Play all audios:
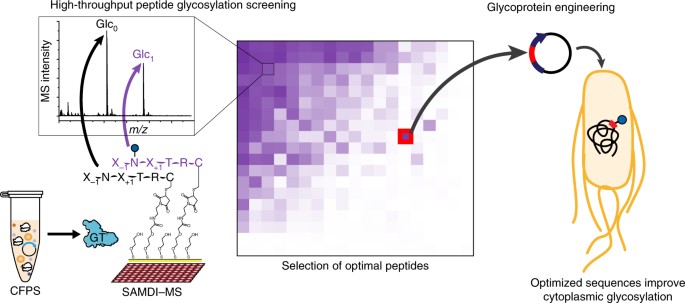
ABSTRACT Glycosylation is an abundant post-translational modification that is important in disease and biotechnology. Current methods to understand and engineer glycosylation cannot
sufficiently explore the vast experimental landscapes required to accurately predict and design glycosylation sites modified by glycosyltransferases. Here we describe a systematic platform
for glycosylation sequence characterization and optimization by rapid expression and screening (GlycoSCORES), which combines cell-free protein synthesis and mass spectrometry of
self-assembled monolayers. We produced six N- and O-linked polypeptide-modifying glycosyltransferases from bacteria and humans in vitro and rigorously determined their substrate
specificities using 3,480 unique peptides and 13,903 unique reaction conditions. We then used GlycoSCORES to optimize and design small glycosylation sequence motifs that directed efficient
N-linked glycosylation in vitro and in the _Escherichia coli_ cytoplasm for three heterologous proteins, including the human immunoglobulin Fc domain. We find that GlycoSCORES is a broadly
applicable method to facilitate fundamental understanding of glycosyltransferases and engineer synthetic glycoproteins. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more
Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS:
* Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A UNIVERSAL GLYCOENZYME BIOSYNTHESIS PIPELINE THAT
ENABLES EFFICIENT CELL-FREE REMODELING OF GLYCANS Article Open access 24 October 2022 RESTORING PROTEIN GLYCOSYLATION WITH GLYCOSHAPE Article Open access 14 October 2024 AN EFFICIENT
_C_-GLYCOSIDE PRODUCTION PLATFORM ENABLED BY RATIONALLY TUNING THE CHEMOSELECTIVITY OF GLYCOSYLTRANSFERASES Article Open access 15 October 2024 REFERENCES * Khoury, G. A., Baliban, R. C.
& Floudas, C. A. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. _Sci. Rep._ 1, 90 (2011). Article CAS PubMed
Central Google Scholar * Helenius, A. & Aebi, M. Intracellular functions of N-linked glycans. _Science_ 291, 2364–2369 (2001). Article CAS PubMed Google Scholar * Sethuraman, N.
& Stadheim, T. A. Challenges in therapeutic glycoprotein production. _Curr. Opin. Biotechnol._ 17, 341–346 (2006). Article CAS PubMed Google Scholar * Elliott, S. et al. Enhancement
of therapeutic protein in vivo activities through glycoengineering. _Nat. Biotechnol._ 21, 414–421 (2003). Article CAS PubMed Google Scholar * Chung, C. H. et al. Cetuximab-induced
anaphylaxis and IgE specific for galactose-α-1,3-galactose. _N. Engl. J. Med._ 358, 1109–1117 (2008). Article CAS PubMed PubMed Central Google Scholar * Lin, C.-W. et al. A common
glycan structure on immunoglobulin G for enhancement of effector functions. _Proc. Natl Acad. Sci. USA_ 112, 10611–10616 (2015). Article CAS PubMed PubMed Central Google Scholar *
Clausen, H., Wandall, H.H., Steentoft, C., Stanley, P. & Schnaar, R.L. in Essentials of Glycobiology. (eds. A. Varki et al.) 713–728 (Cold Spring Harbor Laboratory Press, Cold Spring
Harbor, NY, 2015). * Valderrama-Rincon, J. D. et al. An engineered eukaryotic protein glycosylation pathway in _Escherichia coli_. _Nat. Chem. Biol._ 8, 434–436 (2012). Article CAS PubMed
PubMed Central Google Scholar * Keys, T. G. & Aebi, M. Engineering protein glycosylation in prokaryotes. _Curr. Opin. Syst. Biol._ 5, 23–31 (2017). Article Google Scholar * Wang,
L.-X. & Davis, B. G. Realizing the promise of chemical glycobiology. _Chem. Sci._ 4, 3381–3394 (2013). Article CAS PubMed Google Scholar * Yang, Z. et al. Engineered CHO cells for
production of diverse, homogeneous glycoproteins. _Nat. Biotechnol._ 33, 842–844 (2015). Article CAS PubMed Google Scholar * Li, H. et al. Optimization of humanized IgGs in
glycoengineered _Pichia pastoris_. _Nat. Biotechnol._ 24, 210–215 (2006). Article CAS PubMed Google Scholar * Xu, Y. et al. A novel enzymatic method for synthesis of glycopeptides
carrying natural eukaryotic N-glycans. _Chem. Commun. (Camb.)_ 53, 9075–9077 (2017). Article CAS Google Scholar * Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M. &
Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. _Nucleic Acids Res._ 42, D490–D495 (2014). Article CAS PubMed Google Scholar * Ban, L. et al. Discovery of
glycosyltransferases using carbohydrate arrays and mass spectrometry. _Nat. Chem. Biol._ 8, 769–773 (2012). Article CAS PubMed PubMed Central Google Scholar * Pathak, S. et al. The
active site of O-GlcNAc transferase imposes constraints on substrate sequence. _Nat. Struct. Mol. Biol._ 22, 744–750 (2015). Article CAS PubMed PubMed Central Google Scholar *
Ortiz-Meoz, R. F., Merbl, Y., Kirschner, M. W. & Walker, S. Microarray discovery of new OGT substrates: the medulloblastoma oncogene OTX2 is O-GlcNAcylated. _J. Am. Chem. Soc._ 136,
4845–4848 (2014). Article CAS PubMed PubMed Central Google Scholar * Robinson, P. V., Tsai, C. T., de Groot, A. E., McKechnie, J. L. & Bertozzi, C. R. Glyco-seek: ultrasensitive
detection of protein-specific glycosylation by proximity ligation polymerase chain reaction. _J. Am. Chem. Soc._ 138, 10722–10725 (2016). Article CAS PubMed PubMed Central Google Scholar
* Naegeli, A. et al. Substrate specificity of cytoplasmic N-glycosyltransferase. _J. Biol. Chem._ 289, 24521–24532 (2014). Article CAS PubMed PubMed Central Google Scholar * Naegeli,
A. et al. Molecular analysis of an alternative N-glycosylation machinery by functional transfer from _Actinobacillus pleuropneumoniae_ to _Escherichia coli_. _J. Biol. Chem._ 289, 2170–2179
(2014). Article CAS PubMed Google Scholar * Keys, T. G. et al. A biosynthetic route for polysialylating proteins in _Escherichia coli_. _Metab. Eng._ 44, 293–301 (2017). Article CAS
PubMed Google Scholar * Cuccui, J. et al. The N-linking glycosylation system from _Actinobacillus pleuropneumoniae_ is required for adhesion and has potential use in glycoengineering.
_Open Biol._ 7, 160212 (2017). Article CAS PubMed PubMed Central Google Scholar * Schwarz, F., Fan, Y. Y., Schubert, M. & Aebi, M. Cytoplasmic N-glycosyltransferase of
_Actinobacillus pleuropneumoniae_ is an inverting enzyme and recognizes the NX(S/T) consensus sequence. _J. Biol. Chem._ 286, 35267–35274 (2011). Article CAS PubMed PubMed Central Google
Scholar * Song, Q. et al. Production of homogeneous glycoprotein with multi-site modifications by an engineered N-glycosyltransferase mutant. _J. Biol. Chem._ 292, 8856–8863 (2017).
Article CAS PubMed PubMed Central Google Scholar * Gross, J. et al. The Haemophilus influenzae HMW1 adhesin is a glycoprotein with an unusual N-linked carbohydrate modification. _J.
Biol. Chem._ 283, 26010–26015 (2008). Article CAS PubMed PubMed Central Google Scholar * Kawai, F. et al. Structural insights into the glycosyltransferase activity of the
_Actinobacillus pleuropneumoniae_ HMW1C-like protein. _J. Biol. Chem._ 286, 38546–38557 (2011). Article CAS PubMed PubMed Central Google Scholar * Lomino, J. V. et al. A two-step
enzymatic glycosylation of polypeptides with complex N-glycans. _Bioorg. Med. Chem._ 21, 2262–2270 (2013). Article CAS PubMed PubMed Central Google Scholar * Chen, M. M., Glover, K. J.
& Imperiali, B. From peptide to protein: comparative analysis of the substrate specificity of N-linked glycosylation in _C. jejuni_. _Biochemistry_ 46, 5579–5585 (2007). Article CAS
PubMed Google Scholar * Fisher, A. C. et al. Production of secretory and extracellular N-linked glycoproteins in _Escherichia coli_. _Appl. Environ. Microbiol._ 77, 871–881 (2011). Article
CAS PubMed Google Scholar * Carlson, E. D., Gan, R., Hodgman, C. E. & Jewett, M. C. Cell-free protein synthesis: applications come of age. _Biotechnol. Adv._ 30, 1185–1194 (2012).
Article CAS PubMed Google Scholar * Kuo, H. Y., DeLuca, T. A., Miller, W. M. & Mrksich, M. Profiling deacetylase activities in cell lysates with peptide arrays and SAMDI mass
spectrometry. _Anal. Chem._ 85, 10635–10642 (2013). Article CAS PubMed PubMed Central Google Scholar * Kornacki, J. R., Stuparu, A. D. & Mrksich, M. Acetyltransferase p300/CBP
associated Factor (PCAF) regulates crosstalk-dependent acetylation of histone H3 by distal site recognition. _ACS Chem. Biol._ 10, 157–164 (2015). Article CAS PubMed Google Scholar *
Kim, J. & Mrksich, M. Profiling the selectivity of DNA ligases in an array format with mass spectrometry. _Nucleic Acids Res._ 38, e2 (2010). Article CAS PubMed Google Scholar *
Laurent, N. et al. Enzymatic glycosylation of peptide arrays on gold surfaces. _ChemBioChem_ 9, 883–887 (2008). Article CAS PubMed PubMed Central Google Scholar * Laurent, N. et al.
SPOT synthesis of peptide arrays on self-assembled monolayers and their evaluation as enzyme substrates. _ChemBioChem_ 9, 2592–2596 (2008). Article CAS PubMed Google Scholar * Hussain,
M. R., Hoessli, D. C. & Fang, M. N-acetylgalactosaminyltransferases in cancer. _Oncotarget_ 7, 54067–54081 (2016). Article PubMed PubMed Central Google Scholar * Schjoldager, K. T.
et al. Probing isoform-specific functions of polypeptide GalNAc-transferases using zinc finger nuclease glycoengineered SimpleCells. _Proc. Natl Acad. Sci. USA_ 109, 9893–9898 (2012).
Article PubMed PubMed Central Google Scholar * Yoshida, A., Suzuki, M., Ikenaga, H. & Takeuchi, M. Discovery of the shortest sequence motif for high level mucin-type O-glycosylation.
_J. Biol. Chem._ 272, 16884–16888 (1997). Article CAS PubMed Google Scholar * Gerken, T. A., Raman, J., Fritz, T. A. & Jamison, O. Identification of common and unique peptide
substrate preferences for the UDP-GalNAc:polypeptide α-N-acetylgalactosaminyltransferases T1 and T2 derived from oriented random peptide substrates. _J. Biol. Chem._ 281, 32403–32416 (2006).
Article CAS PubMed Google Scholar * Kong, Y. et al. Probing polypeptide GalNAc-transferase isoform substrate specificities by in vitro analysis. _Glycobiology_ 25, 55–65 (2015). Article
CAS PubMed Google Scholar * Steentoft, C. et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. _EMBO J._ 32, 1478–1488 (2013). Article CAS
PubMed PubMed Central Google Scholar * Wang, A. C., Jensen, E. H., Rexach, J. E., Vinters, H. V. & Hsieh-Wilson, L. C. Loss of _O_-GlcNAc glycosylation in forebrain excitatory neurons
induces neurodegeneration. _Proc. Natl Acad. Sci. USA_ 113, 15120–15125 (2016). Article CAS PubMed PubMed Central Google Scholar * Yang, X. et al. Phosphoinositide signalling links
O-GlcNAc transferase to insulin resistance. _Nature_ 451, 964–969 (2008). Article CAS PubMed Google Scholar * Liu, X. et al. A peptide panel investigation reveals the acceptor
specificity of O-GlcNAc transferase. _FASEB J._ 28, 3362–3372 (2014). Article CAS PubMed Google Scholar * Chalkley, R. J., Thalhammer, A., Schoepfer, R. & Burlingame, A. L.
Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. _Proc. Natl Acad. Sci. USA_ 106, 8894–8899 (2009). Article PubMed
PubMed Central Google Scholar * Lazarus, M. B., Nam, Y., Jiang, J., Sliz, P. & Walker, S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. _Nature_
469, 564–567 (2011). Article CAS PubMed PubMed Central Google Scholar * Choi, K. J., Grass, S., Paek, S., St Geme, J. W. III & Yeo, H. J. The _Actinobacillus pleuropneumoniae_
HMW1C-like glycosyltransferase mediates N-linked glycosylation of the _Haemophilus influenzae_ HMW1 adhesin. _PLoS One_ 5, e15888 (2010). Article CAS PubMed PubMed Central Google Scholar
* Haselberg, R., de Jong, G. J. & Somsen, G. W. Low-flow sheathless capillary electrophoresis-mass spectrometry for sensitive glycoform profiling of intact pharmaceutical proteins.
_Anal. Chem._ 85, 2289–2296 (2013). Article CAS PubMed Google Scholar * Schoborg, J. A. et al. A cell-free platform for rapid synthesis and testing of active oligosaccharyltransferases.
_Biotechnol. Bioeng._ 115, 739–750 (2018). Article CAS PubMed Google Scholar * Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using
Clustal Omega. _Mol. Syst. Biol._ 7, 539 (2011). Article PubMed PubMed Central Google Scholar * Gurard-Levin, Z. A., Scholle, M. D., Eisenberg, A. H. & Mrksich, M. High-throughput
screening of small molecule libraries using SAMDI mass spectrometry. _ACS Comb. Sci._ 13, 347–350 (2011). Article CAS PubMed PubMed Central Google Scholar * Goerke, A. R. & Swartz,
J. R. Development of cell-free protein synthesis platforms for disulfide bonded proteins. _Biotechnol. Bioeng._ 99, 351–367 (2008). Article CAS PubMed Google Scholar * Espah Borujeni,
A., Channarasappa, A. S. & Salis, H. M. Translation rate is controlled by coupled trade-offs between site accessibility, selective RNA unfolding and sliding at upstream standby sites.
_Nucleic Acids Res._ 42, 2646–2659 (2014). Article CAS PubMed Google Scholar * Martin, R. W. _et al_. Cell-free protein synthesis from genomically recoded bacteria enables multisite
incorporation of noncanonical amino acids. _Nat. Commun_. 9, 1203 (2018). Article CAS PubMed PubMed Central Google Scholar * Lajoie, M. J. _et al_. Genomically recoded organisms expand
biological functions. _Science_ 342, 357–360 (2013). Article CAS PubMed PubMed Central Google Scholar * Kwon, Y.-C. & Jewett, M. C. High-throughput preparation methods of crude
extract for robust cell-free protein synthesis. _Sci. Rep_. 5, 8663 (2015). Article CAS PubMed Google Scholar * Jewett, M. C. & Swartz, J. R. Mimicking the _Escherichia coli_
cytoplasmic environment activates long-lived and efficient cell-free protein synthesis. _Biotechnol. Bioeng_. 86, 19–26 (2004). Article CAS PubMed Google Scholar * Jewett, M. C.,
Calhoun, K. A., Voloshin, A., Wuu, J. J. & Swartz, J. R. An integrated cell-free metabolic platform for protein production and synthetic biology. _Mol. Syst. Biol_. 4, 220 (2008).
Article CAS PubMed Google Scholar * Jewett, M. C. & Swartz, J. R. Rapid expression and purification of 100 nmol quantities of active protein using cell-free protein synthesis.
_Biotechnol_. _Prog_. 20, 102–109 (2004). Article CAS PubMed Google Scholar * Hong, S. H. _et al_. Cell-free protein synthesis from a release factor 1 deficient _Escherichia coli_
activates efficient and multiple site-specific nonstandard amino acid incorporation. _ACS Synth_. _Biol_. 3, 398–409 (2014). * Jian, W., Edom, R. W., Wang, D., Weng, N. & Zhang, S. W.
Relative quantitation of glycoisoforms of intact apolipoprotein C3 in human plasma by liquid chromatography-high-resolution mass spectrometry. _Anal_. _Chem_. 85, 2867–2874 (2013). Download
references ACKNOWLEDGEMENTS The authors acknowledge J.C. Stark and J. Hershewe for assistance with western blotting, helpful discussions, and sharing of reagents and ideas; S. Habibi for
assistance with LC-TOF instrumentation; and A. Karim for helpful conversations. The authors also thank J. Kath for supply of plasmids, advice on protein expression, and critical reading of
the manuscript. We also thank A. Natarajan of the Department of Microbiology at Cornell University, T. Jaroentomeechai of the Robert Frederick Smith School of Chemical and Biomolecular
Engineering at Cornell University, and J. Janetzko of the Department of Chemistry and Chemical Biology at Harvard University for sharing the ppGalNAcT, Im7, and hOGT source plasmids,
respectively. This work made use of the Integrated Molecular Structure Education and Research Center at Northwestern University, which has received support from the state of Illinois, the
Northwestern University Office of Research and the Chemistry Department for LC-TOF instrumentation. This material is based upon work supported by the Defense Threat Reduction Agency
(HDTRA1-15-10052/P00001), the David and Lucile Packard Foundation, the Dreyfus Teacher-Scholar program, and the National Science Foundation (Graduate Research Fellowship under Grant No.
DGE-1324585 and MCB-1413563). AUTHOR INFORMATION Author notes * These authors contributed equally: Weston Kightlinger, Liang Lin. AUTHORS AND AFFILIATIONS * Department of Chemical and
Biological Engineering, Northwestern University, Evanston, IL, USA Weston Kightlinger, Milan Mrksich & Michael C. Jewett * Center for Synthetic Biology, Northwestern University,
Evanston, IL, USA Weston Kightlinger, Liang Lin, Milan Mrksich & Michael C. Jewett * Department of Biomedical Engineering, Northwestern University, Evanston, IL, USA Liang Lin, Madisen
Rosztoczy, Wenhao Li & Milan Mrksich * Robert Frederick Smith School of Chemical and Biomolecular Engineering, Cornell University, Ithaca, NY, USA Matthew P. DeLisa * Department of
Microbiology, Cornell University, Ithaca, NY, USA Matthew P. DeLisa * Department of Chemistry, Northwestern University, Evanston, IL, USA Milan Mrksich Authors * Weston Kightlinger View
author publications You can also search for this author inPubMed Google Scholar * Liang Lin View author publications You can also search for this author inPubMed Google Scholar * Madisen
Rosztoczy View author publications You can also search for this author inPubMed Google Scholar * Wenhao Li View author publications You can also search for this author inPubMed Google
Scholar * Matthew P. DeLisa View author publications You can also search for this author inPubMed Google Scholar * Milan Mrksich View author publications You can also search for this author
inPubMed Google Scholar * Michael C. Jewett View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS W.K. and L.L. designed, performed, and analyzed
experiments. M.R. designed and optimized experimental protocols. W.L. helped to synthesize peptide libraries. M.M. and M.C.J. directed the studies and interpreted the data. W.K., L.L.,
M.P.D., M.M., and M.C.J. conceived of the study and wrote the manuscript with assistance from M.R. and W.L. CORRESPONDING AUTHORS Correspondence to Milan Mrksich or Michael C. Jewett. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Tables 1–5, Supplementary Figures 1–27, Supplementary Note 1 REPORTING
SUMMARY RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kightlinger, W., Lin, L., Rosztoczy, M. _et al._ Design of glycosylation sites by rapid synthesis
and analysis of glycosyltransferases. _Nat Chem Biol_ 14, 627–635 (2018). https://doi.org/10.1038/s41589-018-0051-2 Download citation * Received: 24 October 2017 * Accepted: 07 March 2018 *
Published: 07 May 2018 * Issue Date: June 2018 * DOI: https://doi.org/10.1038/s41589-018-0051-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative