neurotherapeutics. _J. Exp. Neurosci_. 12, 1179069518800508 (2018). Article PubMed PubMed Central Google Scholar * Ly, C. et al. Psychedelics promote structural and functional neural
plasticity. _Cell Rep_. 23, 3170–3182 (2018). Article CAS PubMed PubMed Central Google Scholar * Bogenschutz, M. P. & Johnson, M. W. Classic hallucinogens in the treatment of
addictions. _Prog. Neuropsychopharmacol. Biol. Psychiatry_ 64, 250–258 (2016). Article CAS PubMed Google Scholar * Wender, P. A., Verma, V. A., Paxton, T. J. & Pillow, T. H.
Function-oriented synthesis, step economy, and drug design. _Acc. Chem. Res_. 41, 40–49 (2008). Article CAS PubMed Google Scholar * Gassaway, M. M. et al. Deconstructing the iboga
alkaloid skeleton: potentiation of FGF2-induced glial cell line-derived neurotrophic factor release by a novel compound. _ACS Chem. Biol_. 11, 77–87 (2016). Article CAS PubMed Google
Scholar * Wager, T. T., Hou, X., Verhoest, P. R. & Villalobos, A. Central nervous system multiparameter optimization desirability: application in drug discovery. _ACS Chem. Neurosci_.
7, 767–775 (2016). Article CAS PubMed Google Scholar * Glennon, R. A., Young, R., Jacyno, J. M., Slusher, M. & Rosecrans, J. A. DOM-stimulus generalization to LSD and other
hallucinogenic indolealkylamines. _Eur. J. Pharmacol_. 86, 453–459 (1983). Article CAS PubMed Google Scholar * Dunlap, L. E. et al. Identification of psychoplastogenic
_N_,_N_-dimethylaminoisotryptamine (isoDMT) analogs through structure–activity relationship studies. _J. Med. Chem_. 63, 1142–1155 (2020). Article CAS PubMed PubMed Central Google
Scholar * Halberstadt, A. L., Chatha, M., Klein, A. K., Wallach, J. & Brandt, S. D. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their
behavioral and subjective effects in other species. _Neuropharmacology_ 167, 107933 (2020). Article CAS PubMed Google Scholar * McCarroll, M. N. et al. Zebrafish behavioural profiling
identifies GABA and serotonin receptor ligands related to sedation and paradoxical excitation. _Nat. Commun_. 10, 4078 (2019). Article ADS PubMed PubMed Central CAS Google Scholar *
Breuer, L. et al. “Herbal seizures” – atypical symptoms after ibogaine intoxication: a case report. _J. Med. Case Rep_. 9, 243 (2015). Article PubMed PubMed Central Google Scholar *
Dach, K. et al. Teratological and behavioral screening of the national toxicology program 91-compound library in zebrafish (_Danio rerio_). _Toxicol. Sci_. 167, 77–91 (2019). Article CAS
PubMed Google Scholar * Rothman, R. B. & Baumann, M. H. Serotonergic drugs and valvular heart disease. _Expert Opin. Drug Saf_. 8, 317–329 (2009). Article CAS PubMed PubMed Central
Google Scholar * Phoumthipphavong, V., Barthas, F., Hassett, S., Kwan, A. C. Longitudinal effects of ketamine on dendritic architecture in vivo in the mouse medial frontal cortex.
_eNeuro_ 3, 0133-15 (2016). Article Google Scholar * Moda-Sava, R. N. et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. _Science_ 364,
eaat8078 (2019). Article CAS PubMed PubMed Central Google Scholar * Cameron, L. P. & Olson, D. E. Dark classics in chemical neuroscience: _N_,_N_-dimethyltryptamine (DMT). _ACS
Chem. Neurosci_. 9, 2344–2357 (2018). Article CAS PubMed Google Scholar * Warnault, V., Darcq, E., Levine, A., Barak, S. & Ron, D. Chromatin remodelling — a novel strategy to control
excessive alcohol drinking. _Transl. Psychiatry_ 3, e231 (2013). Article CAS PubMed PubMed Central Google Scholar * Glick, S. D. et al. Effects of iboga alkaloids on morphine and
cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum. _Brain Res_. 657, 14–22 (1994). Article CAS
PubMed Google Scholar * Giannotti, G., Barry, S. M., Siemsen, B. M., Peters, J. & McGinty, J. F. Divergent prelimbic cortical pathways interact with BDNF to regulate cocaine-seeking.
_J. Neurosci_. 38, 8956–8966 (2018). Article CAS PubMed PubMed Central Google Scholar * Glick, S. D., Kuehne, M. E., Maisonneuve, I. M., Bandarage, U. K. & Molinari, H. H.
18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. _Brain Res_. 719, 29–35 (1996).
Article CAS PubMed Google Scholar * Carnicella, S., He, D. Y., Yowell, Q. V., Glick, S. D. & Ron, D. Noribogaine, but not 18-MC, exhibits similar actions as ibogaine on GDNF
expression and ethanol self-administration. _Addict. Biol_. 15, 424–433 (2010). Article CAS PubMed PubMed Central Google Scholar * Bandarage, U. K., Kuehne, M. E. & Glick, S. D.
Total syntheses of racemic albifloranine and its anti-addictive congeners, including 18-methoxycoronaridine. _Tetrahedron_ 55, 9405–9424 (1999). Article CAS Google Scholar * Langheinrich,
U., Vacun, G. & Wagner, T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. _Toxicol. Appl.
Pharmacol_. 193, 370–382 (2003). Article CAS PubMed Google Scholar * Sampurna, B. P., Audira, G., Juniardi, S., Lai, Y.-H. & Hsiao, C.-D. A simple ImageJ-based method to measure
cardiac rhythm in zebrafish embryos. _Inventions_ 3, 21 (2018). Article Google Scholar * Westerfield, M. _The zebrafish book. A guide for the laboratory use of zebrafish (_Danio rerio_)_
5th edn (Univ. Oregon Press, 2007). * Ahrens, M. B., Orger, M. B., Robson, D. N., Li, J. M. & Keller, P. J. Whole-brain functional imaging at cellular resolution using light-sheet
microscopy. _Nat. Methods_ 10, 413–420 (2013). Article CAS PubMed Google Scholar * Kroeze, W. K. et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human
GPCRome. _Nat. Struct. Mol. Biol_. 22, 362–369 (2015). Article CAS PubMed PubMed Central Google Scholar * Barupal, D. K. et al. A comprehensive plasma metabolomics dataset for a cohort
of mouse knockouts within the international mouse phenotyping consortium. _Metabolites_ 9, 101 (2019). Article CAS PubMed Central Google Scholar * Feng, G. et al. Imaging neuronal
subsets in transgenic mice expressing multiple spectral variants of GFP. _Neuron_ 28, 41–51 (2000). Article CAS PubMed Google Scholar * Xu, T. et al. Rapid formation and selective
stabilization of synapses for enduring motor memories. _Nature_ 462, 915–919 (2009). Article ADS CAS PubMed PubMed Central Google Scholar * Chen, C. C., Lu, J., Yang, R., Ding, J. B.
& Zuo, Y. Selective activation of parvalbumin interneurons prevents stress-induced synapse loss and perceptual defects. _Mol. Psychiatry_ 23, 1614–1625 (2018). Article CAS PubMed
Google Scholar * Vazquez, M., Frazier, J. H., Reichel, C. M. & Peters, J. Acute ovarian hormone treatment in freely cycling female rats regulates distinct aspects of heroin seeking.
_Learn. Mem_. 27, 6–11 (2020). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by funds from the National Institutes of
Health (NIH) (R01GM128997 to D.E.O.; R37AA01684 to D.R.; R01AA022583 to D.K.; R01MH109475, R01MH104227 and R01NS104950 to Y.Z.; R01DA045836 to J.P.; and U19AG023122 to O.F.), a Hellman
Fellowship (D.E.O.), UC Davis STAIR and STAIR Plus grants (D.E.O.), a Max Planck Fellowship at MPFI (Y.Z.), four NIH training grants (T32GM113770 to R.J.T., T32MH112507 to L.P.C.,
5T32GM099608 to M.V.V., and 4T32GM6754714 to D.M.-T.), two UC Davis Provost’s Undergraduate Fellowships (to J.V. and A.J.P.), the Paul G. Allen Family Foundation (M.N.M. and D.K.), the
Genentech Fellowship Program (D.M.-T.), and a Medical College of Wisconsin Research Affairs Counsel Pilot Grant (J.D.M.). B.M.B. was supported by the National Center for Advancing
Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award TL1 TR001861. Delix Therapeutics funded the large receptor screen conducted at
Eurofins Discovery. We thank F. F. Wagner for help in coordinating with Eurofins Discovery. This project used the Biological Analysis Core of the UC Davis MIND Institute Intellectual and
Development Disabilities Research Center (U54 HD079125). The Olympus FV1000 confocal microscope used in this study was purchased using NIH Shared Instrumentation Grant 1S10RR019266-01. We
thank the MCB Light Microscopy Imaging Facility, which is a UC Davis Campus Core Research Facility, for the use of this microscope. Several of the drugs used in this study were provided by
the NIDA Drug Supply Program. We thank D. R. Carty for assistance with larval zebrafish toxicity assays. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Neuroscience Graduate Program,
University of California, Davis, Davis, CA, USA Lindsay P. Cameron & Maxemiliano V. Vargas * Department of Chemistry, University of California, Davis, Davis, CA, USA Robert J. Tombari,
Alexander J. Pell, Zefan Q. Hurley, Guoliang Zhang, Jayashri Viswanathan, Lee E. Dunlap & David E. Olson * Department of Molecular, Cell and Developmental Biology, University of
California, Santa Cruz, Santa Cruz, CA, USA Ju Lu, Taohui Liu, Michelle Tjia & Yi Zuo * Department of Neurology, University of California, San Francisco, San Francisco, CA, USA Yann
Ehinger & Dorit Ron * Institute for Neurodegenerative Diseases, University of California, San Francisco, San Francisco, CA, USA Matthew N. McCarroll, Jack C. Taylor, Douglas
Myers-Turnbull & David Kokel * Quantitative Biosciences Consortium, University of California, San Francisco, San Francisco, CA, USA Douglas Myers-Turnbull * Department of Molecular
Biosciences, School of Veterinary Medicine, University of California, Davis, Davis, CA, USA Bianca Yaghoobi & Pamela J. Lein * Department of Cell Biology, Neurobiology, and Anatomy,
Medical College of Wisconsin, Milwaukee, WI, USA Lauren J. Laskowski, Emilie I. Anderson & John D. McCorvy * Department of Pharmacology, School of Medicine, University of California,
Davis, Davis, CA, USA Brandon M. Brown & Heike Wulff * West Coast Metabolomics Center, University of California, Davis, Davis, CA, USA Zachary T. Rabow & Oliver Fiehn * Department of
Physiology, University of California, San Francisco, San Francisco, CA, USA David Kokel * Department of Anesthesiology, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO,
USA Jamie Peters * Department of Pharmacology, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO, USA Jamie Peters * Department of Biochemistry and Molecular Medicine,
School of Medicine, University of California, Davis, Sacramento, CA, USA David E. Olson * Center for Neuroscience, University of California, Davis, Davis, CA, USA David E. Olson * Delix
Therapeutics, Inc., Palo Alto, CA, USA David E. Olson Authors * Lindsay P. Cameron View author publications You can also search for this author inPubMed Google Scholar * Robert J. Tombari
View author publications You can also search for this author inPubMed Google Scholar * Ju Lu View author publications You can also search for this author inPubMed Google Scholar * Alexander
J. Pell View author publications You can also search for this author inPubMed Google Scholar * Zefan Q. Hurley View author publications You can also search for this author inPubMed Google
Scholar * Yann Ehinger View author publications You can also search for this author inPubMed Google Scholar * Maxemiliano V. Vargas View author publications You can also search for this
author inPubMed Google Scholar * Matthew N. McCarroll View author publications You can also search for this author inPubMed Google Scholar * Jack C. Taylor View author publications You can
also search for this author inPubMed Google Scholar * Douglas Myers-Turnbull View author publications You can also search for this author inPubMed Google Scholar * Taohui Liu View author
publications You can also search for this author inPubMed Google Scholar * Bianca Yaghoobi View author publications You can also search for this author inPubMed Google Scholar * Lauren J.
Laskowski View author publications You can also search for this author inPubMed Google Scholar * Emilie I. Anderson View author publications You can also search for this author inPubMed
Google Scholar * Guoliang Zhang View author publications You can also search for this author inPubMed Google Scholar * Jayashri Viswanathan View author publications You can also search for
this author inPubMed Google Scholar * Brandon M. Brown View author publications You can also search for this author inPubMed Google Scholar * Michelle Tjia View author publications You can
also search for this author inPubMed Google Scholar * Lee E. Dunlap View author publications You can also search for this author inPubMed Google Scholar * Zachary T. Rabow View author
publications You can also search for this author inPubMed Google Scholar * Oliver Fiehn View author publications You can also search for this author inPubMed Google Scholar * Heike Wulff
View author publications You can also search for this author inPubMed Google Scholar * John D. McCorvy View author publications You can also search for this author inPubMed Google Scholar *
Pamela J. Lein View author publications You can also search for this author inPubMed Google Scholar * David Kokel View author publications You can also search for this author inPubMed Google
Scholar * Dorit Ron View author publications You can also search for this author inPubMed Google Scholar * Jamie Peters View author publications You can also search for this author inPubMed
Google Scholar * Yi Zuo View author publications You can also search for this author inPubMed Google Scholar * David E. Olson View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS A.J.P., Z.Q.H. and G.Z. synthesized the ibogalogs. L.E.D. synthesized 5-MeO-DMT fumarate and performed the CNS MPO calculations. L.P.C. performed the
dendritogenesis and spinogenesis assays. L.P.C. and J.V. performed the head-twitch response experiments. M.N.M. performed the zebrafish heart-rate and seizure experiments. J.C.T., D.M.-T.
and R.J.T. performed the zebrafish behavioural experiments. R.J.T. and B.Y. performed the zebrafish toxicity assays. B.M.B. and L.P.C. performed the hERG inhibition studies. L.P.C. performed
the solubility studies and conditioned place preference experiments. J.L., T.L. and L.P.C. performed the experiments assessing in vivo spine dynamics. L.J.L., E.I.A. and J.D.M. performed
the receptor functional assays. J.L. and M.T. performed the forced swim test following UMS. M.V.V. performed the forced swim test study without UMS with assistance from L.E.D. Z.T.R. and
L.P.C. performed the pharmacokinetic studies. J.P. performed the heroin self-administration experiments. Y.E. performed the alcohol consumption assays. L.P.C. performed the sucrose
preference assay. O.F., H.W., J.D.M., P.J.L., D.K., D.R., J.P., Y.Z. and D.E.O. supervised various aspects of this project and assisted with data analysis. D.E.O. conceived the project and
wrote the manuscript with input from all authors. CORRESPONDING AUTHOR Correspondence to David E. Olson. ETHICS DECLARATIONS COMPETING INTERESTS D.E.O. is the president and chief scientific
officer of Delix Therapeutics. Delix Therapeutics has licensed TBG-related technology from the University of California, Davis. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature_ thanks
Amy Newman, Yavin Shaham and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIG. 1 SYNTHESIS OF IBOGALOGS. A, Ibogalogs lacking the
tetrahydroazepine of ibogaine were synthesized in only a few steps. In brief, acylation of pyridine 1 under reductive conditions yielded the carboxybenzyl (Cbz)-protected dihydropyridine 2,
which was immediately subjected to a Diels–Alder reaction with methyl vinyl ketone (3) followed by an in situ epimerization with NaOMe to afford an inseparable 1:1 mixture of _exo_ (4A) and
_endo_ (4B) isomers (73% over 3 steps). Reaction of 4A and 4B with tosylhydrazide yielded the hydrazones 5A and 5B, which were separable via a combination of selective crystallization and
chromatography (total yield of the two isomers, 75%). Caglioti reduction of the tosylhydrazones yielded 6A or 6B, which were readily converted to a variety of analogues via reaction
sequences involving hydrogenolysis of the Cbz group, hydrogenation of the olefin, and C–N bond formation (Supplementary Information). B, Ibogalogs lacking the isoquinuclidine of ibogaine
were synthesized in a single step through Fischer indole cyclization. See Supplementary Information for details. EXTENDED DATA FIG. 2 THE EFFECTS OF IBOGALOGS ON DENDRITOGENESIS. A,
Representative images of rat embryonic cortical neurons (DIV6) treated with the indicated compounds. Scale bar, 10 μm. B, Maximum numbers of crossings (_N_max) of the Sholl plots demonstrate
that tetrahydroazepine-containing ibogalogs are more effective at increasing dendritic arbor complexity than are isoquinuclicine-containing ibogalogs. C, Sholl analysis (circle radii,
1.34-μm increments) demonstrates that cultured cortical neurons treated with several ibogalogs have more complex dendritic arbors compared to vehicle control (_n_ = 52–83 neurons per
treatment). The shaded area surrounding each line represents 95% confidence intervals. Control compounds, isoquinuclidines and tetrahydroazepines are shown in blue, purple and red,
respectively. Exact _n_ values for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact _P_ values are
reported in Methods and Supplementary Table 1. EXTENDED DATA FIG. 3 TBG IS SAFER THAN IBOGAINE. A, Unlike ibogaine, IBG and TBG do not induce bradycardia in larval zebrafish. Sertindole (SI)
was used as a positive control. B, Heat maps are shown representing aggregate larval zebrafish locomotor activity per well compared to vehicle controls (pseudo-_Z_-score). Red and blue
indicate higher and lower activity than the mean of vehicle controls, respectively, while white indicates activity within ±1 s.d. of the control. Stimuli applied over time are indicated
under the heat maps. Colours indicate bright LED light of respective colours, black traces represent the waveforms of acoustic stimuli, and grey vertical lines indicate physical tapping as
secondary acoustic stimuli. C, Confusion matrix for classification of compounds (200 μM) plus vehicle and lethal controls. D, Concentration–response curves are shown for treated zebrafish
subjected to the series of stimuli depicted in B. Lower percentages indicate treatments that were more often classified as vehicle (blue) or lethal (red). The solid line denotes the median
and the shading denotes a 95th percentile confidence interval calculated by bootstrap. _n_ = 8 wells per condition (64 zebrafish per condition). Blue lines indicate that all compounds
produce behavioural phenotypes more distinct from vehicle at higher concentrations. Red lines indicate that known toxins (for example, PTZ, picrotoxin, endosulfan), known hERG inhibitors
(sertindole, haloperidol, terfenadine) and iboga alkaloids (IBO, NOR) produce behavioural phenotypes more closely resembling a lethal phenotype as their concentrations are increased.
Increasing concentrations of IBG or TBG do not produce lethal-like behavioural phenotypes. E, Transgenic larval zebrafish expressing GCaMP5G were immobilized in agarose, treated with
compounds, and imaged over time. The known seizure-inducing compound PTZ was used as a positive control. Ibogaine and TBG were treated at 50 μM (_n_ = 2 per condition). F, Proportion of
viable and non-viable (malformed + dead) zebrafish following treatment with vehicle and TBG (66 μM) for 5 dpf (Fisher’s exact test: _P_ = 0.3864). Representative images of zebrafish treated
with vehicle and TBG (66 μM) for 2 and 5 dpf are shown. Scale bar, 2 mm. Exact _n_ values for each experimental condition are reported in Supplementary Table 1. Specific statistical tests,
information on reproducibility and exact _P_ values are reported in the Methods and Supplementary Table 1. EXTENDED DATA FIG. 4 CONCENTRATION–RESPONSE CURVES DEMONSTRATING THE ABILITIES OF
IBOGALOGS AND RELATED COMPOUNDS TO ACTIVATE 5-HT AND OPIOID RECEPTORS. All compounds were assayed in parallel using the same drug dilutions. Graphs reflect representative
concentration–response curves plotting mean and s.e.m. of data points performed in duplicate or triplicate. Assay details are described in Methods. Exact _n_ values for each experimental
condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility and exact _P_ values are reported in Methods and in Supplementary Table 1.
EXTENDED DATA FIG. 5 PHARMACOLOGICAL PROFILES OF IBOGALOGS AND RELATED COMPOUNDS. EC50 and _E_max estimates from at least two independent concentration-response curves performed in duplicate
or triplicate. log(_E_max/EC50) activity relative to the system _E_max. Inactive, inactive in agonist mode; N.D., not determined; blue boxes indicate that the compound exhibits antagonist
activity; dark grey boxes indicate that the compound is inactive in agonist mode but not tested in antagonist mode; orange boxes indicate that the compound is an inverse agonist. Ibogalogs
are more selective 5-HT2A agonists than is 5-MeO-DMT. Exact _n_ values for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on
reproducibility and exact _P_ values are reported in Methods and in Supplementary Table 1. EXTENDED DATA FIG. 6 HIGH DOSES OF TBG DO NOT PRODUCE A CONDITIONED PLACE PREFERENCE. A, Schematic
of the design of the conditioned place preference experiments. On day 1, the amount of time the mice spent in each distinct side of a two-chamber apparatus was recorded. Next, vehicle and
TBG were administered to mice on alternating days while they were confined to chamber A (white box) or chamber B (grey parallel lines), respectively. Conditioning lasted for a total of 6
days. On day 8, preference for each distinct side of the two-chamber apparatus was assessed. B, A low dose of TBG (1 mg kg−1) did not produce conditioned place preference or conditioned
place aversion. Higher doses (10 and 50 mg kg−1) produce a modest conditioned place aversion. C, TBG does not produce any long-lasting (>24 h) effects on locomotion. There is no
statistical difference in locomotion between any pre- or post-conditioning groups (_P_ = 0.9985, one-way ANOVA). White bars indicate groups before receiving TBG (that is, pre-conditioning),
and blue bars indicate groups 24 h after the last TBG administration (that is, post-conditioning). Exact _n_ values for each experimental condition are reported in Supplementary Table 1.
Specific statistical tests, information on reproducibility and exact _P_ values are reported in Methods and in Supplementary Table 1. EXTENDED DATA FIG. 7 TBG PRODUCES ANTIDEPRESSANT EFFECTS
IN MICE. A, Schematic illustrating the stressors used as part of the 7-day UMS protocol. White and grey boxes represent the light and dark phases of the light cycle, respectively. B, TBG
rescues the effects of UMS on immobility. C, TBG (50 mg kg−1) reaches high brain concentrations and is rapidly eliminated from the body. Mice were administered 3 different doses of TBG via
i.p. injection and euthanized either 15 min or 3 h later. Whole brains and livers were collected, dried, homogenized and extracted with MTBE. Quantification was accomplished using LC–MS and
concentrations of TBG in the two organs were calculated. Several samples for the 10 and 1 mg kg−1 doses at the 3 h time point had TBG at levels below the limit of quantification (around 5
nmol g−1). In those cases, the values were recorded as 0. Exact _n_ numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on
reproducibility and exact _P_ values are reported in Methods and in Supplementary Table 1. EXTENDED DATA FIG. 8 EFFECTS OF TBG ON LOCOMOTION AND SUCROSE-SEEKING BEHAVIOUR IN RATS. A, Acute
administration of TBG does not impair locomotion in the open field. Rats were subjected to novelty-induced locomotion (baseline) for 30 min. At that time, cocaine was administered and
psychostimulant-induced locomotion (+cocaine) was assessed for 60 min. There were no differences between the vehicle- and TBG-treated groups with respect to total distance travelled or
average velocity. Furthermore, there was no difference in thigmotaxis measured during the baseline period (that is, the percentage of time in the centre of the open field). B–E, A sucrose
self-administration experiment was conducted in a similar manner to the heroin self-administration experiment in Fig. 4. Doses in mg kg−1 are shown in parentheses. B, Sucrose seeking over
time is shown. Coloured arrows indicate when each group received TBG. VEH was administered at all other time points to each group. C, TBG acutely reduces sucrose-seeking behaviour in a
dose-dependent manner when administered during self-administration. D, TBG acutely reduces sucrose seeking when administered immediately before the first extinction session. The CUE
(injection 1 = vehicle; injection 2, vehicle) and EXT (injection 1, vehicle; injection 2, TBG) groups were compared, as they were matched for the number of withdrawal days between the last
self-administration and first extinction session. E, TBG does not have long-lasting effects on sucrose-seeking behaviour, as it does not reduce active lever pressing during the cued
reinstatement when administered 12–14 days previously during self-administration (SA) or immediately before extinction (EXT). Exact _n_ values for each experimental condition are reported in
Supplementary Table 1. Specific statistical tests, information on reproducibility and exact _P_ values are reported in Methods and in Supplementary Table 1. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplemental methods describing the synthesis of key compounds. Spectral data are provided. REPORTING SUMMARY SUPPLEMENTARY TABLE Supplementary Table 1: The number
of replicates for each experimental condition, statistical parameters for each experiment, and information on reproducibility are provided. VIDEO 1 : Ibogaine induces bradycardia. Larval
zebrafish were immobilized in agarose and treated with either vehicle (VEH), ibogaine (IBO), ibogainalog (IBG), or tabernanthalog (TBG). Sertindole (SI) was used as a positive control. VIDEO
2 : PTZ induces excessive neural activity. Zebrafish larvae expressing GCaMP5G were immobilized in agarose and treated with either vehicle (VEH), ibogaine (IBO), or tabernanthalog (TBG).
The seizure-inducing compound pentylenetetrazol (PTZ) was used as a positive control. SOURCE DATA SOURCE DATA FIG. 2 SOURCE DATA FIG. 3 SOURCE DATA FIG. 4 RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cameron, L.P., Tombari, R.J., Lu, J. _et al._ A non-hallucinogenic psychedelic analogue with therapeutic potential. _Nature_ 589, 474–479
(2021). https://doi.org/10.1038/s41586-020-3008-z Download citation * Received: 17 January 2020 * Accepted: 30 October 2020 * Published: 09 December 2020 * Issue Date: 21 January 2021 * DOI:
https://doi.org/10.1038/s41586-020-3008-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative