Play all audios:
ABSTRACT Large negative differential conductance (NDC) at lower bias regime is a very desirable functional property for single molecular device. Due to the non-conjugated segment separating
two conjugated branches, the single thiolated arylethynylene molecule with 9,10-dihydroanthracene core (denoted as TADHA) presents excellent NDC behavior in lower bias regime. Based on the
_ab initio_ calculation and non-equilibrium Green’s function formalism, the NDC behavior of TADHA molecular device and the H2O-molecule-adsorption effects are studied systematically. The
numerical results show that the NDC behavior of TADHA molecular junction originates from the Stark effect of the applied bias which splits the degeneration of the highest occupied molecular
orbital (HOMO) and HOMO-1. The H2O molecule adsorbed on the terminal sulphur atom strongly suppresses the conductance of TADHA molecular device and destroys the NDC behavior in the lower
bias regime. Single or separated H2O molecules adsorbed on the backbone of TADHA molecule can depress the energy levels of molecular orbitals, but have little effects on the NDC behavior of
the TADHA molecular junction. Aggregate of several H2O molecules adsorbed on one branch of TADHA molecule can dramatically enhance the conductance and NDC behavior of the molecular junction,
and result in rectifier behavior. SIMILAR CONTENT BEING VIEWED BY OTHERS LOCAL CATION-TUNED REVERSIBLE SINGLE-MOLECULE SWITCH IN ELECTRIC DOUBLE LAYER Article Open access 09 June 2023
GATE-SWITCHABLE RECTIFICATION IN ISOTYPE VAN DER WAALS HETEROSTRUCTURE OF MULTILAYER MOTE2/SNS2 WITH LARGE BAND OFFSETS Article Open access 12 June 2020 HIGHLY EFFICIENT HOLE INJECTION FROM
AU ELECTRODE TO FULLERENE-DOPED TRIPHENYLAMINE DERIVATIVE LAYER Article Open access 04 May 2022 INTRODUCTION Utilizing single molecule as functional device in electronic circuit is an
ultimate goal of molecular electronics, which has motivated scientists to devote themselves to the investigations of molecular devices for tens of years1,2,3,4,5,6,7,8,9,10. Due to the rapid
development of single molecular technologies11,12,13,14, great progresses have been achieved for single-molecule-device fabrications in recent years15,16,17,18. At the meantime, different
strategies are designed to control and improve the functional properties of single molecular device19,20,21,22. In order to gain insights into the controlling mechanism of single molecular
functional characteristics23,24,25, the effects of external ambient26,27,28, electrode distance29, 30, molecule-electrode interface31,32,33,34,35, molecular anchor36,37,38,39,40,41,42,43,
side group44,45,46,47, doping48, 49 and external field50,51,52,53,54,55 have been studied intensively. In experimental studies, molecular devices are often fabricated in solution and
measured in vacuum or in gas circumstance, thus the effect of the surrounding molecule on the functional properties of molecular device should also be discussed56,57,58,59. Generally, the
surrounding molecules play negative effects on the molecular junction, for example, aqueous solution or the water vapor can suppress the electronic transport of molecular junction
dramatically28, 56,57,58. However, sometimes the surrounding molecules can also have positive effects, our recent research reveals that the small ambient molecules can make molecular
junction more stable due to their suppressing the thermal vibrations of the molecular junction14. Therefore, studying the influence of surrounding molecules on molecular device is very
helpful to improve the functional characteristics of the molecular device by properly applying surrounding molecules. Due to the non-conjugated segment separating two conjugated arms, the
single thiolated arylethynylene molecule with 9,10-dihydroanthracene core (denoted as TADHA) shows pronounced negative differential conductance (NDC) behavior, which has recent been detected
by Perrin _et al_.60. Although the NDC behavior of single molecular device is often presented in theoretical studies, it is very scarce in experimental findings, and the NDC often shows
very small for most cases. Thus, it is significant that Perrin _et al_. fabricated single TADHA molecular junction with large NDC behavior, especially the NDC behavior was occurred at lower
bias regime because high bias voltage may deform the configuration of molecular junction14. Considering that the molecular junction was fabricated in solution, we are very interested in that
if the junction is not absolutely dried, _i.e_., if one or several H2O molecules are left and adsorbed on the functional molecule, whether the NDC behavior can be destroyed or be enhanced?
In order to answer this question, according to the density functional theory (DFT), we simulated the adsorptions of H2O molecules on different positions of the TADHA molecule. Then by
applying non-equilibrium Green’s function (NEGF) formalism, the electronic transport properties of TADHA molecular junction were studied with the influences of H2O adsorbates. Our study
shows that, the H2O molecules adsorbed on the terminal sulphur atoms not only destroy the NDC behavior of TADHA molecular device, but also depress the current of the molecular junction
distinctly at lower bias regime. The NDC behavior of TADHA molecular device seems to show some water-immunity character for single or separated H2O molecules adsorbed on the backbone of
TADHA molecule, but it is very sensitive to the aggregate of several H2O molecules. Our findings are valuable to the fabrication of TADHA molecular junction in solvent, _i.e_., one can
determine whether there are H2O molecules adsorbed on the terminal S atoms or aggregate of H2O molecules adsorbed on the backbone of functional molecule by the electronic transport
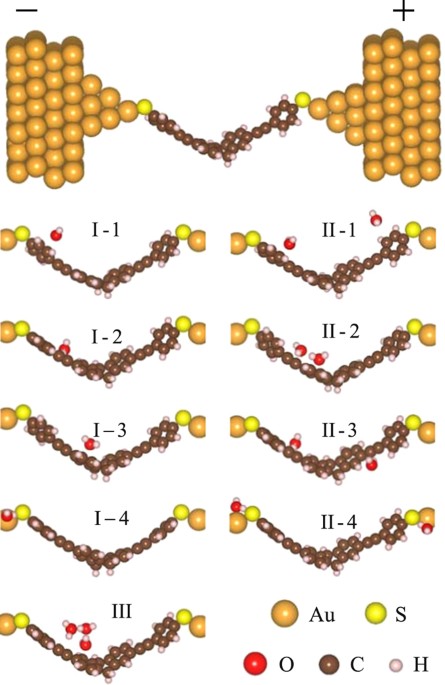
properties of molecular junction in experiment. RESULTS ONE H2O MOLECULE ADSORPTION In order to simulate the TADHA molecular junction and study the H2O effect, we constructed Au-Molecule-Au
systems by sandwiching TADHA molecule between two gold electrodes with one or several H2O molecules adsorbed on different sites of TADHA molecule as shown in Fig. 1. As for
one-H2O-adsorption samples, based on the geometric optimizations, we found that there are four typical sites for H2O molecule to be adsorbed on, _i.e_., (1) the aryl ring site on one arm of
TADHA molecule near the electrode, denoted as Type I-1; (2) the ethynylene site on one arm of TADHA molecule, denoted as Type I-2; (3) 9,10-dihydroanthracene core site, denoted as Type I-3;
(4) the terminal sulphur atom site, denoted as Type I-4 (See Fig. 1). The calculations of ground-state-energy show that, the affinities for the H2O molecule adsorbed as Type I-1, 2, 3 and 4
are about 0.09, 0.2, 0.15 and 0.78 eV, respectively, which suggests that the H2O molecule is more likely to be adsorbed on the terminal S atoms, and then is the triple bond of TADHA
molecule. The affinities also show that the adsorptions are very weak compared to the covalent bond. Figure 2 shows the current and the differential conductance as functions of applied bias
for the TADHA molecular junctions without H2O adsorbate or with one H2O molecule being adsorbed on different sites of TADHA molecule. The figure shows that for the molecular junction without
H2O molecule adsorbate, the molecular system shows symmetric electronic transport characters with respect to the positive and negative bias. Since the current shows peak values at about
±0.25 V, the NDC behavior appears in the differential conductance curve when the absolute value of the applied bias is larger than 0.25 V. The peak values of the NDC are presented at about
±0.35 V, which is a lower-bias NDC behavior as the experiment detected60. Since the molecular junction may be destroyed and the interface configuration may be deformed by high bias voltage
and thermal vibration at room temperature14, one can expect that the low-bias NDC behavior is a very desirable function of molecular device in the future. When one H2O molecule is adsorbed
on the TADHA molecule, the current and the differential conductance in the negative bias regime for the Type I-1, 2 and 3 molecular junctions show a little difference compared with those of
the molecular junctions without the H2O adsorbate. However, in the positive bias regime for the voltage larger than 0.2 V, the TADHA molecular system seems more conductive with Type I-3
configuration (see Fig. 2). At the meantime, the peak values of the current and the differential conductance are shifted slightly to higher bias regime as well as the peak values of the NDC.
It is noticeable that the NDC behavior of the molecular junction in the low bias regime is destroyed when the H2O is adsorbed on the terminal S site (Type I-4). At the same time, the
low-bias current is depressed by about one order in the magnitude, which is similar to the molecular junctions probed by Long _et al_.28. In order to understand the NDC behavior of the TADHA
molecular junction and the H2O-adsorbate effects, we presented the transmission spectra for the bias voltages of 0.0 V, ±0.25 V, ±0.5 V and the voltage of peak-current in Fig. 3. Due to the
contributions of the highest occupied molecular orbital (HOMO) and HOMO-1 to the electronic transmission and the degenerate of these two molecular orbitals, a high transmission peak is
presented at about −0.2 eV for the transmission curve with zero bias as Fig. 3(a) shows. When the bias is applied, the degenerate of the two molecular orbitals is destroyed by the Stark
effect of the bias, which further splits the high transmission peak into two lower transmission peaks. Because of the split of the transmission peak, the transmission probability at Fermi
energy level slightly increases with the increase of the bias in the lower bias regime. However, due to the rapid decrease of the height of the split transmission peaks with the increase of
the bias, the area under the transmission curve in the bias window increases at first and reaches a peak value at about 0.25 V, and then decreases with the increase of the bias, which
depends on the rivalry of the width of the bias window and the mean height of transmission spectra in bias window. Thus a current peak appears at about 0.25 V as well as the NDC behavior for
the TADHA molecular junction without H2O adsorbate. As for one H2O molecule adsorbed on the TADHA molecular junction, due to the strong electronegativity of the O atom, the TADHA molecular
orbitals are modulated differently with the H2O molecule adsorbed on different positions, which further induces different changes of the transmission spectra as well as the conductance of
the molecular system. In detail, for Type I-1 adsorption, since the H2O molecule is very close to the electrode and the affinity is very weak, the applied bias dominates the split of the
energy levels and suppresses the effect of the H2O molecule by the electrodes, hence the transmission spectra show little difference from those of the TADHA molecular junction without H2O
adsorbate (Fig. 3(b)). However, for Type I-2 and Type I-3 adsorption, the strong electronegativity of the O atom depresses the energy of the molecular orbital which close to it (Fig. 3(f)).
Taking Type I-3 system as an example, since the H2O molecule is closer to the left branch of TADHA molecule, the energy of the orbital which locates on the left arm of TADHA molecule is
lowered down, while the energy of the orbital which locates on the right branch of TADHA molecule is almost unaltered by the H2O molecule. As for positive bias, the HOMO is lowered, whereas
the HOMO-1 is little influenced. Thus the transmission peaks are less separated with positive bias compared with that of the molecular junction without the H2O adsorbate. Consequently, the
heights of the transmission peaks decrease a little more slowly in the split process with the increase of the positive bias than with negative bias, which can be easily seen by comparing the
transmission curve of Type I-3 molecular system with that of the molecular system without H2O adsorbate at 0.25 V (Fig. 3(d)). Therefore, due to the effect of the H2O molecule on the HOMO
and further on the transmission spectra, the TADHA molecular system with Type I-3 configuration shows more conductive when the bias is larger than 0.25 V, and the NDC behavior begins from a
little higher bias voltage compared with that of the molecular system without H2O. On the contrary, for the negative bias, when the transmission peak is split, the H2O adsorbate is closer to
HOMO-1, which results in a little red-shift of transmission peak corresponding to HOMO-1, while the transmission peak which corresponds to HOMO is almost unaltered. Since the HOMO-1 is
shifted to the lower energy area and is apart from the bias window, one can easily understand why the current is changed very little by the H2O molecule in the negative bias regime.
Different from the transmissions of Type I-1, 2 and 3 molecular systems, the transmission peaks are shifted to much lower energy regime by the strong influence of H2O adsorbate for Type I-4
system at lower bias. As Fig. 3(e) shows, the transmission peak related to the HOMO appears at about −0.4 eV, which is about 0.2 eV lower than those of other type molecular junctions. Thus,
with lower bias, the transmissions of Type I-4 system in the bias windows are evidently smaller than the other cases, which results in the poor current of Type I-4 molecular junction in the
lower bias regime. With the increase of the negative bias, the transmission peak corresponding to the HOMO is blue-shifted and gradually enters bias window (Fig. 3(f)). However, with the
increase of the positive bias, the transmission peak corresponding to the HOMO is shifted very little. Therefore, the current begins increasing from relatively lower bias in the negative
bias regime compared with positive bias. In order to gain deep insight into the NDC behavior, we presented spatial distributions of the HOMOs and HOMOs-1 for TADHA molecular systems at 0.0
V, ±0.5 V and at the peak-current voltages in Fig. 4. The figure shows that, at 0.0 V, due to the approximate degeneration, the HOMO and HOMO-1 are both delocalized over the whole TADHA
molecule not only for the molecular junction without H2O molecule, but also for the molecular junction with Type I-1, 2 and 3 configurations, which results in the high transmission peaks at
about −0.2 eV for these molecular junctions at 0.0 V. However, there are still some differences for the effects of the H2O molecule on the orbital distributions at 0.0 V, such as for Type
I-2 and 3 molecular systems. Attributing to the depressing of the H2O molecule, the gaps between the HOMO and HOMO-1 are slightly enlarged compared with the molecular system without the H2O
molecule, and the spatial distributions of the orbitals are obviously asymmetric to the two branches of TADHA molecule. However, for Type I-1 molecular system, the orbitals are little
influenced by the H2O molecule since the adsorption of H2O molecule is very weak. For the molecular junctions at peak-current voltage or at ±0.5 V, the HOMOs and the HOMOs-1 have been pulled
apart by bias and each only locates on one branch of the TADHA molecule. Thus one can easily understand why the height of the transmission peaks decrease very quickly in the split process
with the increase of the bias voltage. We should mention that the orbitals presented in the figure are the molecular projected self-consistent Hamiltonian (MPSH) eigenstates, which are often
used to discuss the electronic transport properties of molecular junctions in literatures7, 23. The MPSH is the self-consistent Hamiltonian of the functional molecule including the H2O
adsorbates with the influence of the electrode, which contains the electrode-molecule coupling effects but does not contain the Hamiltonian of the gold electrode, so the energies of the MPSH
eigenstates are not perfectly consistent with the positions of the transmission peaks7. MORE H2O MOLECULES OR H2O AGGREGATE ADSORPTION In order to understand the effect of H2O molecules on
the electronic transports more explicitly, we further set two or more H2O molecules adsorbed on the TADHA molecule, which are shown in Fig. 1, where Type II-1, 2 and 3 are the molecular
junctions with two H2O molecules being adsorbed on TADHA molecule, Type III is the molecular junction with three H2O adsorbates. After geometric optimizations, we calculated the electronic
transport properties for the molecular systems with Type II and Type III configurations. Figure 5 shows the current and the differential conductance of TADHA molecular junctions with two or
three H2O adsorbates as functions of applied bias. From the figure one can see that, TADHA molecular junctions still show low-bias NDC behaviors with the influence of two or three H2O
molecules adsorbed on the backbone of TADHA molecule. For the Type II-1 and Type II-2 molecular junctions, the electronic transport properties show similar characteristics to Type I
molecular systems. In detail, due to the influences of the H2O molecules adsorbed near the electrodes being suppressed by bias voltage, the electronic transport properties of Type II-1 (see
Figs 5 and 6(a)) are very similar to those of Type I-1 molecular system and the molecular system without H2O adsorbate. For the Type II-2 molecular system, since the H2O molecule adsorbed on
the ethynylene site can depress the energy of the frontier molecular orbital which close to the H2O molecule and consequently induces red-shift of the transmission peaks, the peak value of
the current is shifted to higher bias voltage compared to the ones without H2O adsorbate (see Figs 5 and 6(b)). Since the influences of the two H2O molecules are nearly symmetric to the
TADHA molecular junctions, the current curves and the conductance curves are both approximately symmetric to the positive and negative bias for the junctions with Type II-1 and Type II-2
configuration. Attributing to the approximately symmetric effects of the two H2O molecules, at 0.0 V, the spatial distributions of HOMO and HOMO-1 for Type II-1 and Type II-2 molecular
systems also present approximately symmetric character to the two branches of the TADHA molecule (Figure S1). Our calculations show that, if we first set two H2O molecules on the two
conjugated rings of the 9,10-dihydroanthracene core respectively and perform geometric optimization, the two H2O molecules will move and aggregate with each other, until at last formed Type
II-3 configuration, which obviously due to the electrostatic attraction originated from the strong polarity of H2O molecule. From Fig. 5 one can see that, the current and the differential
conductance curves of Type II-3 system are more asymmetric than those of Type I-3. The peak current value is further enhanced and the peak NDC value is more than doubled of that without H2O
adsorbate for the junction in the positive bias regime, whereas for the negative bias, which is obviously depressed by the influence of the H2O adsorbates. Thus, for TADHA molecular junction
with Type II-3 configuration, the adsorption of H2O molecules induces apparent rectifier behavior with the maximum rectification ratio of 2.74 at 0.35 V. Figure 6(c) shows that, for Type
II-3 molecular system, due to the depressing of the two H2O molecules to the molecular orbitals, the HOMO and HOMO-1 are not degenerated at zero bias, thus the transmission spectrum shows
two peaks at about −0.15 eV and −0.24 eV corresponding to the HOMO and HOMO-1. With the increase of the positive bias, the HOMO is depressed and simultaneously the HOMO-1 is enhanced. When
the bias is increased to about 0.15 V, the HOMO and HOMO-1 are re-degenerated, which results in the combination of the two transmission peaks into one higher transmission peak at 0.19 eV.
Hence, the TADHA molecular junction with Type II-3 configuration is more conductive in the positive bias regime. Different from positive bias, the negative bias further splits and depresses
the two transmission peaks which makes the molecular junction less conductive when the bias less than −0.20 V, so the TADHA molecular junction shows rectifier behavior. In fact, from the
asymmetric evolution of the HOMO and HOMO-1, especially, the degeneration point of the HOMO and HOMO-1 obviously deviates from 0.0 V (Figure S2), one can also understand the rectifier
behavior of Type II-3 molecular system, because the degeneration of the HOMO and HOMO-1 results in the delocalization of the HOMO and HOMO-1 (Figure S1), and consequently enhances the
current of positive bias regime. Similar to one-H2O-molecule adsorption on S atom site, for the Type II-4 molecular system that each terminal S atom adsorbs one H2O molecule, the NDC
behavior of TADHA molecular junction has been destroyed absolutely in the lower bias regime. Due to the strong influence of the adsorbates, the conducting orbitals have been further
depressed to lower than −0.5 eV (Fig. 6(d) and Figure S1), which results in much poorer conductance of Type II-4 molecular system compared with the molecular system without H2O adsorbate or
with one H2O molecule adsorbed on the terminal S atom. The numerical results show that, for the bias lower than 0.25 V, the current of Type II-4 molecular system is about two orders lower
than that of the system without the H2O adsorbate in the magnitudes, which is in good agreement with Long’s experiment28. Although with the increase of the positive bias, the transmission
peak corresponding to the HOMO is shifted to higher energy regime, it is still out of the bias window when the bias is enhanced to 0.5 V (Figure S2). So the current is very weak for the Type
II-4 molecular junction. DISCUSSION It is interesting that, for the H2O molecules being adsorbed on the backbone of TADHA molecule, the aggregate of H2O molecules seems to have stronger
influence on the electronic transport properties of TADHA molecular junction than single or separated H2O molecules. Especially, with the influence of the aggregate of H2O molecules adsorbed
on one branch of TADHA molecule, the values of the current and NDC peaks are both enhanced dramatically. Taking the aggregate of three H2O molecules as an example, after geometric
optimization we obtained the configuration of Type III molecular junction (Fig. 1). From Fig. 5 one can see that the peak-current value is up to about 21 nA, which is more than four times
than that for the system without H2O adsorbate. Similar to Type II-3 molecular system, the depression of the H2O aggregate destroys the degeneration of the HOMO and HOMO-1 at zero bias, and
consequently splits the high transmission peak at about 0.2 eV (Fig. 6(e)). Since the transmission spectra near Fermi energy is changed very slightly, the current is slightly changed in the
lower bias regime. However, when the HOMO and HOMO-1 are re-degenerated at the positive bias of about 0.2 V, the two transmission peaks which are related to the HOMO and HOMO-1 are combined
into one high peak. Further enhancing the positive bias after the two orbitals re-degeneration, the combined transmission peak is re-split, and compared with former cases, the transmission
peak corresponding to HOMO enters bias window with a higher height. For example, at 0.25 V and 0.4 V, the height of the transmission peak related to the HOMO is about 0.05 and 0.01,
respectively. However, except for the Type II-3 with an aggregate of two H2O molecules, the heights of the transmission peaks corresponding to the HOMO are all no more than 0.004 for the
other cases at 0.25 V, not to mention at 0.4 V. Thus, when the currents of other cases reach the peak values at about 0.25–0.30 V, the current of Type III system still increases rapidly
until reaches a much higher current peak at 0.4 V. Then due to the rapid shrink of the transmission peak which is induced by the further split of the HOMO and the HOMO-1, the current
decreases quickly and results in larger NDC behavior. As to the accuracy of our work, we found that our calculations successfully reproduced the low-bias NDC behavior of TADHA molecular
junction that was investigated experimental by Perrin _et al_.60. However, the absolute values of the current in the lower bias regime are about one order of magnitude larger than the
experimental results. According to the studies of Quek _et al_.61,62,63, the HOMO-LUMO (LUMO: lowest unoccupied molecular orbital) gap is usually underestimated in standard density
functional theory (DFT), which results in overestimate of conductance of single molecular junction. Thus an alternative DFT-based approach61,62,63,64,65 (e.g. DFT + Σ approach) is needed to
give more accurate and physically meaningful understandings of electron-transport properties of TADHA molecular junction in the future. Since the HOMO is much closer to the Fermi level than
the LUMO, the electron-transport properties are mainly governed by the HOMO. According to the DFT + Σ method in the literatures61,62,63 and the experimental results60, we can roughly
estimate that, for the TADHA molecular junction without H2O adsorbate, the HOMO is overestimated by not more than 0.3 eV relative to the Fermi level, which is much smaller than those of weak
coupling systems61,62,63. In summary, the NDC behavior of TADHA molecular junctions and the H2O adsorption effect are investigated by applying the density-functional theory and
non-equilibrium Green’s function method. In the lower bias regime, the TADHA molecular junction exhibits excellent NDC behavior, which is attributed to the splitting of the degenerated
transmission channels at applied bias voltage. The H2O molecules may depress the energy of the transmission channel, and further influence the electronic transport properties of the
molecular junction. The influences of the H2O molecules are closely related to the H2O molecule affinity. Although the single H2O molecule or the separated H2O molecules adsorbed on the
backbone of TADHA molecule can lower the energies of the TADHA molecular orbitals and shift the positions of the transmission peaks, they have relatively smaller effects on the current and
NDC behavior of the TADHA molecular junction. The aggregate of several H2O molecules adsorbed on one branch of the TADHA molecule can strongly enhance the current and the NDC behavior of the
molecular junction. The H2O molecule adsorbed on the terminal S atom strongly depresses the conductive orbitals, which further dramatically suppresses the current of TADHA molecular
junction in the lower bias regime and consequently destroys the low-bias NDC behavior of the molecular junction. METHODS The geometric structures of TADHA molecular systems without or with
H2O molecules were optimized using the SIESTA package with a maximum force 0.02 eV/Å66, 67. The Troullier–Martin type norm-conserving pseudopotentials are applied to represent the core
electrons68, the generalized gradient approximation (GGA) with Perdew–Burke–Ernzerhof (PBE) formulation is applied as the exchange-correlation functional69. For Au atoms, a single-_ζ_ plus
polarization basis set is used, and for other atoms, a double-_ζ_ plus polarization basis set is employed. The current through the molecular device with different bias voltage is obtained
according to the Landauer–Buttiker formula70 $$I=\frac{2e}{h}\int T(E,V)\,[f(E-{\mu }_{L})-f(E-{\mu }_{R})]dE,$$ (1) which was calculated with the TranSIESTA module of the SIESTA package. In
Eq. (1), _T_(_E_, _V_) is the transmission probability, which depends on the incident energy _E_ of the transmission electrons and the applied bias voltage _V_. \({\mu }_{L}\) and \({\mu
}_{R}\) in the Fermi–Dirac distribution functions _f_(_E_) are the electrochemical potentials of the two electrodes. The transmission probability _T_(_E_, _V_) is calculated by NEGF method.
The differential conductance is defined as \(G=\partial I/\partial V\). In the electron transport calculations, a 300 Ry mesh cutoff for the real space grid was chosen. The convergence
criterion for density matrix was set to 1.0 × 10−4. A 4 × 4 _k_-point grid was used for the Brillouin-zone (BZ) sampling in the transverse directions. A 300 K smearing was applied for the
electronic Fermi-Dirac distribution. REFERENCES * Xiang, D., Wang, X. L., Jia, C. C., Lee, T. & Guo, X. F. Molecular-scale electronics: from concept to function. _Chem. Rev._ 116,
4318–4440 (2016). Article CAS PubMed Google Scholar * Wang, C. K. & Luo, Y. Current–voltage characteristics of single molecular junction: dimensionality of metal contacts. _J. Chem.
Phys._ 119, 4923–4928 (2003). Article ADS CAS Google Scholar * Jiang, J., Kula, M. & Luo, Y. A Generalized quantum chemical approach for elastic and inelastic electron transports in
molecular electronics devices. _J. Chem. Phys._ 124, 034708 (2006). Article ADS PubMed Google Scholar * Zhang, X. J., Chen, K. Q., Tang, L. M. & Long, M. Q. Electronic transport
properties on V-shaped-notched zigzag graphene nanoribbons junctions. _Phys. Lett. A_ 375, 3319–3324 (2011). Article ADS CAS Google Scholar * Liu, R., Wang, C. K. & Li, Z. L. A
method to study electronic transport properties of molecular junction: one-dimension transmission combined with three-dimension correction approximation (OTCTCA). _Sci. Rep._ 6, 21946
(2016). Article ADS CAS PubMed PubMed Central Google Scholar * Dou, K. P., Fu, X. X., De Sarkar, A. & Zhang, R. Q. Dual response of graphene-based ultra-small molecular junctions
to defect engineering. _Nano Res._ 9, 1480–1488 (2016). Article CAS Google Scholar * Zhang, Z. H. _et al_. A Dramatic Odd-Even Oscillating Behavior for the Current Rectification and
Negative Differential Resistance in Carbon-Chain-Modified Donor-Acceptor Molecular Devices. _Adv. Funct. Matr._ 23, 2765–2774 (2013). Article CAS Google Scholar * Hu, G. C., Zhang, Z.,
Li, Y., Ren, J. F. & Wang, C. K. Length dependence of rectification in organic co-oligomer spin rectifiers. _Chin. Phys. B_ 25, 057308 (2016). Article ADS Google Scholar * Zhang, X.
J., Chen, K. Q., Long, M. Q., He, J. & Gao, Y. L. Effect of length and negative differential resistance behavior in conjugated molecular wire tetrathiafulvalene devices. _Mod. Phys.
Lett. B_ 29, 1550106 (2015). Article ADS CAS Google Scholar * Li, Z. L. Theoretical study on electronic transport properties of oligothiophene molecular devices. _Chin. J. Chem. Phys._
24, 194–198 (2011). Article Google Scholar * Cui, X. D. _et al_. Reproducible measurement of single-molecule conductivity. _Science_ 294, 571–574 (2001). Article ADS CAS PubMed Google
Scholar * Sun, M., Zhang, Z., Kim, Z. H., Zheng, H. & Xu, H. Plasmonic scissors for molecular design. _Chem. Eur. J._ 19, 14958–14962 (2013). Article CAS PubMed Google Scholar *
Xiang, D., Jeong, H., Lee, T. & Mayer, D. Mechanically controllable break junctions for molecular electronics. _Adv. Mater._ 25, 4845–4867 (2013). Article CAS PubMed Google Scholar *
Wang, Q. _et al_. Single-atom switches and single-atom gaps using stretched metal nanowires. _ACS Nano_ 10, 9695–9702 (2016). Article CAS Google Scholar * Taniguchi, M., Tsutsui, M.,
Shoji, K., Fujiwara, H. & Kawai, T. Single-molecule junctions with strong molecule-electrode coupling. _J. Am. Chem. Soc._ 131, 14146–14147 (2009). Article CAS PubMed Google Scholar
* Taniguchi, M. _et al_. Dependence of single-molecule conductance on molecule junction symmetry. _J. Am. Chem. Soc._ 133, 11426–11429 (2011). Article CAS PubMed Google Scholar * Xiang,
D., Zhang, Y., Pyatkov, F., Offenhäusser, A. & Mayer, D. Gap size dependent transition from direct tunneling to field emission in single molecule junctions. _Chem. Commun._ 47, 4760–4762
(2011). Article CAS Google Scholar * Xu, B. Q. & Tao, N. J. Measurement of single-molecule resistance by repeated formation of molecular junctions. _Science_ 301, 1221–1223 (2003).
Article ADS CAS PubMed Google Scholar * Liu, R. _et al_. Study on force sencitivity of electronic transport properties of 1,4-butanedithiol molecular device. _Acta Phys. Sin._ 63,
068501 (2014). Google Scholar * Li, Y., Feng, Y. & Sun, M. Photoinduced charge transport in a BHJ solar cell controlled by an external electric field. _Sci. Rep._ 5, 13970 (2015).
Article ADS PubMed PubMed Central Google Scholar * Zhang, Y. F., Yi, X. H., Zhang, Z., Sun, J. X. & Li, Z. L. Theoretical studies on electronic transport properties of
2,5-dimercapto-pyridazin molecular junctions: influence of CO and H2O molecules. _J. At. Mol. Sci._ 6, 263–271 (2015). Google Scholar * Xiang, D., Lee, T., Kim, Y., Mei, T. T. & Wang,
Q. L. Origin of discrete current fluctuations in a single molecule junction. _Nanoscale_ 6, 13396–13401 (2014). Article ADS CAS PubMed Google Scholar * Song, Y., Xie, Z., Ma, Y., Li, Z.
L. & Wang, C. K. Giant Rectification Ratios of Azulene-like Dipole Molecular Junctions Induced by Chemical Doping in Armchair-Edged Graphene Nanoribbon Electrodes. _J. Phys. Chem. C_
118, 18713–18720 (2014). Article CAS Google Scholar * Zhao, W. K. _et al_. Rectification inversion in oxygen substituted graphyne–graphene-based heterojunctions. _Phys. Chem. Chem. Phys._
17, 3115–3122 (2015). Article CAS PubMed Google Scholar * Zou, D., Zhao, W., Fang, C., Cui, B. & Liu, D. The electronic transport properties of zigzag silicene nanoribbon slices
with edge hydrogenation and oxidation. _Phys. Chem. Chem. Phys._ 18, 11513–11519 (2016). Article CAS PubMed Google Scholar * Li, Z. L., Li, H. Z., Ma, Y., Zhang, G. P. & Wang, C. K.
Hydration effect on the electronic transport properties of oligomeric phenylene ethynylene molecular junctions. _Chin. Phys. B_ 19, 067305 (2010). Article ADS Google Scholar * Lin, X. N.,
Zhang, G. P., Ren, J. F., Yuan, X. B. & Hu, G. C. Electronic transport properties of oligophenyleneethynylene molecular junctions in alkaline and acid solutions. _Acta Phys. Sin._ 63,
068502 (2014). Google Scholar * Long, D. P. _et al_. Effects of hydration on molecular junction transport. _Nature Mater._ 5, 901–908 (2006). Article ADS CAS Google Scholar * Yi, X. H.
_et al_. Low-Bias negative differential conductance controlled by electrode separation. _Chin. Phys. B_ 25, 128503 (2016). Article ADS Google Scholar * Zhang, G. P., Hu, G. C., Song, Y.,
Xie, Z. & Wang, C. K. Stretch or contraction induced inversion of rectification in diblock molecular junctions. _J. Chem. Phys._ 139, 094702 (2013). Article ADS PubMed Google Scholar
* Zhao, W. K., Ji, G. M. & Liu, D. S. Contact position and width effect of graphene electrode on the electronic transport properties of Dehydrobenzoannulenne molecule. _Phys. Lett. A_
378, 446–452 (2014). Article ADS CAS Google Scholar * Li, Z. L., Zou, B., Wang, C. K. & Luo, Y. Electronic transport properties of molecular bipyridine junctions: effects of isomer
and contact structures. _Phys. Rev. B_ 73, 075326 (2006). Article ADS Google Scholar * Li, Y. _et al_. Spin Polarization at Organic-Ferromagnetic Interface: Effect of Contact
Configuration. _Chin. J. Chem. Phys._ 29, 344–348 (2016). Article CAS Google Scholar * Jiang, Z. L., Wang, H., Shen, Z. Y., Sanvito, S. & Hou, S. M. Effects of the molecule-electrode
interface on the low-bias conductance of Cu-H2-Cu single-molecule junctions. _J. Chem. Phys._ 145, 044701 (2016). Article ADS PubMed Google Scholar * Hu, G. C. _et al_. Effect of
interfacial coupling on the rectification in organic spin rectifiers. _Chin. Phys. B_ 24, 077308 (2015). Article ADS Google Scholar * Li, M. J., Xu, H., Chen, K. Q. & Long, M. Q.
Electronic transport properties in benzene-based heterostructure: Effects of anchoring groups. _Phys. Lett. A_ 376, 1692–1697 (2012). Article ADS CAS Google Scholar * Hong, W. _et al_.
Single molecular conductance of tolanes: experimental and theoretical study on the junction evolution dependent on the anchoring group. _J. Am. Chem. Soc._ 134, 2292–2304 (2012). Article
CAS PubMed Google Scholar * Hong, W. _et al_. Trimethylsilyl-terminated oligo(phenylene ethynylene)s: an approach to single-molecule junctions with covalent Au−C σ-Bonds. _J. Am. Chem.
Soc._ 134, 19425–19431 (2012). Article CAS PubMed Google Scholar * Bao, D. L. _et al_. Theoretical study on mechanical and electron-transport properties of conjugated molecular junctions
with carboxylic or methyl sulfide links. _Phys. Lett. A_ 378, 1290–1295 (2014). Article ADS CAS Google Scholar * Li, X. L. _et al_. Conductance of single alkanedithiols: Conduction
mechanism and effect of molecule-electrode contacts. _J. Am. Chem. Soc._ 128, 2135–2141 (2006). Article CAS PubMed Google Scholar * Chen, F., Li, X., Hihath, J., Huang, Z. & Tao, N.
Effect of anchoring groups on single-molecule conductance: comparative study of thiol-, amine-, and carboxylic-acid-terminated molecules. _J. Am. Chem. Soc._ 128, 15874–15881 (2006). Article
CAS PubMed Google Scholar * Park, Y. S. _et al_. Contact chemistry and single-molecule conductance: A comparison of phosphines, methyl sulfides, and amines. _J. Am. Chem. Soc._ 129,
15768–15769 (2007). Article CAS PubMed Google Scholar * Yokota, K., Taniguchi, M., Tsutsui, M. & Kawai, T. Molecule-electrode bonding design for high single-molecule conductance. _J.
Am. Chem. Soc._ 132, 17364–17365 (2010). Article CAS PubMed Google Scholar * Fu, X. X., Zhang, L. X., Li, Z. L. & Wang, C. K. Switching properties of bi-OPE-monothiol molecular
junctions: Substituent effects and improvement of open-close ratio. _Chin. Phys. B_ 22, 028504 (2013). Article ADS Google Scholar * Xiang, D. _et al_. Molecular Junctions Bridged by Metal
Ion Complexes. _Chem. Eur. J._ 17, 13166–13169 (2011). Article CAS PubMed Google Scholar * Fu, X. X., Zhang, R. Q., Zhang, G. P. & Li, Z. L. Rectifying properties of oligo(phenylene
ethynylene) heterometallic molecular junctions: molecular length and side group effects. _Sci. Ref._ 4, 06357 (2014). CAS Google Scholar * Di Ventra, M., Kim, S. G., Pantelides, S. T.
& Lang, N. D. Temperature effects on the transport properties of molecules. _Phys. Rev. Lett._ 86, 288–291 (2001). Article ADS PubMed Google Scholar * Zou, D. Q., Song, Y., Xie, Z.,
Li, Z. L. & Wang, C. K. Large rectification ratio induced by nitrogen (boron) doping in graphene nanoribbon electrodes for OPE junctions. _Phys. Lett. A_ 379, 1842–1846 (2015). Article
ADS CAS Google Scholar * Yang, Z., Lang, N. D. & Di Ventra, M. Effects of geometry and doping on the operation of molecular transistors. _Appl. Phys. Lett._ 82, 1938–1940 (2003).
Article ADS CAS Google Scholar * Xu, B. Q., Xiao, X. Y., Yang, X. M., Zang, L. & Tao, N. J. Large gate modulation in the current of a room temperature single molecule transistor. _J.
Am. Chem. Soc._ 127, 2386–2387 (2005). Article CAS PubMed Google Scholar * Li, Z. L., Fu, X. X., Zhang, G. P. & Wang, C. K. Effect of gate electric field on single organic molecular
devices. _Chin. J. Chem. Phys._ 26, 185–190 (2013). Article CAS Google Scholar * Su, W., Jiang, J., Lu, W. & Luo, Y. First-Principles Study of Electrochemical Gate-Controlled
Conductance in Molecular Junctions. _Nano Lett._ 6, 2091–2094 (2006). Article ADS CAS PubMed Google Scholar * Xu, B. Q., Li, X. L., Xiao, X. Y., Sakaguchi, H. & Tao, N. J.
Electromechanical and conductance switching properties of single oligothiophene molecules. _Nano Lett._ 5, 1491–1495 (2005). Article ADS CAS PubMed Google Scholar * Li, Z. L., Zhang, G.
P. & Wang, C. K. First-principles study on formation and electron transport properties of single oligothiophene molecular junctions. _J. Phys. Chem. C_ 115, 15586–15591 (2011). Article
CAS Google Scholar * Xiang, D. _et al_. Three-terminal single-molecule junctions formed by mechanically controllable break junctions with side gating. _Nano Lett._ 13, 2809–2813 (2013).
Article ADS CAS PubMed Google Scholar * Li, X. L. _et al_. Thermally activated electron transport in single redox molecules. _J. Am. Chem. Soc._ 129, 11535–11542 (2007). Article CAS
PubMed Google Scholar * Na, J. S., Ayres, J., Chandra, K. L., Gorman, C. B. & Parsons, G. N. Real-time conductivity analysis through single-molecule electrical junctions.
_Nanotechnology_ 18, 424001 (2007). Article PubMed Google Scholar * Cao, H., Jiang, J., Ma, J. & Luo, Y. Temperature-dependent statistical behavior of single molecular conductance in
aqueous solution. _J. Am. Chem. Soc._ 130, 6674–6675 (2008). Article CAS PubMed Google Scholar * Cao, H., Ma, J. & Luo, Y. Field effects on the statistical behavior of the molecular
conductance in a single molecular junction in aqueous solution. _Nano Res._ 3, 350–355 (2010). Article ADS CAS Google Scholar * Perrin, M. L. _et al_. Large negative differential
conductance in single-molecule break junctions. _Nature Nanotech._ 9, 830–834 (2014). Article ADS CAS Google Scholar * Quek, S. Y., Choi, H. J., Louie, S. G. & Neaton, J. B. Length
dependence of conductance in aromatic single-molecule junctions. _Nano Lett._ 9, 3949–3953 (2009). Article ADS CAS PubMed Google Scholar * Quek, S. Y. _et al_. Amine-gold linked
single-molecule circuits: experiment and theory. _Nano Lett._ 7, 3477–3482 (2007). Article ADS CAS PubMed Google Scholar * Quek, S. Y. & Khoo, K. H. Predictive DFT-based approaches
to charge and spin transport in single-molecule junctions and two-dimensional materials: successes and challenges. _Acc. Chem. Res._ 47, 3250–3257 (2014). Article CAS PubMed Google
Scholar * Koentopp, M., Burke, K. & Evers, F. Zero-bias molecular electronics: Exchange-correlation corrections to Landauer’s formula. _Phys. ReV. B_ 73, 121403 (2006). Article ADS
Google Scholar * Darancet, P., Ferretti, A., Mayou, D. & Olevano, V. _Ab initio_ GW electron-electron interaction effects in quantum transport. _Phys. ReV. B_ 75, 075102 (2007). Article
ADS Google Scholar * Brandbyge, M., Mozos, J. L., Ordejόn, P., Taylor, J. & Stokbro, K. Density-functional method for nonequilibrium electron transport. _Phys. Rev. B_ 65, 165401
(2002). Article ADS Google Scholar * Soler, J. M. _et al_. The SIESTA method for ab initio order-N materials simulation. _J. Phys.: Condens. Matter_ 14, 2745 (2002). ADS CAS Google
Scholar * Troullier, N. & Martins, J. L. A straightforward method for generating soft transferable pseudopotentials. _Solid State Commun._ 74, 613–616 (1990). Article ADS Google
Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. _Phys. Rev. Lett._ 77, 3865 (1996). Article ADS CAS PubMed Google Scholar *
Buttiker, M., Imry, Y., Landauer, R. & Pinhas, S. Generalized many-channel conductance formula with application to small rings. _Phys. Rev. B_ 31, 6207 (1985). Article ADS CAS Google
Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (Grant Nos. 11374195 and 11304185) and the Natural Science
Foundation of Shandong province, China (Grant No. ZR2013FM006). Thanks to the supporting of Taishan scholar project of Shandong Province. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School
of Physics and Electronics, Shandong Normal University, Jinan, 250014, China Zong-Liang Li, Xiao-Hua Yi, Ran Liu, Jun-Jie Bi, Huan-Yan Fu, Guang-Ping Zhang, Yu-Zhi Song & Chuan-Kui Wang
Authors * Zong-Liang Li View author publications You can also search for this author inPubMed Google Scholar * Xiao-Hua Yi View author publications You can also search for this author
inPubMed Google Scholar * Ran Liu View author publications You can also search for this author inPubMed Google Scholar * Jun-Jie Bi View author publications You can also search for this
author inPubMed Google Scholar * Huan-Yan Fu View author publications You can also search for this author inPubMed Google Scholar * Guang-Ping Zhang View author publications You can also
search for this author inPubMed Google Scholar * Yu-Zhi Song View author publications You can also search for this author inPubMed Google Scholar * Chuan-Kui Wang View author publications
You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors provided essential contributions to the manuscript and the project. All authors have given approval to
the final version of the manuscript. Z.L.L., X.H.Y. and R.L. contributed the ideas and discussed the manuscript in detail. X.H.Y., R.L., J.J.B. and H.Y.F. performed the calculations and the
data analysis. G.P.Z., Y.Z.S. and C.K.W. discussed some details with the corresponding author. CORRESPONDING AUTHOR Correspondence to Zong-Liang Li. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Li, ZL., Yi, XH., Liu, R. _et al._ Effect of H2O Adsorption on Negative
Differential Conductance Behavior of Single Junction. _Sci Rep_ 7, 4195 (2017). https://doi.org/10.1038/s41598-017-04465-3 Download citation * Received: 28 December 2016 * Accepted: 16 May
2017 * Published: 23 June 2017 * DOI: https://doi.org/10.1038/s41598-017-04465-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative