Play all audios:
ABSTRACT We aimed to investigate the association between nonalcoholic fatty liver disease (NAFLD) and cerebral small vessel disease (CSVD) burden, especially according to the NAFLD severity.
A total of 1,260 participants were included. The CSVD burden was assessed with white matter hyperintensities (WMH), lacunes, and microbleeds (MBs) on brain MRI. An ultrasound diagnosis of
fatty liver was made based on standard criteria, and the Fibrosis-4 (FIB-4) index was used to classify participants with NAFLD with having a high-intermediate (FIB-4 ≥1.45) or low (FIB-4
< 1.45) probability of advanced fibrosis. A multivariable logistic regression analysis was used to assess the association between NAFLD and the presence of moderate to severe WMH,
lacunes, and MBs. NAFLD had a significant association only with moderate to severe WMH (OR: 1.64, 95% CI: 1.10–2.42), even after controlling for cardiometabolic risk factors. A linear trend
test showed a significant association between the severity of NAFLD fibrosis and the presence of moderate to severe WMH (_p_ for trend <0.001). Our findings suggest that NAFLD, especially
NAFLD with fibrosis, has a significant association with the presence of moderate to severe WMH in cognitively normal individuals, and NAFLD severity predicted more frequent moderate to
severe WMH. SIMILAR CONTENT BEING VIEWED BY OTHERS AMYLOID DEPOSITION AND SMALL VESSEL DISEASE ARE ASSOCIATED WITH COGNITIVE FUNCTION IN OLDER ADULTS WITH TYPE 2 DIABETES Article Open access
01 February 2024 LOBAR MICROBLEEDS ARE ASSOCIATED WITH COGNITIVE IMPAIRMENT IN PATIENTS WITH LACUNAR INFARCTION Article Open access 02 October 2020 ASSOCIATION BETWEEN TOTAL CEREBRAL SMALL
VESSEL DISEASE SCORE AND COGNITIVE FUNCTION IN PATIENTS WITH VASCULAR RISK FACTORS Article 09 March 2023 INTRODUCTION Nonalcoholic fatty liver disease (NAFLD) is the most common cause of
chronic liver disease, and the prevalence is rapidly increasing worldwide1,2. NAFLD has attracted growing attention in terms of its relation with not only hepatic complications, but also
with cardiometabolic risk factors, such as hypertension, insulin resistance, and obesity3. There is increasing evidence that NAFLD may affect brain health. A recent study showed that NAFLD
is associated with learning and memory, as measured by symbol digit learning tests4. However, the pathobiology of this association remains unclear. Cerebral small vessel diseases (CSVD),
such as white matter hyperintensities (WMH), lacunes, and microbleeds (MBs), are major causes of cognitive impairment. Studies have demonstrated an association between certain
cardiometabolic risk factors, such as hypertension and diabetes, with CSVD development. Considering the role of NAFLD as an independent cardiometabolic risk factor5,6,7, it is reasonable to
expect NAFLD to be associated with the development of CSVD. In fact, a recent study revealed that the fibrosis severity of NAFLD predicted the presence of WMH8. However, this study
investigated a small number of study subjects, and the relationships between NAFLD and other CSVD markers, such as lacunes and MBs, which have different pathobiologies from WMH, have not
been thoroughly studied. Therefore, in this study, we aimed to investigate the association between NALFD, as assessed by ultrasonography (US), and CSVD burden, measured by magnetic resonance
imaging (MRI), in a large sample of cognitively normal individuals. We hypothesized that: (1) NAFLD is associated with the presence of lacunes and MBs, as well as WMH, independent of other
cardiometabolic risk factors; and (2) their significant association might depend on NAFLD severity. RESULTS SUBJECTS CHARACTERISTICS Among 1,260 patients, 498 patients (39.6%) had NAFLD at
baseline and 239 patients (19.0%) were categorized as having NAFLD with an intermediate to high FIB-4 (≥1.45) (Table 1). The demographic data of categorized patients according to NFS is
shown in Table 2. Participants with NAFLD were more likely to be older, male, smokers, moderate alcohol drinkers, and to have a higher BMI and a higher frequency of hypertension and diabetes
compared to those without NAFLD. In terms of CSVD markers, while only 9.6% of patients without NAFLD had moderate to severe WMH, 13.1% of patients with NAFLD with a low FIB-4 (<1.45) and
18.8% of NAFLD patients with an intermediate to high FIB-4 (≥1.45) had moderate to severeWMH. These results were statistically different. However, the prevalence of MBs and lacunes did not
vary among the three groups. ASSOCIATION BETWEEN NAFLD AND CSVD MARKERS First, the crude odds ratio (OR) for moderate to severe WMH comparing participants with NAFLD to those without it was
1.78 (95% confidence interval (CI): 1.27–2.50). (Model 1). This association remained significant after adjusting for age, sex, smoking, alcohol, obesity, hypertension, diabetes, and
hyperlipidemia (OR: 1.64; 95% CI: 1.10–2.42). However, the associations between NAFLD and the presence of lacunes and MBs were not significant, as crude ORs for lacunes and MB were 1.19 (95%
CI: 0.81–1.76) and 1.15 (95% CI: 0.66–1.55), respectively. When we assessed these associations according to the severity of NAFLD, the OR (95% CI) for moderate to severe WMH in participants
with a low FIB-4 (<1.45) and with intermediate to high FIB-4 (≥1.45) were 1.14 (0.72–1.82) and 1.77 (1.13–2.78) compared to participants without NAFLD, respectively. Especially, the
linear trend test showed a significant association between the severity of NAFLD fibrosis (non-NAFLD, NAFLD with FIB-4 < 1.45 or NAFLD with FIB-4 ≥1.45) and the presence of moderate to
severe WMH (_p_ for trend = 0.016) (Table 3). When the analyses were conducted with NAFLD categories using NFS, the results were same as those seen with FIB-4 index, as the association
between the presence of moderate to severe WMH and NAFLD with a low NFS (<−1.455) was not significant (OR: 0.92; 95% CI: 0.53–1.59), while its association with an intermediate to high NFS
(≥−1.455) was significant (OR: 2.05; 95% CI: 1.34–3.14). A linear trend test showed a significant association between the severity of NAFLD fibrosis and the presence of moderate to severe
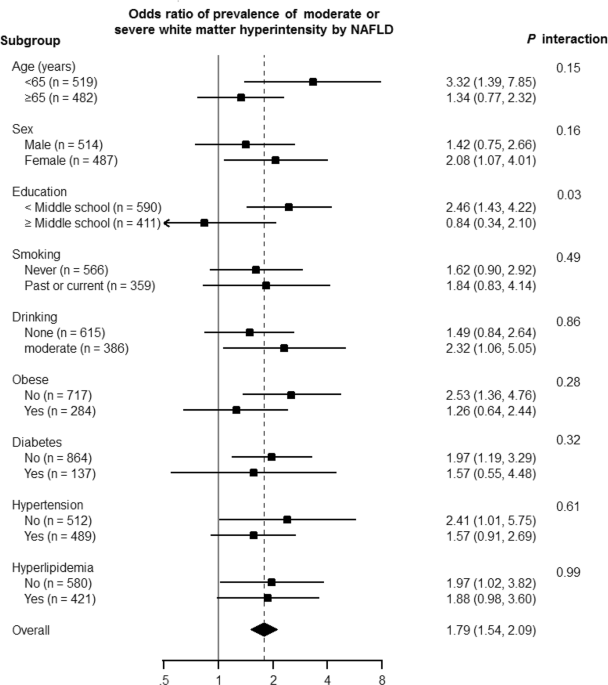
WMH as well (_p_ for trend = 0.002) (Table 4). Additionally, we evaluated the association between NAFLD with FIB-4 ≥1.45 with the prevalence of moderate or severe WMH in different clinical
subgroups (Fig. 1). The level of association between NAFLD with FIB-4 ≥1.45 and the presence of moderate to severe WMH was significantly different according to the educational level (_p_ for
interaction = 0.03). DISCUSSION This study determined that NAFLD has a significant association with the presence of WMHs, even after controlling for cardiometabolic risk factors. This study
included a large number of participants (n = 1,260) and extensive data about comorbid cardiometabolic risk factors and various CSVD MRI markers, key issues in older patients. These
strengths enabled us to examine the independent relationship between NAFLD and CSVD, regardless of various cardiometabolic risk factors. Therefore, our findings provide insight into the
contribution of NAFLD to development of CSVD. Our major finding was that NAFLD has a significant association only with WMH among different CSVD markers, even after controlling for
cardiometabolic risk factors. Especially, when we divided patients into two groups according to fibrosis score, association with WMH was maintained only in NAFLD with an intermediate to
severe fibrosis score, suggesting that the severity of NAFLD, rather than the presence of NAFLD itself, might be more importantly related to the development of CSVD. This finding is
consistent with a recent study which reported that patients with NAFLD with fibrosis have a higher risk of having WMH than patients without NALFD or patients with NALFD without fibrosis8.
However, our findings showed that there was an increase in the frequency of moderate to severe WMH with increasing fibrosis severity. It is difficult to clarify the exact pathomechanism of
the association between NAFLD and WMH. However, there are several explanations for this finding. First, NAFLD contributes to decreased cerebrovascular reactivity, causing chronic
hypoperfusion and subsequent WMH. The pathogenesis of WMH has not been identified, but endothelial dysfunction could decrease cerebrovascular reactivity9 and chronic hypoperfusion. NAFLD is
closely related to insulin resistance10 and metabolic risk factors, which is significantly associated with endothelial dysfunction11. Second, NAFLD could be related to subclinical
inflammation, similar to other metabolic syndromes which contribute to blood brain barrier (BBB) disruption12, and progress to WMH13, despite the unknown relationship between systemic
inflammation and CSVD. NAFLD with advanced fibrosis might contribute to systemic inflammation more than NAFLD without fibrosis. Finally, NAFLD is related to carotid atherosclerosis14, which
might cause microembolic events, which could in turn lead to increased WMH. The aforementioned processes could also be interrelated, rather than occurring independently. The level of
association between NAFLD and the presence of moderate to severe WMH was significantly different according to the educational level of the patients (_p_ for interaction = 0.03). This
observation has led us to consider the reason for the effect of different educational levels on the NAFLD-WMH association. Interestingly, the lower educational group had a stronger
association between NAFLD and WMH. One of possible explanations for this is that education is a representative measure of socioeconomic status (SES)15. Therefore, patients with a lower SES
might have more health-related risk factors, including cardiovascular risk factors16 and other unmeasured confounding variables, such as dietary and lifestyle risk factors, which make
patients in this group more vulnerable to developing WMH in response to the same metabolic stress. In this study, NAFLD was not associated with the formation of lacunes or MBs. Unlike our
findings, a recent study showed fatty liver disease is reported to be associated with lacunar infarct especially in non-obese population17. However, they included alcoholic fatty liver
disease as well as NAFLD. WMH, lacunes, and MBs are CSVD MRI markers, but they have slightly different pathogeneses. WMH are related to chronic, diffuse, and subclinical ischemia, and BBB
disruption18,19. Lacunes are attributed to acute, severe, or localized ischemia18, and MBs result from focal bleeding in small vessels that are either damaged by lipohyalinosis or by amyloid
angiopathy20. Therefore, we can assume that NAFLD may lead to increased inflammation or cerebrovascular reactivity, and cause diffuse and chronic hypoperfusion or BBB disruption, rather
than focal ischemia or microhemorrhages. In this study, we investigated the relationship between CSVD and NAFLD in a large study with cognitively normal individuals. However, there were
several limitations to this study. First, our study was cross-sectional, precluding claims of causality. The temporal relationship between NAFLD and CSVD markers remains unclear.
Longitudinal studies are needed to determine the impact of NAFLD on the incidence of CSVD. Second, we defined NAFLD by US, which cannot detect mild steatosis and is operator-dependent. We
also calculated the NAFLD FIB-4/NFS index to represent the severity of NAFLD, because simple US cannot differentiate steatohepatitis from simple steatosis21. In addition, while the accuracy
of US for establishing the presence of fatty liver is high, it is subject to measurement error22. Finally, this study was conducted in Koreans who underwent health screening exams, and thus
may not be generalizable to other settings or to other ethnicities. In conclusion, our findings suggest that NAFLD might be a potential independent risk factor for the presence of moderate
to severe WMH. Therefore, evaluation of conventional risk factors in patients with NAFLD warrants the presence of CSVD. This is of particular importance, given that there is an increasing
prevalence of NAFLD, which can be potentially controlled by lifestyle modification, such as weight reduction, regular exercise, and changes in dietary patterns. Therefore, additional efforts
to evaluate and manage NAFLD as one of several important cardiometabolic risk factors in patients with CSVD are needed. METHODS STUDY PARTICIPANTS The study population was comprised of men
and women 40 years of age or older who underwent a health screening exam at the Health Promotion Center of the Samsung Medical Center in Seoul, Korea from October 1, 2008 to December 31,
2013. We included 2,320 subjects who had undergone at least one neurological and neurocognitive screening test, including brain MRI and the Mini-Mental Status Examination (MMSE), as well as
an abdominal US. We excluded participants who had any of the following conditions: history of cancer (n = 210), history of liver cirrhosis/positive hepatitis B surface antigen/hepatitis C
virus antibodies (n = 165), alcohol intake ≥30 g/day in men or ≥20 g/day in women (n = 489), or that scored below the 16th percentile in age-, sex-, and education-matched norms according to
the MMSE (n = 108). Patients that had structural lesions, including territorial cerebral infarctions, brain tumors, or intracranial hemorrhage on brain MRI were also excluded (n = 23). In
addition, we excluded participants who had missing data on alcohol intake (n = 117), education, or MMSE score (n = 89), resulting in a final sample size of 1,260 (640 men and 620 women).
(Fig. 2). This study was approved by the Institutional Review Board of the Samsung Medical Center. The requirement for informed consent was waived because we only used de-identified data
collected for clinical purposes during health screening exams. DATA COLLECTION To evaluate the presence of NAFLD, an abdominal US was performed using the LogiQ E9 (GE Healthcare, Milwaukee,
WI, USA), iU22 xMatrix (Philips Medical Systems, Cleveland, OH, USA) or ACUSON Sequoia 512 (Siemens, Issaquah, WA, USA) US machine, and the procedure was performed by experienced
radiologists blinded to the study aims. Images were captured in a standard fashion with the patients in the supine position with their right arm raised above their head. A US diagnosis of
fatty liver was made based on standard criteria, including parenchymal brightness, liver-to-kidney contrast, deep beam attenuation, and bright vessel walls23,24. Because we excluded
participants with excessive alcohol use (≥30 g/day for men and ≥20 g/day for women), as well as other identifiable causes of fatty liver at baseline, a US-diagnosed fatty liver was
considered NAFLD. To assess the severity of fibrosis, we calculated Fibrosis-4 (FIB-4) index as (age (years) x AST (U/L))/(platelet (109/L) x (ALT(U/L)1/2), and stratified NAFLD patients
according to their FIB-4 index as having an intermediate to high (FIB-4 ≥1.45) or low (FIB-4 < 1.45) probability of advanced fibrosis25. A low FIB-4 (<1.45) is also a strong predictor
of the absence of liver fibrosis. For sensitivity analysis, we also classified patients into two groups using NAFLD fibrosis score (NFS), which was calculated as −1.675 + 0.037 × age (years)
+ 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet count (×109/l) − 0.66 × albumin (g/dl)26, with subjects having
either a high-intermediate (NFS ≥ −1.455) or low (NFS < −1.455) probability of advanced fibrosis. BRAIN MRI All participants underwent brain MRI, including T2* gradient echo (GRE) and
three-dimensional (3D) Fluid-attenuated inversion recovery (FLAIR) imaging, using the same type of 3.0 T MRI scanner (Philips 3.0 T Achieva, Best, the Netherlands). The following parameters
were used for the T2* GRE images: axial slice thickness, 5.0 mm; inter-slice thickness, 2 mm; repetition time (TR), 669 ms; echo time (TE) 16 ms; flip angle, 18°; matrix size, 560 × 560
pixels. 3D FLAIR images were acquired with the following imaging parameters: axial slice thickness, 2 mm; no gap; TR, 11,000 ms; TE, 125 ms; flip angle, 90°; and matrix size, 512 × 512
pixels. CSVD MARKERS ON MRI We used a modified Fazekas scale to visually rate WMH27. Periventricular WMH (PWMH) were classified as P1 (cap or band <5 mm), P2 (5 mm ≤ cap or band <10
mm), or P3 (cap or band ≥10 mm); deep WMH (DWMH) were classified into D1 (maximum diameter of deep white matter lesion <10 mm), D2 (10 mm ≤ lesion <25 mm), or D3 (≥λ25 mm). The
intra-class correlation coefficients for the inter-rater reliability of the WMH visual rating scale ranged from 0.73 and 0.91. PWMH and DWMH ratings were combined to give a final WMH
classification of minimal (D1P1 or D1P2), moderate (D2P1, D3P1, D2P2, D3P2, D1P3, and D2P3), or severe (D3P3). In this study, the presence of WMH was determined when patients had moderate or
severe WMH. This classification discriminates the presence of vascular risk factors and the severity of cerebrovascular disease markers28. Two experienced neurologists blinded to patient
data reviewed images to determine the number and location of lacunes and MBs according to neuroimaging standards29. The kappa values for agreement between the two neurologists for the
presence of lacunes and MBs were 0.78 and 0.92, respectively. CARDIOMETABOLIC RISK FACTORS Serum total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, and low-density
lipoprotein (LDL) cholesterol levels were determined using an enzymatic colorimetric method. Hyperlipidemia was defined according to the Adult Treatment Panel III30 criteria as triglyceride
levels ≥150 mg/dl, HDL-cholesterol levels <40 mg/dl, or use of medication for dyslipidemia. Glucose was measured in blood samples that were collected after at least 10 hours of fasting.
Diabetes was defined as a fasting serum glucose ≥126 mg/dL or self-reported use of insulin or antidiabetic medications. The Department of Laboratory Medicine and Genetics at the Samsung
Medical Center has participated in several proficiency testing programs operated by the Korean Association of Quality Assurance for Clinical Laboratory, the Asian Network of Clinical
Laboratory Standardization and Harmonization, and the College of American Pathologists. Smoking status was categorized as never, past, or current smoker. Alcohol intake was categorized as
either none or moderate (<30 g/day in men and <20 g/day in women). Trained nurses measured the height, weight, and sitting blood pressure of participants wearing a lightweight hospital
gown and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. BMI was classified according to Asian-specific criteria31, and obesity
was defined as a BMI ≥25 kg/m2. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medications.
STATISTICAL ANALYSIS An analysis of variance was used to compare the demographics and clinical characteristics of three groups: no NAFLD, NAFLD with a low FIB-4 (<1.45) and NAFLD with an
intermediate to high FIB-4 (≥1.45). After the test, we conducted post hoc test using Scheffé's method. A multivariable logistic regression analysis was performed to assess the
association between NAFLD and CSVD markers. We also conducted the same analysis for three groups categorized by NFS: no NAFLD, NAFLD with a low NFS (< −1.455) and NAFLD with an
intermediate to high NFS (≥−1.455). We used three models with increasing degrees of adjustment to account for potential confounding factors at baseline. To address missing data, we used a
missing-indicator method to create a missing value category for each incomplete, independent, categorical variable32. The crude model was not adjusted for confounders. Model 1 was adjusted
for age and sex. Model 2 was further adjusted for smoking (never vs. past or current smokers), alcohol consumption (none vs. moderate), obesity (not obese vs. obese), and metabolic factors,
including hypertension, diabetes, and hyperlipidemia. Additionally, to test for linear trends according to NAFLD severity, we included the NAFLD severity category (no NAFLD, NAFLD with a low
probability of fibrosis (FIB-4 < 1.45 or NFS < −1.455) and NAFLD with an intermediate to high probability of fibrosis (FIB-4 ≥ 1.45 or NFS ≥−1.455)) as a continuous variable in the
logistic regression models. In addition, we explored the association between NAFLD with FIB-4 ≥1.45 and the CSVD markers in pre-specified clinically relevant subgroups defined by age (<65
vs. ≥65 years), sex (women vs. men), education (<middle school vs. ≥middle school), smoking (never vs. past or current smokers), alcohol consumption (none vs. moderate), obesity (not
obese vs. obese), hypertension (yes vs. no), diabetes (yes vs. no), and hyperlipidemia (yes vs.no). We tested for the interaction of NAFLD with clinical characteristics using Wald tests for
cross-product terms in regression models. All reported p-values were two-sided, and the significance level was set at 0.05. All analyses were performed using STATA version 14 (StataCorp LP,
College Station, TX, USA). CHANGE HISTORY * _ 10 OCTOBER 2019 An amendment to this paper has been published and can be accessed via a link at the top of the paper. _ REFERENCES * Chitturi,
S., Wong, V. W. & Farrell, G. Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. _J Gastroenterol Hepatol_ 26(Suppl 1), 163–172,
https://doi.org/10.1111/j.1440-1746.2010.06548.x (2011). Article PubMed Google Scholar * Chalasani, N. _et al_. The diagnosis and management of non-alcoholic fatty liver disease: practice
Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. _Hepatology_ 55, 2005–2023,
https://doi.org/10.1002/hep.25762 (2012). Article PubMed Google Scholar * Byrne, C. D. & Targher, G. NAFLD: a multisystem disease. _J Hepatol_ 62, S47–64,
https://doi.org/10.1016/j.jhep.2014.12.012 (2015). Article PubMed Google Scholar * Seo, S. W. _et al_. Nonalcoholic fatty liver disease is associated with cognitive function in adults.
_Neurology_ 86, 1136–1142, https://doi.org/10.1212/wnl.0000000000002498 (2016). Article CAS PubMed PubMed Central Google Scholar * Chang, Y. _et al_. Cohort study of non-alcoholic fatty
liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. _Am J Gastroenterol_ 108, 1861–1868, https://doi.org/10.1038/ajg.2013.349 (2013). Article ADS
CAS PubMed Google Scholar * Ryoo, J. H. _et al_. The clinical availability of non alcoholic fatty liver disease as an early predictor of the metabolic syndrome in Korean men: 5-year’s
prospective cohort study. _Atherosclerosis_ 227, 398–403, https://doi.org/10.1016/j.atherosclerosis.2013.01.002 (2013). Article CAS PubMed Google Scholar * Lonardo, A., Ballestri, S.,
Marchesini, G., Angulo, P. & Loria, P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. _Dig Liver Dis_ 47, 181–190, https://doi.org/10.1016/j.dld.2014.09.020
(2015). Article PubMed Google Scholar * Petta, S. _et al_. The Presence of White Matter Lesions Is Associated With the Fibrosis Severity of Nonalcoholic Fatty Liver Disease. _Medicine
(Baltimore)_ 95, e3446, https://doi.org/10.1097/md.0000000000003446 (2016). Article CAS Google Scholar * Knottnerus, I. L., Ten Cate, H., Lodder, J., Kessels, F. & van Oostenbrugge,
R. J. Endothelial dysfunction in lacunar stroke: a systematic review. _Cerebrovasc Dis_ 27, 519–526, https://doi.org/10.1159/000212672 (2009). Article CAS PubMed Google Scholar * Sinn,
D. H. _et al_. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic
middle-aged Asian adults. _Am J Gastroenterol_ 107, 561–567, https://doi.org/10.1038/ajg.2011.400 (2012). Article ADS PubMed Google Scholar * Thomas, G. N. _et al_. Deleterious impact of
“high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: the CATHAY study.
_Arterioscl Throm Vas_ 24, 739–743, https://doi.org/10.1161/01.ATV.0000118015.26978.07 (2004). Article CAS Google Scholar * Hamann, G. F., Okada, Y., Fitridge, R. & del Zoppo, G. J.
Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. _Stroke_ 26, 2120–2126 (1995). Article CAS PubMed Google Scholar * Sam, K. _et al_. Development of
White Matter Hyperintensity Is Preceded by Reduced Cerebrovascular Reactivity. _Ann Neurol_ 80, 277–285, https://doi.org/10.1002/ana.24712 (2016). Article CAS PubMed Google Scholar *
Targher, G. _et al_. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. _Diabetes Care_ 29, 1325–1330,
https://doi.org/10.2337/dc06-0135 (2006). Article PubMed Google Scholar * Winkleby, M. A., Jatulis, D. E., Frank, E. & Fortmann, S. P. Socioeconomic-Status and Health - How Education,
Income, and Occupation Contribute to Risk-Factors for Cardiovascular-Disease. _Am J Public Health_ 82, 816–820, https://doi.org/10.2105/Ajph.82.6.816 (1992). Article CAS PubMed PubMed
Central Google Scholar * Smith, G. D., Hart, C., Blane, D., Gillis, C. & Hawthorne, V. Lifetime socioeconomic position and mortality: prospective observational study. _BMJ_ 314,
547–552 (1997). Article CAS PubMed PubMed Central Google Scholar * Kwak, M. S. _et al_. Non-obese fatty liver disease is associated with lacunar infarct. _Liver international: official
journal of the International Association for the Study of the Liver_ 38, 1292–1299, https://doi.org/10.1111/liv.13663 (2018). Article CAS Google Scholar * Pantoni, L. Cerebral small
vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. _The Lancet. Neurology_ 9, 689–701, https://doi.org/10.1016/S1474-4422(10)70104-6 (2010). Article
PubMed Google Scholar * Farrall, A. J. & Wardlaw, J. M. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. _Neurobiol Aging_ 30, 337–352,
https://doi.org/10.1016/j.neurobiolaging.2007.07.015 (2009). Article CAS PubMed Google Scholar * Fazekas, F. _et al_. Histopathologic analysis of foci of signal loss on gradient-echo
T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. _AJNR Am J Neuroradiol_ 20, 637–642 (1999). CAS PubMed PubMed
Central Google Scholar * Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. _Clin Mol Hepatol_ 19, 325–348,
https://doi.org/10.3350/cmh.2013.19.4.325 (2013). Article Google Scholar * Hernaez, R. _et al_. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a
meta-analysis. _Hepatology_ 54, 1082–1090, https://doi.org/10.1002/hep.24452 (2011). Article PubMed Google Scholar * Saverymuttu, S. H., Joseph, A. E. & Maxwell, J. D. Ultrasound
scanning in the detection of hepatic fibrosis and steatosis. _British medical journal_ 292, 13–15 (1986). Article CAS PubMed PubMed Central Google Scholar * Mathiesen, U. L. _et al_.
Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases.
_Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver_ 34, 516–522 (2002). Article CAS Google
Scholar * Shah, A. G. _et al_. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. _Clinical gastroenterology and hepatology: the official
clinical practice journal of the American Gastroenterological Association_ 7, 1104–1112, https://doi.org/10.1016/j.cgh.2009.05.033 (2009). Article CAS Google Scholar * Angulo, P. _et al_.
The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. _Hepatology_ 45, 846–854, https://doi.org/10.1002/hep.21496 (2007). Article CAS
PubMed Google Scholar * Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I. & Zimmerman, R. A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. _AJR Am J
Roentgenol_ 149, 351–356, https://doi.org/10.2214/ajr.149.2.351 (1987). Article CAS PubMed Google Scholar * Noh, Y. _et al_. A new classification system for ischemia using a combination
of deep and periventricular white matter hyperintensities. _Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association_ 23, 636–642,
https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.06.002 (2014). Article Google Scholar * Wardlaw, J. M. _et al_. Neuroimaging standards for research into small vessel disease and its
contribution to ageing and neurodegeneration. _The Lancet. Neurology_ 12, 822–838, https://doi.org/10.1016/S1474-4422(13)70124-8 (2013). Article PubMed PubMed Central Google Scholar *
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult
Treatment Panel III). _Jama_ 285, 2486–2497 (2001). Article Google Scholar * Pan, W.-H. & Yeh, W.-T. How to define obesity? _Evidence-based multiple action points for public awareness,
screening, and treatment: an extension of Asian-Pacific recommendations._ 17, 370–374 (2008). Google Scholar * Jones, M. P. Indicator and stratification methods for missing explanatory
variables in multiple linear regression. _J Am Stat Assoc_ 91, 222–230, https://doi.org/10.2307/2291399 (1996). Article MathSciNet MATH Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2017R1A2B2005081) and the Brain Research Program
through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1913844). AUTHOR INFORMATION Author notes * Hyemin Jang, Danbee
Kang, Juhee Cho and Sang Won Seo contributed equally. AUTHORS AND AFFILIATIONS * Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, 06351,
Korea Hyemin Jang, Yeshin Kim, Young Kyoung Jang, Hee Jin Kim, Duk L. Na & Sang Won Seo * Neuroscience Center, Samsung Medical Center, 06351, Seoul, Korea Hyemin Jang, Yeshin Kim, Young
Kyoung Jang, Hee Jin Kim, Duk L. Na & Sang Won Seo * Center for Clinical Epidemiology, Samsung Medical Center, 06351, Seoul, Korea Danbee Kang, Eliseo Guallar & Juhee Cho * Health
Promotion Center, Samsung Medical Center, 06351, Seoul, Korea Hee Young Shin & Mira Kang * Department of Occupational and Environmental Medicine, Kangbuk Samsung Hospital, Sungkyunkwan
University, School of Medicine, Seoul, Korea Yoosoo Chang * Department of Neurology, Kyung Hee University Hospital, Seoul, Korea Jin San Lee * Department of Neurology, Chonbuk National
University Hospital, Chonbuk National University Medical School, Jeonju, Korea Ko Woon Kim * Department of Health Sciences and Technology, Sungkyunkwan University, Seoul, 06351, Korea Juhee
Cho * Clinical Research Design and Evaluation, SAIHST, Sungkyunkwan University, Seoul, 06351, Korea Eliseo Guallar & Juhee Cho * Department of Epidemiology, Johns Hopkins Medical
Institutions, Baltimore, USA Eliseo Guallar * Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, USA Eliseo Guallar * Welch Center for Prevention, Epidemiology and
Clinical Research, Johns Hopkins Medical Institutions, Baltimore, USA Eliseo Guallar Authors * Hyemin Jang View author publications You can also search for this author inPubMed Google
Scholar * Danbee Kang View author publications You can also search for this author inPubMed Google Scholar * Yoosoo Chang View author publications You can also search for this author
inPubMed Google Scholar * Yeshin Kim View author publications You can also search for this author inPubMed Google Scholar * Jin San Lee View author publications You can also search for this
author inPubMed Google Scholar * Ko Woon Kim View author publications You can also search for this author inPubMed Google Scholar * Young Kyoung Jang View author publications You can also
search for this author inPubMed Google Scholar * Hee Jin Kim View author publications You can also search for this author inPubMed Google Scholar * Duk L. Na View author publications You can
also search for this author inPubMed Google Scholar * Hee Young Shin View author publications You can also search for this author inPubMed Google Scholar * Mira Kang View author
publications You can also search for this author inPubMed Google Scholar * Eliseo Guallar View author publications You can also search for this author inPubMed Google Scholar * Juhee Cho
View author publications You can also search for this author inPubMed Google Scholar * Sang Won Seo View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS H.J., D.K.: design of the study, acquisition and analysis of the data, drafting the manuscript. Y.K., K.W.K., J.S.L., Y.K.J., H.J.K., H.Y.S., M.K.: acquisition of the data.
Y.C., D.L.N., E.G.: interpretation of the data, revising the manuscript. S.W.S., J.C.: design of the study, acquisition and analysis of the data, interpretation of the data, revising the
manuscript, study supervision. CORRESPONDING AUTHORS Correspondence to Juhee Cho or Sang Won Seo. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format,
as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jang, H., Kang, D., Chang, Y. _et
al._ Non-alcoholic fatty liver disease and cerebral small vessel disease in Korean cognitively normal individuals. _Sci Rep_ 9, 1814 (2019). https://doi.org/10.1038/s41598-018-38357-x
Download citation * Received: 24 April 2018 * Accepted: 15 December 2018 * Published: 12 February 2019 * DOI: https://doi.org/10.1038/s41598-018-38357-x SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative