Play all audios:
ABSTRACT The purpose of this study was to investigate a preclinical spectral photon-counting CT (SPCCT) prototype compared to conventional CT for pulmonary imaging. A custom-made lung
phantom, including nodules of different sizes and shapes, was scanned with a preclinical SPCCT and a conventional CT in standard and high-resolution (HR-CT) mode. Volume estimation was
evaluated by linear regression. Shape similarity was evaluated with the Dice similarity coefficient. Spatial resolution was investigated via MTF for each imaging system. _In-vivo_ rabbit
lung images from the SPCCT system were subjectively reviewed. Evaluating the volume estimation, linear regression showed best results for the SPCCT compared to CT and HR-CT with a root mean
squared error of 21.3 mm3, 28.5 mm3 and 26.4 mm3 for SPCCT, CT and HR-CT, respectively. The Dice similarity coefficient was superior for SPCCT throughout nodule shapes and all nodule sizes
(mean, SPCCT: 0.90; CT: 0.85; HR-CT: 0.85). 10% MTF improved from 10.1 LP/cm for HR-CT to 21.7 LP/cm for SPCCT. Visual investigation of small pulmonary structures was superior for SPCCT in
the animal study. In conclusion, the SPCCT prototype has the potential to improve the assessment of lung structures due to higher resolution compared to conventional CT. SIMILAR CONTENT
BEING VIEWED BY OTHERS EVALUATION OF RADIOMICS FEATURE STABILITY IN ABDOMINAL MONOENERGETIC PHOTON COUNTING CT RECONSTRUCTIONS Article Open access 15 November 2022 PHANTOM AND CLINICAL
ASSESSMENT OF SMALL PULMONARY NODULES USING Q.CLEAR RECONSTRUCTION ON A SILICON-PHOTOMULTIPLIER-BASED TIME-OF-FLIGHT PET/CT SYSTEM Article Open access 14 May 2021 MICRO-CT ACQUISITION AND
IMAGE PROCESSING TO TRACK AND CHARACTERIZE PULMONARY NODULES IN MICE Article 09 December 2022 INTRODUCTION Over the last decades, high-resolution computed tomography (HR-CT) has demonstrated
to be a valuable tool for detection of lung diseases and exploration of the lung1,2,3,4,5. While air is carried to the lungs, it passes several structures, including trachea, bronchi, and
bronchioles, which have features and structures within – or currently below – the spatial resolution of HR-CT systems. When it comes to pathological changes in the lung, HR-CT has a
significant role in the diagnostic evaluation and therapy design6. One example is the detection and classification of lung nodules. Lung cancer is one of the most common diseases worldwide7.
Siegel _et al_. estimate that in 2018 25% of all cancer deaths in the United States of America will be caused by lung cancer8. For classification of lung nodules, apart from growth rate,
the shape and surface of the nodule is a clinically accepted marker to distinguish between benign and cancerous nodules. In comparison, malignant nodules are more likely to present
themselves with irregular shapes, rougher surfaces, and speckled patterns9. A superior spatial resolution could not only improve the classification of small pulmonary nodules (≥4 mm) during
the clinical routine10 but also improve the performance of software-based classification systems11. A different example is the early diagnosis of chronic obstructive pulmonary disease
(COPD), which is gaining in importance worldwide12. In COPD airflow obstruction and airway inflammation frequently lead to a destruction of alveolar architecture with enlargement of distal
airspaces. For early detection, HR-CT allows the clinician to assess wall thickness, which is currently only possible for larger airways13,14. Next generation HR-CT systems would allow a
more robust evaluation of the larger and small airways. Thus, an earlier detection of COPD could become feasible. Present clinical computed tomography (CT) systems are equipped with
energy-integrating detectors with detector pixel dimensions in the range of approximately 1.0 mm. Recently, an ultra-high resolution CT – based on present detector technology – with pixel
dimensions of 0.25 mm has been introduced with a focus on pulmonary and cardiovascular applications15,16,17. A different detection concept, which is currently investigated for its diagnostic
range, are photon-counting detectors (PCD)18,19. The essential advantage of a spectral photon-counting CT (SPCCT) system is that incoming x-ray photons are directly converted in electronic
signals and spectrally binned by analyzing the pulse heights generated in a semiconductor detection layer20. Recent developments showed promising results in the areas of
abdominal21,22,23,24,25, cardiovascular25,26,27,28,29,30, neurological31,32,33, and nanoparticle imaging34. In addition to those possibilities, SPCCT will offer an improved spatial
resolution due to smaller detector pixel sizes compared to the current clinical standard. The influence of electronic noise is significantly reduced in the direct-converting PCDs and can be
considered as eliminated for the energy levels of incoming x-ray photons35. Hence, the reduced pixel dimension in PCDs comes along with a lower radiation exposure compared to (a similar
reduction of detector pixel size with) energy-integrating detectors. In this study, we investigate the resolution capabilities of a preclinical SPCCT prototype compared to a conventional CT
by evaluating size and shape of lung nodules in a phantom model, measuring the modulation transfer function (MTF) and demonstrating lung structure visualization in an _in-vivo_ acquisition
of a rabbit. MATERIALS AND METHODS CT ACQUISITION Images were acquired with a commercial 3rd generation 256-row clinical CT scanner (iCT, Philips Healthcare, Best, The Netherlands) and a
preclinical SPCCT prototype scanner. The clinical CT scans were matched to a CTDIvol of 7 mGy. The CT was operated with 120 kVp, 107 mAs, and two different focal spot sizes: a small focal
spot resulting in high-resolution CT (HR-CT) and a standard focal spot (CT). The SPCCT was operated with a step and shoot acquisition protocol with 120 kVp, 100 mAs and 1 s gantry rotation
time. The x-ray exposures of the CT and the SPCCT were chosen to equalize the Air KERMA. Acquisition parameters are listed in Table 1. Images were reconstructed with standard filtered
backprojection (FBP). SPECTRAL PHOTON COUNTING CT The preclinical SPCCT scanner (Philips Healthcare, Haifa, Israel) is based on a clinical CT system (Brilliance iCT, Philips Healthcare,
Haifa, Israel) providing a conventional x-ray tube and standard beam filtration but with a limited in-plane field of view of 168 mm and a z-coverage of 2.5 mm at isocenter. The scanner is
equipped with hybrid multi-bin photon counting detectors, based on ChromAIX2 ASICs (application specific integrated circuit)36 combined with cadmium zinc telluride (CZT) as sensor material.
The physical pitch of the detector pixels is 500 µm × 500 µm. The projected focal spot size is 600 µm in-plane and 700 µm in the z-direction. LUNG PHANTOM Patient data acquired with a
conventional clinical CT system were used to build a digital model of a healthy lung. A threshold was applied to binarize the CT images and to differentiate the complex lung structure from
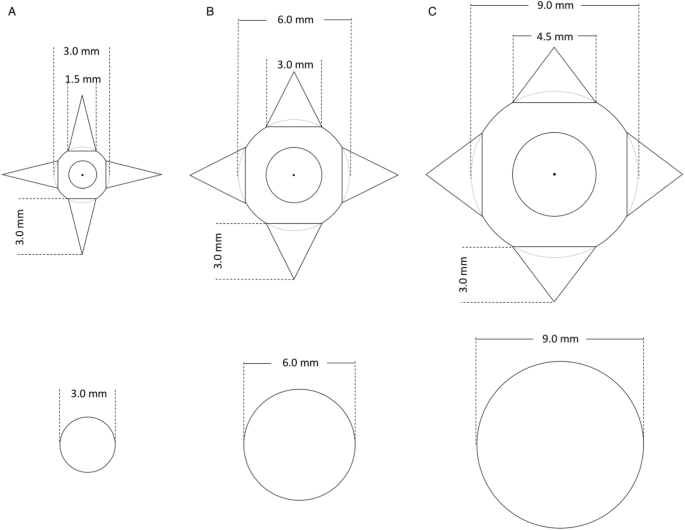
the background. Lung nodules with two different geometries were simulated and inserted in the digital model–spheres to mimic benign nodules and spheres with spikes to mimic malignant nodules
(Fig. 1), similar to the FDA lung-phantom inserts37. The three different sphere sizes had a diameter of 3, 6 and 9 mm. A board-certified radiologist assisted in the design process of the
nodules and determined the location in the model for a realistic representation. The customized lung phantom was fabricated using an additive manufacturing technique of selective laser
sintering based on polyamide. Measured Hounsfield Units (HU) of the vessels and surrounding walls of the lung phantom ([−_130_,−_90_] HU) were similar to values measured in clinical CT
images ([_−130_, +_50_] HU). Due to the manufacturing process, the lung phantom was filled with powdered polyamide resulting in elevated HUs (about −580 HU). The background in the lung of
the patient data was about −875 HU. NODULE SEGMENTATION Lung nodules were segmented from the reconstructed image data (CT, HR-CT, SPCCT) for the evaluation of their volume and shape. The
segmentation was performed with an in-house developed tool based on a numerical computing environment (MATLAB version R2017b, MathWorks, Massachusetts, USA). In each reconstruction, the
estimated center of mass of each nodule was selected. A spherical volume of interest (VOI) around the selected center of mass was extracted from the images, with diameters of _d_ + _1_._5_
_mm_ for spheres and _d_ + _6_._5_ _mm_ for spheres with spikes (with _d_ being the nodule size). The MATLAB-internal function _kmeans_, which is an implementation of the k-means clustering
algorithm, was used with two clusters to separate the nodule structure from the background. Then, any non-connected component to the center of mass was removed from the segmentation. For
each nodule, the segmentation was repeatedly performed three times to reduce the impact of the chosen center of mass on the measurements. Reported results for volume and shape quantification
are the average over the three repeated segmentations. NODULE VOLUME QUANTIFICATION The nodule volume was determined by multiplication of the voxel count in one segmentation with the
corresponding voxel size. The standard of reference was the segmentation performed on the digital lung phantom. Due to the realistic placement of the nodules inside the lung, connected parts
to the center of mass of the nodules were included within a certain VOI (see section _Nodule segmentation_). Nodule volumes were evaluated with linear regression analysis and by comparing
the different modalities to the standard of reference in a Bland-Altman plot. NODULE SHAPE QUANTIFICATION Due to different positioning of the lung phantom during scanning the images are not
registered to each other and also not to the reference image. Therefore, nodule segmentations were semi-automatically registered to the reference segmentation of the three-dimensional (3D)
printing template. In a first step, each segmentation was upscaled with cubic interpolation to isotropic voxel sizes of 0.14 × 0.14 × 0.14 mm3. In a second step, an expert in medical image
processing measured rotation angles of the segmentations with respect to the reference. The segmentations were rotated around the x-, y- and z-axes to be in the same orientation as the
reference. In a final step, two-dimensional (2D) cross-correlation of the mid-slices of the segmentation was used to shift to the same position as the reference. After registration, the Dice
similarity coefficient was computed to determine how well each modality can represent the reference nodules. The Dice similarity coefficient is given by $$dice(A,\,{B}_{m})=2\cdot
\frac{|A\,{\cap }^{}\,{B}_{m}|}{|A|+|{B}_{m}|},$$ (1) where _A_ is the reference template, _B__m_ is the segmentation for the different modalities _m_, \({\cap }^{}\) denotes the
intersection of two sets and \(|\,\cdot \,|\) is the cardinal of a set. This results in the ratio of how many voxels in \({B}_{m}\) are correctly segmented. The Kolmogorov-Smirnov test
showed a standard normal distribution for the differences between Dice coefficients of the different modalities. Thus, Dice coefficients for each of the nodules were compared between the
different modalities with a paired-sample t-test (two-tail, significance level: 0.05). SPATIAL RESOLUTION To evaluate the in-plane resolution of the CT and HR-CT images, the vendor specific
phantom (Philips iCT head system, Philips Healthcare, Haifa, Israel) with a tungsten wire diameter of 200 µm was scanned at 120 kVp in the conventional CT. For the evaluation of the in-plane
resolution of the SPCCT prototype scanner a comparable self-made phantom with a wire thickness of 100 µm was applied. The phantoms were aligned so that the wire was parallel to the rotation
axis of the system and close to the rotation center. The resolution was evaluated quantitatively utilizing the MTF. A small region-of-interest (ROI) around the wire was reconstructed using
the same reconstruction filters and processing as for the images of the lung phantom. The MTF was then determined similar to the approach by Yu _et al_.38 Several image slices were averaged
to reduce noise. The background was calculated as the mean of the image region excluding the wire and subtracted from the image. The resulting image was averaged radially around the wire to
calculate a one-dimensional profile. A Hankel transform was applied to the one-dimensional profile to obtain the MTF39. The MTF was corrected for the finite size of the wire as described by
Nickoloff40. Finally, the MTF was normalized to achieve unity at zero frequency. _IN-VIVO_ EXPERIMENT A clinical HR-CT scan, selected from the departments Picture Archiving and Communication
system (PACS), was visually compared to an _in-vivo_ SPCCT acquisition of a New Zealand white rabbit (weight: 3.7 kg). The visual appearance was assessed by one experienced radiologist
(board-certified; 4 years of experience). The study was approved by the French Department of Education and Research under the reference number _APAFIS#1732-2015091411181645 V3_. All
experiments were performed in accordance with relevant guidelines and regulations. PATIENT POPULATION Institutional review board approval was obtained prior to this study. Written informed
consent was waived by the institutional review board (Ethikkommision der medizinischen Fakultät, Technical University of Munich, Germany) as all patients were included retrospectively. All
scans were performed exclusively for clinical use with clinical standard protocols. RESULTS Figure 2 illustrates a sagittal slice of the lung phantom with a magnification of the area around
the 3 mm sphere with spikes. Edges and boundaries were more prominent in images of the SPCCT compared to CT and HR-CT. Moreover, small details such as the spikes are closer in appearance to
the reference. Figure 3 shows exemplary the segmentation for the 6 mm nodules. The spherical VOI is visualized in light transparent red and the segmented nodule is visualized in opaque red.
Visually, one can observe that the 3D renderings from SPCCT data give the closest representation of the ground truth. However, a small blood vessel at the bottom of the sphere with spikes,
indicated by a black arrow in the reference segmentation (Fig. 3A), is lost in every modality. The connection between the vessel and the peak of the bottom spike could not be identified in
any modality. Hence, the vessel is not included in the segmentations of the different modalities. Volume estimation of the nodules showed an underestimation for all modalities. The linear
regression gives the best results for SPCCT (slope: 0.952; intercept: −6.842 mm3) compared to HR-CT (slope: 0.942; intercept: −9.208 mm3) and CT (slope: 0.933; intercept: −8.622 mm3) with a
root mean squared error (RMSE) of 21.3 mm3, 26.4 mm3, and 28.5 mm3 for SPCCT, HR-CT and CT, respectively (Table 2). Figure 4A shows a plot of the linear regression. The blue line for SPCCT
is the closest to the diagonal line. Bland-Altman plots show a mean difference to the reference measurements of −17.68 mm3, −22.23 mm3 and −23.73 mm3 for SPCCT, HR-CT and CT, respectively.
Ranges of differences are given by the 95% limits of agreement \([{\rm{\Delta }}-1.96\cdot \delta ,\,{\rm{\Delta }}+1.96\cdot \delta ]\), where Δ is the mean difference and \(\delta \) is
the standard deviation of the differences to the reference measurements. The ranges of differences were [−43.30; 7.94] mm3, [−52.91; 8.44] mm3 and [−57.59; 10.14] mm3 for SPCCT, HR-CT and
CT, respectively. Dice similarity coefficients were consistently superior for SPCCT (mean: 0.90) compared to HR-CT and CT (both, mean: 0.85), Table 3. The two-tail paired t-test showed a
significant difference (P < 0.05) between the Dice coefficients of SPCCT and the values of HR-CT and CT (Table 3). The standard deviation of the Dice coefficients from three repeated
segmentations indicate no substantial difference. The MTF measurements are reported in Fig. 5. The 50% (10%) MTF cutoff was 6.7 (10.5), 6.1 (9.8) and 11.0 (21.7) LP/cm for CT, HR-CT and
SPCCT, respectively. Figure 6 shows a comparison between images acquired of an _in-vivo_ rabbit with SPCCT and a patient acquired with HR-CT. With HR-CT, bronchi and bronchioles down to a
diameter of 1.5–2 mm could be identified. An identification of smaller bronchioles (terminal, respiratory and lobular bronchioles) is not possible due to limited resolution. Some secondary
pulmonary lobes and lobular arteries (1 mm in size) can be identified. With SPCCT, very small bronchioles with a diameter of below 1 mm (corresponding to a wall thickness of 0.15 mm) can be
clearly identified (Fig. 6E, marked with arrow _a_). The branching of the dorsal bronchiole (Fig. 6E, arrow _b_) shows a typical separation of lobular bronchioles, suggesting that even
lobular bronchioles can be visualized. Vessels to a diameter of below 0.4 mm can be identified (Fig. 6E, arrow _c_). Comparing images of HR-CT and SPCCT adjusted to the same size, vessels
and walls of bronchioles are visualized more distinctively. Overall, the subjective image quality of SPCCT-images is superior to HR-CT images regarding resolution and detectability of
structures. DISCUSSION In this work, we investigated high-resolution imaging with a preclinical SPCCT prototype for pulmonary imaging in comparison to a commercially available CT system. We
showed that the higher spatial resolution of SPCCT leads to a more precise assessment of lung nodules. Moreover, the visual investigation of small pulmonary structures was superior for SPCCT
in the phantom and animal study. Pourmorteza _et al_. illustrated that photon-counting detector CT (PCD-CT, a synonym for SPCCT) has the potential to provide high-resolution images with
lower image noise compared to conventional CT35. On this note, various academic-industrial research collaborations are developing and evaluating multiple photon-counting detector concepts.
While basic concepts and the ultimate goal between the different platforms are similar, individual parameters vary from concept to concept, e.g. detector pixel-size. As this is not the focus
of this work, we would like to refer interested readers to the work of Willemink _et al_.41 In our study, we also observed superior high-resolution capabilities of SPCCT compared to
conventional CT. However, we did not compare the noise levels of the different systems, because a fair comparison of the image noise would require the same spatial resolution for both
systems. This would imply to reduce the spatial resolution of the SPCCT images, what is not intended in this study. It is known that higher spatial resolution results in more image noise
given the same radiation dose. Reduced detector pixel sizes lead to a decreased number of photons reaching each detector element. The reduced statistics at the detector generates an uptake
in noise. Moreover, in SPCCT high-energy photons and low-energy photons are weighted to contribute equally to the signal. In contrast, in conventional CT high-energy photons contribute
relatively more to the signal than low-energy photons resulting in an uptake in image noise41. When it comes to low-dose CT another effect contributes to an increased noise level. The
contribution of electronic detector noise increases in conventional CT. SPCCT, on the other hand, eliminates electronic detector noise to a certain extent by counting the photons resulting
in lower image noise at same resolution. There were several limitations of this work. We used FBP instead of advanced iterative reconstruction. Iterative reconstruction is known to deliver
improved image quality compared to traditional FBP and could probably improve the results for both scanners, the clinical CT42 and the SPCCT43. However, due to regularization and other
non-linearities, the evaluation of resolution becomes more challenging with iterative reconstruction44. With FBP, on the contrary, a more suitable comparison between conventional CT and
SPCCT is feasible because effects of the reconstruction algorithms are reduced. Another limitation is the uncertainty in the production process of the lung phantom. Synthetic lung nodules
were defined in the digital human lung model. For the 3D printing process, the digital lung model was used as input to the printer. During these processing steps, as well as during 3D
printing, small errors might be propagated to the phantom due to interpolation or manufacturing processes. This might partly contribute to the discrepancy between the measured and the
reference volumes. However, the RMSE in this work (21.3–28.5 mm3) is in the same range as reported by Zhou _et al_. in a similar study assessing lung nodules (21.6–28.3 mm3)45. The presented
results give a promising outlook to the high-resolution capabilities of the SPCCT prototype for pulmonary imaging. Higher spatial resolution, better assessment of lung nodule volume, and
improved visibility of lung vessels compared to conventional CT and HR-CT were achieved. This would not only allow an earlier detection and more precise classifications of lung nodules but
also improve the diagnostic confidence of radiologists assessing other pulmonary abnormalities, like COPD. In conclusion, the assessment of lung nodules could be improved with the presented
preclinical SPCCT prototype. Especially the investigation of small pulmonary structures is improved due to higher resolution and the subjective higher image quality. DATA AVAILABILITY The
datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. REFERENCES * Todo, G. _et al_. High resolution CT
(HR-CT) for the evaluation of pulmonary peripheral disorders. _Rinsho Hoshasen._ 27, 1319–26 (1982). CAS PubMed Google Scholar * Zwirewich, C. V., Vedal, S., Miller, R. R. & Müller,
N. L. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. _Radiology_ 179, 469–76 (1991). Article CAS Google Scholar * Furuya, K. _et al_. New
classification of small pulmonary nodules by margin characteristics on high-resolution CT. _Acta Radiol._ 40, 496–504 (1999). Article CAS Google Scholar * Uchiyama, Y. _et al_.
Quantitative computerized analysis of diffuse lung disease in high-resolution computed tomography. _Med. Phys._ 30, 2440–54 (2003). Article Google Scholar * Webb, W. R., Muller, N. L.
& Naidich, D. P. _High-Resolution CT of the Lun_g. (Wolters Kluwer Health). doi:616.2/407572 (2015) * Schaefer-Prokop, C., Prokop, M., Fleischmann, D. & Herold, C. High-resolution CT
of diffuse interstitial lung disease: key findings in common disorders. _Eur. Radiol._ 11, 373–92 (2001). Article CAS Google Scholar * Ferlay, J. _et al_. Cancer incidence and mortality
worldwide: Sources, methods and major patterns in GLOBOCAN 2012. _Int. J. Cancer_ 136, E359–E386 (2015). Article CAS Google Scholar * Siegel, R. L., Miller, K. D. & Jemal, A. Cancer
statistics, 2018. _CA. Cancer J. Clin._ 68, 7–30 (2018). Article Google Scholar * Verschakelen, J. A. & De Wever, W. _Computed Tomography of the Lun_g. (Springer Berlin Heidelberg)
https://doi.org/10.1007/978-3-642-39518-5 (2007) Book Google Scholar * Liu, Y. _et al_. Radiologic Features of Small Pulmonary Nodules and Lung Cancer Risk in the National Lung Screening
Trial: A Nested Case-Control Study. _Radiology_ 286, 298–306 (2018). Article Google Scholar * Hua, K.-L., Hsu, C.-H., Hidayati, S. C., Cheng, W.-H. & Chen, Y.-J. Computer-aided
classification of lung nodules on computed tomography images via deep learning technique. _Onco. Targets. Ther._ 8, 2015–22 (2015). CAS PubMed PubMed Central Google Scholar * Quaderi, S.
A. & Hurst, J. R. The unmet global burden of COPD. _Glob. Heal. Epidemiol. Genomics_ 3, e4 (2018). Article CAS Google Scholar * Lynch, D. A. _et al_. CT-Definable Subtypes of Chronic
Obstructive Pulmonary Disease: A Statement of the Fleischner Society. _Radiology_ 277, 192–205 (2015). Article Google Scholar * Milne, S. & King, G. G. Advanced imaging in COPD:
insights into pulmonary pathophysiology. _J. Thorac. Dis._ 6, 1570–85 (2014). PubMed PubMed Central Google Scholar * Kakinuma, R. _et al_. Ultra-High-Resolution Computed Tomography of the
Lung: Image Quality of a Prototype Scanner. _PLoS One_ 10, e0137165 (2015). Article Google Scholar * Honda, O. _et al_. Influence of gantry rotation time and scan mode on image quality in
ultra-high-resolution CT system. _Eur. J. Radiol._ 103, 71–75 (2018). Article Google Scholar * Hata, A. _et al_. Effect of Matrix Size on the Image Quality of Ultra-high-resolution CT of
the Lung: Comparison of 512×512, 1024×1024, and 2048×2048. _Acad_. _Radiol_. https://doi.org/10.1016/j.acra.2017.11.017 (2018). Article Google Scholar * McCollough, C. H., Leng, S., Yu, L.
& Fletcher, J. G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. _Radiology_ 276, 637–653 (2015). Article Google Scholar * Taguchi, K. &
Iwanczyk, J. S. Vision 20/20: Single photon counting x-ray detectors in medical imaging. _Med. Phys._ 40, 100901 (2013). Article Google Scholar * Schlomka, J. P. _et al_. Experimental
feasibility of multi-energy photon-counting K-edge imaging in pre-clinical computed tomography. _Phys. Med. Biol._ 53, 4031–4047 (2008). Article CAS Google Scholar * Pourmorteza, A. _et
al_. Abdominal Imaging with Contrast-enhanced Photon-counting CT: First Human Experience. _Radiology_ 279, 239–245 (2016). Article Google Scholar * Muenzel, D. _et al_. Spectral
Photon-counting CT: Initial Experience with Dual–Contrast Agent K-Edge Colonography. _Radiology_ 283, 723–728 (2017). Article Google Scholar * Symons, R. _et al_. Photon-counting CT for
simultaneous imaging of multiple contrast agents in the abdomen: An _in vivo_ study. _Med. Phys._ 44, 5120–5127 (2017). Article Google Scholar * Muenzel, D. _et al_. Simultaneous
dual-contrast multi-phase liver imaging using spectral photon-counting computed tomography: a proof-of-concept study. _Eur. Radiol. Exp._ 1, 25 (2017). Article Google Scholar * Cormode, D.
P. _et al_. Multicolor spectral photon-counting computed tomography: _in vivo_ dual contrast imaging with a high count rate scanner. _Sci. Rep._ 7, 4784 (2017). Article ADS Google Scholar
* Dangelmaier, J. _et al_. Experimental feasibility of spectral photon-counting computed tomography with two contrast agents for the detection of endoleaks following endovascular aortic
repair. _Eur_. _Radiol_. https://doi.org/10.1007/s00330-017-5252-7 (2018). Article Google Scholar * Mannil, M. _et al_. Photon-Counting CT: High-Resolution Imaging of Coronary Stents.
_Invest. Radiol._ 53, 143–149 (2018). Article Google Scholar * Symons, R. _et al_. Dual-contrast agent photon-counting computed tomography of the heart: initial experience. _Int. J.
Cardiovasc. Imaging_ 33, 1253–1261 (2017). Article Google Scholar * Danad, I., Fayad, Z. A., Willemink, M. J. & Min, J. K. New Applications of Cardiac Computed Tomography: Dual-Energy,
Spectral, and Molecular CT Imaging. _JACC. Cardiovasc. Imaging_ 8, 710–23 (2015). Article Google Scholar * Rajendran, K. _et al_. Measuring arterial wall perfusion using photon-counting
computed tomography (CT): improving CT number accuracy of artery wall using image deconvolution. _J. Med. imaging (Bellingham, Wash.)_ 4, 044006 (2017). Google Scholar * Pourmorteza, A. _et
al_. Photon-Counting CT of the Brain: _In Vivo_ Human Results and Image-Quality Assessment. _AJNR. Am. J. Neuroradiol._ 38, 2257–2263 (2017). Article CAS Google Scholar * Gutjahr, R. _et
al_. Human Imaging With Photon Counting-Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. _Invest. Radiol._ 51, 421–9 (2016). Article CAS
Google Scholar * Symons, R. _et al_. Photon-Counting Computed Tomography for Vascular Imaging of the Head and Neck: First _in Vivo_ Human Results. _Invest. Radiol._ 53, 135–142 (2018).
Article Google Scholar * Si-Mohamed, S. _et al_. Evaluation of spectral photon counting computed tomography K-edge imaging for determination of gold nanoparticle biodistribution _in vivo_.
_Nanoscale_ 9, 18246–18257 (2017). Article CAS Google Scholar * Pourmorteza, A., Symons, R., Henning, A., Ulzheimer, S. & Bluemke, D. A. Dose Efficiency of Quarter-Millimeter
Photon-Counting Computed Tomography: First-in-Human Results. _Invest. Radiol._ 53, 365–372 (2018). Article Google Scholar * Steadman, R., Herrmann, C. & Livne, A. ChromAIX2: A large
area, high count-rate energy-resolving photon counting ASIC for a Spectral CT Prototype. _Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip._ 862, 18–24
(2017). Article ADS CAS Google Scholar * Phantom FDA. _The Cancer Imaging Archive (TCIA)_ Available at https://wiki.cancerimagingarchive.net/display/Public/Phantom+FDA (Accessed: 18th
May 2018). * Yu, Z. _et al_. Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array. _Phys. Med. Biol._ 61, 1572–1595 (2016).
Article Google Scholar * Poularikas, A. D. _The Transform and Applications Handbook_. (CRC Press 2000). * Nickoloff, E. L. Measurement of the PSF for a CT scanner: appropriate wire
diameter and pixel size. _Phys. Med. Biol._ 33, 149–155 (1988). Article CAS Google Scholar * Willemink, M. J., Persson, M., Pourmorteza, A., Pelc, N. J. & Fleischmann, D.
Photon-counting CT: Technical Principles and Clinical Prospects. _Radiology_ 172656 https://doi.org/10.1148/radiol.2018172656 (2018). Article Google Scholar * Sauter, A. _et al_. Ultra Low
Dose CT Pulmonary Angiography with Iterative Reconstruction. _PLoS One_ 11, e0162716 (2016). Article Google Scholar * Mechlem, K. _et al_. Joint Statistical Iterative Material Image
Reconstruction for Spectral Computed Tomography Using a Semi-Empirical Forward Model. _IEEE Trans. Med. Imaging_ 37, 68–80 (2018). Article Google Scholar * Pachon, J. H., Yadava, G., Pal,
D. & Hsieh, J. Image quality evaluation of iterative CT reconstruction algorithms: a perspective from spatial domain noise texture measures. _SPIE Med_. _Imaging_ 8313, 83132K (2012).
Google Scholar * Zhou, W. _et al_. Lung nodule volume quantification and shape differentiation with an ultra-high resolution technique on a photon counting detector CT system. _Proc. SPIE
Med. Imaging_ 10132, 101323Q (2017). Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the German Research Foundation (DFG) and the Technical
University of Munich within the funding programme Open Access Publishing. The authors would like to thank Thomas Koehler from Philips Research Hamburg and Kevin Brown from Philips Healthcare
BIU CT Cleveland for their support. We acknowledge support through the German Department of Education and Research (BMBF) under grant IMEDO (13GW0072C), the German Research Foundation (DFG)
within the Research Training Group GRK 2274 and the European Union Horizon 2020 grant under grant No 643694. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of diagnostic and
interventional Radiology, Technische Universität München, Munich, Germany Felix K. Kopp, Andreas P. Sauter, Alexander A. Fingerle, Ernst J. Rummeny, Daniela Pfeiffer & Peter B. Noël *
Philips GmbH Innovative Technologies, Research Laboratories, Hamburg, Germany Heiner Daerr, Bernhard Brendel, Ewald Roessl & Roland Proksa * Department of Interventional Radiology and
Cardio-vascular and Thoracic Diagnostic Imaging, Louis Pradel University Hospital, Bron, France Salim Si-Mohamed & Philippe Douek * CREATIS, CNRS UMR 5220, INSERM U1206, INSA-Lyon,
France Salim Si-Mohamed & Philippe Douek * Chair of Biomedical Physics, Department of Physics & Munich School of BioEngineering, Technische Universität München, 85748, Garching,
Germany Sebastian Ehn & Franz Pfeiffer * Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA Peter B. Noël Authors * Felix K.
Kopp View author publications You can also search for this author inPubMed Google Scholar * Heiner Daerr View author publications You can also search for this author inPubMed Google Scholar
* Salim Si-Mohamed View author publications You can also search for this author inPubMed Google Scholar * Andreas P. Sauter View author publications You can also search for this author
inPubMed Google Scholar * Sebastian Ehn View author publications You can also search for this author inPubMed Google Scholar * Alexander A. Fingerle View author publications You can also
search for this author inPubMed Google Scholar * Bernhard Brendel View author publications You can also search for this author inPubMed Google Scholar * Franz Pfeiffer View author
publications You can also search for this author inPubMed Google Scholar * Ewald Roessl View author publications You can also search for this author inPubMed Google Scholar * Ernst J.
Rummeny View author publications You can also search for this author inPubMed Google Scholar * Daniela Pfeiffer View author publications You can also search for this author inPubMed Google
Scholar * Roland Proksa View author publications You can also search for this author inPubMed Google Scholar * Philippe Douek View author publications You can also search for this author
inPubMed Google Scholar * Peter B. Noël View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS F.K.K., F.P., D.P., R.P., P.D. and P.B.N. designed
the study. F.K.K., D.P., and P.B.N. created the lung phantom. H.D., S.S.M., S.E., B.B., F.P., E.R., D.P., R.P. and P.B.N. acquired the prototype data. F.K.K., A.P.S., A.A.F. and P.B.N.
acquired conventional data. F.K.K., D.P., E.J.R. and P.B.N. wrote the analysis plan. F.K.K., E.R., E.J.R., P.D. and P.B.N. provided data, analysis tools, and coordinated data analysis.
F.K.K., H.D. and A.P.S. carried out experiments and data analysis. All authors reviewed the manuscript before submission. CORRESPONDING AUTHOR Correspondence to Felix K. Kopp. ETHICS
DECLARATIONS COMPETING INTERESTS H.D., B.B., E.R. and R.P. are employees at Philips Research Hamburg. All other authors declare no potential conflict of interest. ADDITIONAL INFORMATION
PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kopp, F.K., Daerr, H., Si-Mohamed, S. _et al._
Evaluation of a preclinical photon-counting CT prototype for pulmonary imaging. _Sci Rep_ 8, 17386 (2018). https://doi.org/10.1038/s41598-018-35888-1 Download citation * Received: 05
September 2018 * Accepted: 09 November 2018 * Published: 26 November 2018 * DOI: https://doi.org/10.1038/s41598-018-35888-1 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * Pulmonary Imaging * Lung Phantom * Dice Similarity Coefficient * Visual Investigation * Lobular Bronchiole