Play all audios:
ABSTRACT The fusion of mononucleated myoblasts produces multinucleated muscle fibers leading to the formation of skeletal muscle. Myomaker, a skeletal muscle-specific membrane protein, is
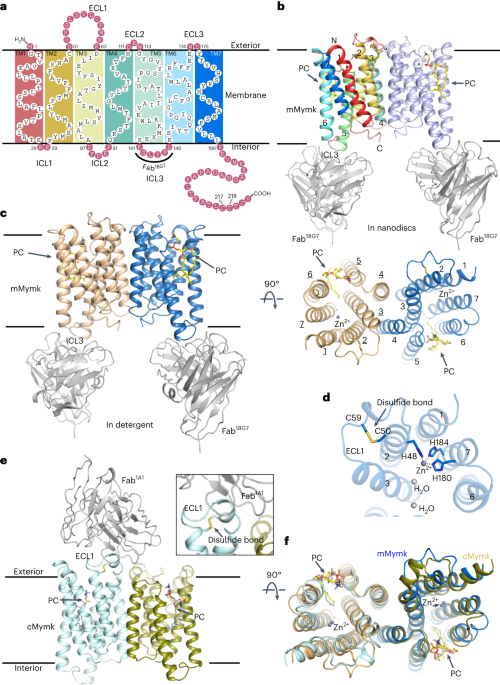
essential for myoblast fusion. Here we report the cryo-EM structures of mouse Myomaker (mMymk) and _Ciona robusta_ Myomaker (cMymk). Myomaker contains seven transmembrane helices (TMs) that
adopt a G-protein-coupled receptor-like fold. TMs 2–4 form a dimeric interface, while TMs 3 and 5–7 create a lipid-binding site that holds the polar head of a phospholipid and allows the
alkyl tails to insert into Myomaker. The similarity of cMymk and mMymk suggests a conserved Myomaker-mediated cell fusion mechanism across evolutionarily distant species. Functional analyses
demonstrate the essentiality of the dimeric interface and the lipid-binding site for fusogenic activity, and heterologous cell–cell fusion assays show the importance of transcellular
interactions of Myomaker protomers for myoblast fusion. Together, our findings provide structural and functional insights into the process of myoblast fusion. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only
$17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MYOMERGER PROMOTES FUSION
PORE BY ELASTIC COUPLING BETWEEN PROXIMAL MEMBRANE LEAFLETS AND HEMIFUSION DIAPHRAGM Article Open access 21 January 2021 WNT-ROR-DVL SIGNALLING AND THE DYSTROPHIN COMPLEX ORGANIZE
PLANAR-POLARIZED MEMBRANE COMPARTMENTS IN _C. ELEGANS_ MUSCLES Article Open access 10 June 2024 TGFΒ SIGNALLING ACTS AS A MOLECULAR BRAKE OF MYOBLAST FUSION Article Open access 02 February
2021 DATA AVAILABILITY Sequences of the anti-Myomaker antibody candidates were analyzed with the IMGT database (http://www.imgt.org/). The 3D cryo-EM density maps have been deposited in the
Electron Microscopy Data Bank under the accession numbers EMD-40933 (mMymk in detergent), EMD-40934 (mMymk in nanodiscs), EMD-40935 (cMymk), EMD-40936 (mMymkR107A) and EMD-40937
(mMymkY118A). Atomic coordinates for the atomic model have been deposited in the Protein Data Bank (PDB) under the accession numbers 8T03 (mMymk in detergent), 8T04 (mMymk in nanodiscs),
8T05 (cMymk), 8T06 (mMymkR107A) and 8T07 (mMymkY118A). Additional data supporting the findings in this study are provided as source data and supplementary information to this paper.
REFERENCES * Kim, J. H., Jin, P., Duan, R. & Chen, E. H. Mechanisms of myoblast fusion during muscle development. _Curr. Opin. Genet Dev._ 32, 162–170 (2015). CAS PubMed PubMed Central
Google Scholar * Buckingham, M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. _Curr. Opin. Genet Dev._ 16, 525–532 (2006). CAS PubMed Google Scholar * Kim, J. H.
& Chen, E. H. The fusogenic synapse at a glance. _J. Cell Sci_. https://doi.org/10.1242/jcs.213124 (2019). * Demonbreun, A. R., Biersmith, B. H. & McNally, E. M. Membrane fusion in
muscle development and repair. _Semin. Cell Dev. Biol._ 45, 48–56 (2015). CAS PubMed PubMed Central Google Scholar * Hindi, S. M., Tajrishi, M. M. & Kumar, A. Signaling mechanisms in
mammalian myoblast fusion. _Sci. Signal_ 6, re2 (2013). PubMed PubMed Central Google Scholar * Leikina, E. et al. Myomaker and Myomerger work independently to control distinct steps of
membrane remodeling during myoblast fusion. _Dev. Cell_ 46, 767–780.e767 (2018). CAS PubMed PubMed Central Google Scholar * Millay, D. P. et al. Myomaker is a membrane activator of
myoblast fusion and muscle formation. _Nature_ 499, 301–305 (2013). CAS PubMed PubMed Central Google Scholar * Millay, D. P., Sutherland, L. B., Bassel-Duby, R. & Olson, E. N.
Myomaker is essential for muscle regeneration. _Genes Dev._ 28, 1641–1646 (2014). CAS PubMed PubMed Central Google Scholar * Di Gioia, S. A. et al. A defect in myoblast fusion underlies
Carey-Fineman-Ziter syndrome. _Nat. Commun._ 8, 16077 (2017). PubMed PubMed Central Google Scholar * Hedberg-Oldfors, C., Lindberg, C. & Oldfors, A. Carey-Fineman-Ziter syndrome with
mutations in the myomaker gene and muscle fiber hypertrophy. _Neurol. Genet._ 4, e254 (2018). CAS PubMed PubMed Central Google Scholar * Jumper, J. et al. Highly accurate protein
structure prediction with AlphaFold. _Nature_ 596, 583–589 (2021). CAS PubMed PubMed Central Google Scholar * Millay, D. P. et al. Structure-function analysis of myomaker domains
required for myoblast fusion. _Proc. Natl Acad. Sci. Acad._ 113, 2116–2121 (2016). CAS Google Scholar * Lee, G. H. et al. A GPI processing phospholipase A2, PGAP6, modulates Nodal
signaling in embryos by shedding CRIPTO. _J. Cell Biol._ 215, 705–718 (2016). CAS PubMed PubMed Central Google Scholar * Zhang, H. et al. Evolution of a chordate-specific mechanism for
myoblast fusion. _Sci. Adv._ 8, eadd2696 (2022). CAS PubMed Google Scholar * Du, J. et al. Structures of human mGlu2 and mGlu7 homo- and heterodimers. _Nature_ 594, 589–593 (2021). CAS
PubMed Google Scholar * Velazhahan, V. et al. Structure of the class D GPCR Ste2 dimer coupled to two G proteins. _Nature_ 589, 148–153 (2021). CAS PubMed Google Scholar * Soderberg, O.
et al. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. _Methods_ 45, 227–232 (2008). PubMed Google Scholar * Holm, L. &
Rosenstrom, P. Dali server: conservation mapping in 3D. _Nucleic Acids Res._ 38, W545–W549 (2010). CAS PubMed PubMed Central Google Scholar * Vasiliauskaite-Brooks, I. et al. Structure
of a human intramembrane ceramidase explains enzymatic dysfunction found in leukodystrophy. _Nat. Commun._ 9, 5437 (2018). CAS PubMed PubMed Central Google Scholar * Tanabe, H. et al.
Human adiponectin receptor AdipoR1 assumes closed and open structures. _Commun. Biol._ 3, 446 (2020). CAS PubMed PubMed Central Google Scholar * Waltenspuhl, Y., Schoppe, J., Ehrenmann,
J., Kummer, L. & Pluckthun, A. Crystal structure of the human oxytocin receptor. _Sci. Adv._ 6, eabb5419 (2020). PubMed PubMed Central Google Scholar * Perez-Vargas, J. et al.
Structural basis of eukaryotic cell-cell fusion. _Cell_ 157, 407–419 (2014). CAS PubMed Google Scholar * Zhang, J. et al. Species-specific gamete recognition initiates fusion-driving
trimer formation by conserved fusogen HAP2. _Nat. Commun._ 12, 4380 (2021). CAS PubMed PubMed Central Google Scholar * Jeong, J. & Conboy, I. M. Phosphatidylserine directly and
positively regulates fusion of myoblasts into myotubes. _Biochem. Biophys. Res. Commun._ 414, 9–13 (2011). CAS PubMed PubMed Central Google Scholar * Tsuchiya, M. et al. Cell surface
flip-flop of phosphatidylserine is critical for PIEZO1-mediated myotube formation. _Nat. Commun._ 9, 2049 (2018). PubMed PubMed Central Google Scholar * Bi, P. et al. Control of muscle
formation by the fusogenic micropeptide myomixer. _Science_ 356, 323–327 (2017). CAS PubMed PubMed Central Google Scholar * Lee, D. M. & Chen, E. H. _Drosophila_ myoblast fusion:
invasion and resistance for the ultimate union. _Annu. Rev. Genet._ 53, 67–91 (2019). CAS PubMed PubMed Central Google Scholar * Srinivas, B. P., Woo, J., Leong, W. Y. & Roy, S. A
conserved molecular pathway mediates myoblast fusion in insects and vertebrates. _Nat. Genet._ 39, 781–786 (2007). CAS PubMed Google Scholar * Long, T., Liu, Y. & Li, X. Molecular
structures of human ACAT2 disclose mechanism for selective inhibition. _Structure_ https://doi.org/10.1016/j.str.2021.07.009 (2021). Article PubMed PubMed Central Google Scholar * Sun,
Y. & Li, X. Cholesterol efflux mechanism revealed by structural analysis of human ABCA1 conformational states. _Nat. Cardiovasc. Res._ 1, 238–245 (2022). PubMed PubMed Central Google
Scholar * Ritchie, T. K. et al. Chapter 11—reconstitution of membrane proteins in phospholipid bilayer nanodiscs. _Methods Enzymol._ 464, 211–231 (2009). CAS PubMed PubMed Central Google
Scholar * Liu, Y. et al. Mechanisms and inhibition of Porcupine-mediated Wnt acylation. _Nature_ 607, 816–822 (2022). CAS PubMed PubMed Central Google Scholar * Guo, X. et al.
Structure and mechanism of human cystine exporter cystinosin. _Cell_ 185, 3739–3752 (2022). CAS PubMed PubMed Central Google Scholar * Wang, Q. et al. A combination of human broadly
neutralizing antibodies against hepatitis B virus HBsAg with distinct epitopes suppresses escape mutations. _Cell Host Microbe_ 28, 335–349.e336 (2020). CAS PubMed PubMed Central Google
Scholar * Rasmussen, S. G. et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. _Nature_ 450, 383–387 (2007). CAS PubMed Google Scholar * Mastronarde, D. N.
Automated electron microscope tomography using robust prediction of specimen movements. _J. Struct. Biol._ 152, 36–51 (2005). PubMed Google Scholar * Li, X. et al. Electron counting and
beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. _Nat. Methods_ 10, 584–590 (2013). CAS PubMed PubMed Central Google Scholar * Zivanov, J. et al. New
tools for automated high-resolution cryo-EM structure determination in RELION-3. _eLife_ https://doi.org/10.7554/eLife.42166 (2018). * Rohou, A. & Grigorieff, N. CTFFIND4: fast and
accurate defocus estimation from electron micrographs. _J. Struct. Biol._ 192, 216–221 (2015). PubMed PubMed Central Google Scholar * Wagner, T. et al. SPHIRE-crYOLO is a fast and
accurate fully automated particle picker for cryo-EM. _Commun. Biol._ 2, 218 (2019). PubMed PubMed Central Google Scholar * Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M.
A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. _Nat. Methods_ 14, 290–296 (2017). CAS PubMed Google Scholar * Emsley, P. & Cowtan, K. Coot:
model-building tools for molecular graphics. _Acta Crystallogr. D_ 60, 2126–2132 (2004). PubMed Google Scholar * Adams, P. D. et al. PHENIX: a comprehensive Python-based system for
macromolecular structure solution. _Acta Crystallogr. D_ 66, 213–221 (2010). CAS PubMed PubMed Central Google Scholar * Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of
macromolecular structures by the maximum-likelihood method. _Acta Crystallogr. D_ 53, 240–255 (1997). CAS PubMed Google Scholar * Croll, T. I. ISOLDE: a physically realistic environment
for model building into low-resolution electron-density maps. _Acta Crystallogr. D. Struct. Biol._ 74, 519–530 (2018). CAS PubMed PubMed Central Google Scholar * Chen, V. B. et al.
MolProbity: all-atom structure validation for macromolecular crystallography. _Acta Crystallogr. D_ 66, 12–21 (2010). CAS PubMed Google Scholar * Pettersen, E. F. et al. UCSF ChimeraX:
structure visualization for researchers, educators, and developers. _Protein Sci._ 30, 70–82 (2021). CAS PubMed Google Scholar * Song, K. et al. Heart repair by reprogramming non-myocytes
with cardiac transcription factors. _Nature_ 485, 599–604 (2012). CAS PubMed PubMed Central Google Scholar * Ramirez-Martinez, A. et al. Impaired activity of the fusogenic micropeptide
Myomixer causes myopathy resembling Carey-Fineman-Ziter syndrome. _J. Clin. Invest._ https://doi.org/10.1172/JCI159002 (2022). Download references ACKNOWLEDGEMENTS The cryo-EM data were
collected at the UT Southwestern Medical Center Cryo-EM Facility (funded in part by the Cancer Prevention and Research Institute of Texas (CPRIT) Core Facility Support Award no. RP170644).
We thank R. Bassel-Duby for guidance and assistance in many aspects of this work, L. Beatty and Y. Qin for cell culture and P. Bi for providing the cDNA of cMymk and helpful discussion. This
work was supported by grant nos. AHA 23EIA1038669 (X.L.), NIH P01 HL160487 (X.L.), R01 GM135343 (X.L.) and R01 AR067294 (E.N.O.), the Robert A. Welch Foundation (grant nos. I-0025 to E.N.O.
and I-1957 to X.L.) and the CPRIT (grant no. RP200103 to E.N.O.). AUTHOR INFORMATION Author notes * These authors contributed equally: Tao Long, Yichi Zhang. AUTHORS AND AFFILIATIONS *
Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX, USA Tao Long, Linda Donnelly & Xiaochun Li * Department of Molecular Biology, University of
Texas Southwestern Medical Center, Dallas, TX, USA Yichi Zhang, Hui Li, Yu-Chung Pien, Ning Liu & Eric N. Olson * Department of Biophysics, University of Texas Southwestern Medical
Center, Dallas, TX, USA Xiaochun Li Authors * Tao Long View author publications You can also search for this author inPubMed Google Scholar * Yichi Zhang View author publications You can
also search for this author inPubMed Google Scholar * Linda Donnelly View author publications You can also search for this author inPubMed Google Scholar * Hui Li View author publications
You can also search for this author inPubMed Google Scholar * Yu-Chung Pien View author publications You can also search for this author inPubMed Google Scholar * Ning Liu View author
publications You can also search for this author inPubMed Google Scholar * Eric N. Olson View author publications You can also search for this author inPubMed Google Scholar * Xiaochun Li
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.L., Y.Z., E.N.O. and X.L. designed the research. T.L. performed the biochemical and
structural experiments. T.L. and L.D. screened the monoclonal antibodies. Y.Z., Y.-C.P. and H.L. performed functional analysis. T.L., Y.Z., N.L., E.N.O. and X.L. analyzed the data and
contributed to paper preparation. T.L., Y.Z., E.N.O. and X.L. wrote the paper. E.N.O. and X.L. supervised the project. CORRESPONDING AUTHORS Correspondence to Eric N. Olson or Xiaochun Li.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Structural & Molecular Biology_ thanks Stephen Muench,
Pier Lorenzo Puri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Katarzyna
Ciazynska, in collaboration with the _Nature Structural & Molecular Biology_ team. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 REPRESENTATIVE GEL-FILTRATION CHROMATOGRAMS OF MYOMAKER COMPLEX WITH FAB. A, Fab18G7-bound mMymkWT
in lipid nanodiscs. B, Fab18G7-bound mMymkWT in detergent. C, Fab18G7-bound mMymkR107A in detergent. D, Fab18G7-bound mMymkY118A in detergent. E, Fab1A1-bound cMymkWT in detergent. The
fraction of each complex is shown on SDS–PAGE with molecular markers and proteins indicated. The peak of each excess fab is indicated. Data shown are representative of two independent
experiments. Source data EXTENDED DATA FIG. 2 CRYO-EM ANALYSES OF MMYMK. A and B, Summary of image processing procedures of mMymk in nanodiscs (A) and detergent (B). The cryo-EM 3D classes
and the final cryo-EM map are shown. C, Fourier shell correlation (FSC) curves between two half maps using cryoSPARC output and Angular distribution of the particles used for the final
reconstructions. D, Local resolution of cryo-EM maps. Maps are colored according to local resolution, estimated using cryoSPARC. E, cryo-EM density of the transmembrane helices (TM) and PC
of mMymk in detergent. The model is shown as cartoon with sticks. F, cryo-EM density of water molecules and zinc ion in TMs at 8σ level. The panel was generated by COOT. EXTENDED DATA FIG. 3
CRYO-EM ANALYSES OF CMYMK. A, Summary of image processing procedures of cMymk in detergent. The cryo-EM 3D classes and the final cryo-EM map are shown. B, Fourier shell correlation (FSC)
curves between two half maps using cryoSPARC output and Angular distribution of the particles used for the final reconstructions. C, Local resolution of cryo-EM map. Map is colored according
to local resolution, estimated using cryoSPARC. D, cryo-EM density of the transmembrane helix (TM) and PC of cMymk in detergent. The model is shown as cartoon with sticks. EXTENDED DATA
FIG. 4 STRUCTURES OF DIMERIC GPCRS. A, Overall structure of mGlu2, a Class-C GPCR (PDB: 7EPA), from the side of the membrane (left) and from the extracellular space (right). One protomer is
colored in pink and the other protomer is colored in purple. TM helices are numbered. B, Overall structure of Ste2, a Class-D GPCR (PDB: 7AD3), from the side of the membrane (left) and from
the extracellular space (right). TMs are labeled. One protomer is colored in blue and the other protomer is colored in green. TM helices are numbered. EXTENDED DATA FIG. 5 SCHEMATIC OF
EXPERIMENTAL DESIGN TO VALIDATE THE FUNCTION OF MMYMK. A, Schematic of experimental design to validate _in cis_ dimerization of mMymk by Proximity Ligation Assay (PLA). Created with
BioRender.com. Two primary antibodies of different species were used to detect two signal peptide and flag-tagged (SF) mMymk monomers. The PLA probe binds the primary antibodies in close
proximity to generate the signal. B and C, Trans proximity ligation assay (PLA) was performed on C2C12 myoblasts that express signal peptide Flag-mMymk and detected using mouse Flag (B) and
rabbit Flag (C) antibodies. Representative images of PLA (red) with Flag (green) expressing mMymkWT or mMymk3M are shown. Nuclei were labeled with DAPI (blue). Scale bar, 10 μm. Experiments
were repeated three times with similar results. D, Schematic of experimental design to validate antibody inhibition of mMymk by Ab15G1. Created with BioRender.com. Ab15G1 occupies mMymk
dimers on either cell and would prevent mMymk interaction across cells, thereby inhibiting myoblast fusion. E, Schematic of experimental design to validate _in trans_ interaction of mMymk by
Proximity Ligation Assay (PLA). Created with BioRender.com. C2C12 myoblasts express either signal peptide and flag-tagged (SF) mMymk monomers or signal peptide and HA-tagged (SHA) mMymk
monomers. Two primary antibodies (anti-HA antibody and anti-Flag antibody) from different species were used to detect two mMymk dimers in the neighboring cells. The PLA probe binds the close
primary antibodies to generate localization of the signal. F, Representative images of HA (green) with DAPI (blue) expressing mMymkWT or mMymk3M on C2C12 myoblasts. The images show that HA
antibody binds the two forms of mMymkWT or mMymk3M similarly. Scale bar, 10 μm. Experiments were repeated three times with similar results. EXTENDED DATA FIG. 6 SEQUENCE ALIGNMENT OF MOUSE,
HUMAN, ZEBRAFISH AND _CIONA_ MYOMAKER. A, Sequence alignment. The transmembrane helices (TM), intracellular loops (ICL), extracellular loops (ECL) and the residue numbers of Myomaker are
indicated above the protein sequence. The conserved residues and disulfide bond are highlighted in green and indicated beneath the protein sequence, respectively. The specific residues
necessary for dimerization and lipid binding and Carey-Fineman-Ziter syndrome (CFZS) causing mutations are indicated by purple, blue and red stars, respectively. m, mouse; h, human; z,
zebrafish; c, _Ciona_. B, The distribution of CFZS-causing mutations. EXTENDED DATA FIG. 7 COMPARISON OF MMYMK WITH ITS STRUCTURAL HOMOLOGUES. A, Monomeric structure of mMymk (blue) viewed
from the side of the membrane. B, Overall structure of ACER3 in green (PDB: 6G7O) viewed from the side of the membrane. C, Structural comparison between mMymk in dark blue and ACER3 in
green. D, Structural comparison between mMymk in dark blue and AdipoR1 in cyan (PDB: 6KS0,). E, Structural comparison between mMymk in dark blue and OTR in magenta (PDB: 6TPK). F, Comparison
between catalytic core of ACER3 and mMymk with related residues labeled. Zinc ion is shown as a sphere in gray (mMymk) and orange (ACER3 and AdipoR1). Calcium ion is shown as a sphere in
green (ACER3). 1-Oleylglycerol, which was co-crystallized with ACER3, is shown as sticks in magenta. The PC of mMymk is shown as sticks in yellow. TM helices are numbered. EXTENDED DATA FIG.
8 CRYO-EM ANALYSES OF MMYMK VARIANTS. A, Summary of image processing procedures of mMymkR107A (left) and mMymkY118A (right) in detergent. The cryo-EM 3D classes and the final cryo-EM map
are shown. B, Fourier shell correlation (FSC) curves between two half maps using cryoSPARC output and Angular distribution of the particles used for the final reconstructions. C, Local
resolution of cryo-EM maps. Maps are colored according to local resolution, estimated using cryoSPARC. D, The cryo-EM maps around the position of putative PC in mMymkWT, mMymkR107A, and
mMymkY118A. The cryo-EM maps of mMymkWT were shown at 2.7 Å and 3.3 Å (low-pass filtered by cryoSPARC) resolution, respectively. There is no notable density around the putative PC position
in the cryo-EM maps of mMymkR107A, and mMymkY118A. The modeled PC is shown as sticks in yellow and its map is colored in red. EXTENDED DATA FIG. 9 STRUCTURAL COMPARISON BETWEEN MMYMKWT,
MMYMKR107A AND MMYMKY118A. A, The hydrophilic interaction network in the extracellular leaflet of TMs 4, 5 and 6 between mMymkWT in blue, mMymkR107A in green, mMymkY118A in salmon. Water
molecule and Zinc ion are shown as sphere in white and gray, respectively. B, Structural comparisons among mMymkWT in blue, mMymkR107A in green, mMymkY118A in salmon and the AlphaFold
prediction model of mMymkWT in gray. The arrow indicates the structural shift of TM5. TMs and related residues are labeled. The PC of mMymkWT is shown as sticks in yellow. C, The cryo-EM
maps of ECL2 in mMymkWT, mMymkR107A, and mMymkY118A. The entire cryo-EM map of each structure is shown and the map of ECL2 is colored in red. EXTENDED DATA FIG. 10 AB15G1 BINDS TO THE
EXTRACELLULAR REGIONS OF MMYMK. A, Pull-down assay of Ab15G1 or control antibody (anti-Hemophilus influenza type B antibody) with mMymk or mMymk-Fab18G7 complex detected by Coomassie
staining. The molecular markers (left) and proteins (right) are indicated. Data shown are representative of two independent experiments. B, Immunofluorescence microscopy of live C2C12
myoblasts expressing GFP (green). Endogenous mMymk is stained with Ab15G1. Scale bar, 10 μm. Experiments were repeated three times with similar results. Source data SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplementary Table 1. REPORTING SUMMARY PEER REVIEW FILE SOURCE DATA SOURCE DATA FIG. 2 Unprocessed western blots. SOURCE DATA FIG. 2 Statistical source data.
SOURCE DATA FIG. 3 Unprocessed western blots. SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 1 Unprocessed gels.
SOURCE DATA EXTENDED DATA FIG. 10 Unprocessed gel. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a
publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing
agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Long, T., Zhang, Y., Donnelly, L. _et al._ Cryo-EM structures of Myomaker reveal a molecular basis
for myoblast fusion. _Nat Struct Mol Biol_ 30, 1746–1754 (2023). https://doi.org/10.1038/s41594-023-01110-8 Download citation * Received: 09 February 2023 * Accepted: 25 August 2023 *
Published: 28 September 2023 * Issue Date: November 2023 * DOI: https://doi.org/10.1038/s41594-023-01110-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative