Play all audios:
ABSTRACT Although the P300 event-related potential (ERP) is the most likely central measure of Sokolov’s Orienting Reflex (OR), there are few systematic comparisons with the skin conductance
response (SCR), the “gold standard” electrodermal OR measure. We examine habituation, stimulus significance, and inter-stimulus interval (ISI) effects in SCRs and components of the P300
from single-trial ERPs in an auditory dishabituation paradigm. Single trial ERP components were separated by temporal principal components analysis, and five components of the P300 were
examined as potential phasic OR measures: P3a, P3b, Novelty P3, and two Slow Waves (SW1, SW2). Across the factors of ISI and significance, SCRs showed decrement over trials, recovery at a
deviant, and dishabituation at the subsequent standard. This general pattern was not present in any of the components of the P300. SCRs were also larger to significant stimuli and at the
long ISI; effects differed between P300 components. The electrodermal SCR showed the complete profile over trials expected of the phasic OR, and was enhanced by stimulus significance,
confirming it as the model measure of Sokolov’s phasic OR. Components of the P300 failed to match this profile, but instead appear to reflect different aspects of the stimulus processing
involved in OR elicitation. SIMILAR CONTENT BEING VIEWED BY OTHERS PREPULSE INHIBITION VS COGNITIVE MODULATION OF THE HAND-BLINK REFLEX Article Open access 25 February 2021 MODULATING
CORTICAL EXCITABILITY AND CORTICAL AROUSAL BY PUPIL SELF-REGULATION Article Open access 16 May 2025 INVOLUNTARY MOTOR RESPONSES ARE ELICITED BOTH BY RARE SOUNDS AND RARE PITCH CHANGES
Article Open access 30 August 2024 INTRODUCTION The Orienting Reflex (OR) is an adaptive mechanism that focuses an organism’s attention towards changes in the environment to facilitate
optimal perceptual processing. The founder of the psychophysiology of the OR, E.N. Sokolov, proposed that for a physiological response to be considered as a measure of the OR, it should
demonstrate habituation and sensitivity to stimulus characteristics (particularly novelty and significance)1,2,3. Habituation is an important fundamental learning mechanism that enables an
organism to maintain homeostasis by reflexively inhibiting responses to irrelevant stimuli. Criteria originally proposed by Thompson and Spencer4, and later revised by Rankin and
colleagues5, specify that for response decrement to be formally labelled as habituation, response recovery (enhanced responding to a change/deviant stimulus) and dishabituation (increased
responding to the habituated stimulus following the deviant), must be evident. These criteria are essential for differentiating habituation from other decrementing response patterns, such as
those due to refractory periods. The skin conductance response (SCR), which reflects bursts in sympathetic sudomotor nerve signalling6, is considered the “yardstick” or gold standard
measure of the OR in humans7,8,9,10. Due to the slow onset, rise-time, and resolution of the SCR, paradigms with long interstimulus intervals (ISIs) of 10–60 s have been considered necessary
to separate SCRs between stimuli11,12,13,14. However, lengthy ISI studies are incongruous with contemporary psychophysiological paradigms, particularly those designed to elicit
event-related potentials (ERPs), relying on averaging responses elicited at short ISIs (~ 1 s duration); here single trials have been thought undesirable due to the poor signal-to-noise
ratio15. In the quest to find a central nervous system index of the OR, research efforts have been made to elicit SCRs at shorter ISIs in a range of contexts12,15,16,17,18, and to assess
corresponding ERP components as potential OR candidates18,19. Gatchel and Lang20 investigated SCRs in an auditory habituation task with mean ISIs of 20, 60, and 100 s and reported smaller
SCRs that habituated more rapidly with shorter ISIs. Berti and colleagues21 recorded SCRs and ERPs during a two-stimulus auditory oddball task (novel target probability = 10%) at 0.5, 1.0,
3.0, and 10.0 s ISIs in younger and older adults. SCRs and target N2 and P3 ERP component amplitudes decreased with shorter ISIs in both groups. Recio and colleagues17 assessed differences
in SCR and Go/NoGo ERP components N2 and P3 whilst manipulating ISI to 2, 5, and 8 s. Increasing the ISI enhanced SCRs, but did not alter the condition-specific effects. The same pattern was
demonstrated by Breska and colleagues12 using a concealed information test to compare SCRs elicited to mean ISIs of 10 and 20 s. They concluded that the ISI should be selected carefully
depending on the aims of the study, indeed an important point demonstrated in Steiner and Barry13,14. In our 2011 investigation into the mechanism of electrodermal dishabituation22,
stimulus-onset asynchrony (SOA) at 5–7 s was too short to allow the complete resolution of the phasic SCR to significant (counted) stimuli. Our follow-up investigation14 found that an SOA of
13–15 s was adequate for SCR resolution (consistent with Barry23). As Sokolov1 noted, significant (task-relevant/salient) stimuli elicit larger ORs that are slower to habituate in the
context of elevated arousal levels, an observation confirmed in our previous investigations13,14,22,23,24. We have also suggested that ISI affects stimulus novelty13,14, with stimuli
presented at shorter ISIs perceived as less novel than at longer ISIs, exhibiting SCRs with smaller amplitudes that decrement more quickly12,13,14,17,20. This may be a potential mechanism
for the well-documented P300-ISI effect, where shorter ISIs elicit P300 ERP components with smaller amplitudes and shorter latencies compared to longer ISIs25,26,27,28,29,30,31. The P300
(Late Positive Complex, LPC) has long been considered the central analogue of the OR19,32,33. This view arose when peak-picking quantification methods were most ubiquitous, where P300 and
its underlying components (P3a, P3b, Novelty P3, and at least one Slow Wave [SW]) were collectively measured as a single entity and shown to mirror the SCR, as demonstrated (for example) by
Rushby and colleagues18. However, once temporal principal components analysis (PCA) is applied to separate temporally overlapping components, not one of the individual members of the “P3
family” are found to parallel the SCR as an OR index18. Each P3 component differs in scalp topography, peak latency, and sensitivity to task requirements and stimulus parameters, indicating
multi-layered cognitive and perceptual processes constituting the OR, with differential cortical sources to match8,9,10,34,35,36,37,38. We have previously reported response decrement for P3b
and Novelty P3 (nP3), response recovery for nP3 only, no components showing dishabituation, and sensitivity to stimulus significance for P3b and SW1 only9,35,38. To the best of our
awareness, ISI has not yet been explored in this context. Here, we varied the ISI to explore whether the rate of stimulus presentation affected stimulus novelty and the OR, in the context of
indifferent (no task instructions) and significant (counting) stimuli. We used an autonomic-style dishabituation paradigm with two different ISIs (short: 5–7 s; long: 13–15 s) and
quantified single trial SCRs and components of the P300, the latter using PCA. It was hypothesised that SCR would “behave” as a typical OR measure: demonstrating habituation, larger
responses to significant than indifferent stimuli, and smaller responses that decremented more quickly to short than long ISIs. Corresponding effects were expected to be seen in P300 ERP
components in line with our previous investigations9,35, with response decrement seen for P3b and nP3, and response recovery and sensitivity to ISI for nP3 only; stimulus significance was
expected to enhance P3b and SW138. The later SW2 has not been examined in this context before. RESULTS All participants correctly reported their number of trials to the experimenter at the
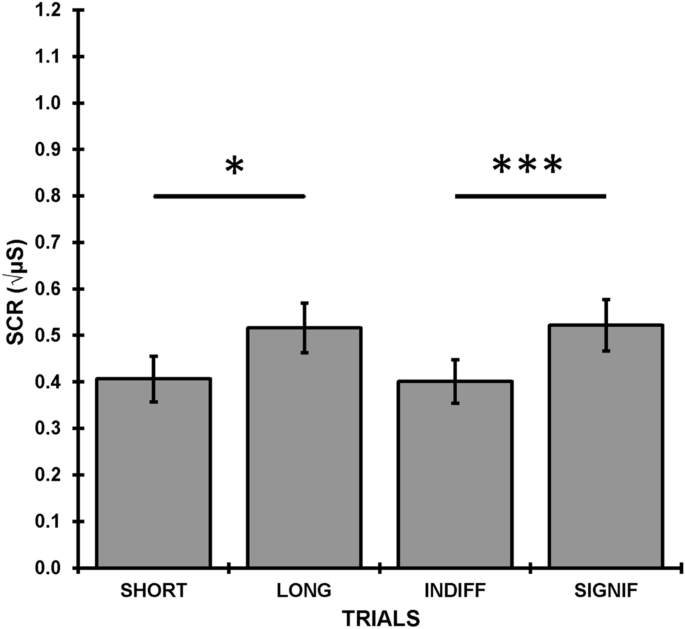
end of the counting condition, confirming compliance with the task. ELECTRODERMAL RESPONSES Figure 1 shows the square-root SCRs averaged over the 12 trials as a function of significance
(significant vs. indifferent) and ISI (short vs. long). Responses to significant stimuli were larger than to indifferent stimuli (_F_(1, 38) = 28.29, _p_ < 0.001, ηp2 = 0.43), and
responses in the long group were larger than in the short group (_F_ = 4.35, _p_ = 0.044, ηp2 = 0.10). There was no interaction between significance and ISI. TRIALS EFFECTS IN ELECTRODERMAL
RESPONSES Square-root SCRs, averaged across significance and ISI, are shown at each trial in Fig. 2A. Overall, a substantial decrement is apparent over the repeated stimuli. This is
interrupted by response recovery to the change stimulus at trial 11. Subsequently, SCRs at trial 12 appear larger than at trial 10 (dishabituation). Figure 2B shows this response profile for
each level of significance at each ISI. Statistically, over trials 1 to 10, decrement was apparent in linear (_F_ = 233.00, _p_ < 0.001, ηp2 = 0.86), and quadratic (_F_ = 127.24, _p_
< 0.001, ηp2 = 0.77) trends. Responses were initially larger in the significant versus indifferent condition, leading to greater decrement in both linear (_F_ = 5.30, _p_ = 0.027, ηp2 =
0.12) and quadratic (_F_ = 7.60, _p_ = 0.009, ηp2 = 0.17) trends. Greater decrement in the short _cf._ long group was apparent in a linear trend difference (_F_ = 9.25, _p_ = 0.004, ηp2 =
0.20). There was an interaction between significance and ISI, with the linear decrement being greatest to significant stimuli at the short ISI (_F_ = 4.65, _p_ = 0.038, ηp2 = 0.11). Across
trials 10 and 11, there was recovery at the change tone (_F_ = 48.68, _p_ < 0.001, ηp2 = 0.56). Recovery was larger for the significant condition (_F_ = 9.14, _p_ = 0.004, ηp2 = 0.19),
and for the short _cf._ long ISI (_F_ = 7.05, _p_ = 0.012, ηp2 = 0.16). These interacted, with the significance enhancement larger at the short ISI (_F_ = 4.47, _p_ = 0.041, ηp2 = 0.11).
Across trials 10 and 12, dishabituation was apparent (_F_ = 13.06, _p_ < 0.001, ηp2 = 0.26), but this did not differ with significance. Dishabituation was somewhat larger at short ISI
(_F_ = 3.78, _p_ = 0.059, ηp2 = 0.09), but there was no interaction of this with significance. ERP COMPONENTS Figure 3A shows the grand mean ERPs at the midline sites averaged over trials,
significance, and ISI. There was a small Na and P1, followed by a large N1 complex and P2. A large P3 complex followed, then two slow waves (SW1 and SW2). Figure 3B shows the morphology at
the midline sites for each combination of condition and ISI. As shown in Fig. 4, a similar series of components was apparent in each separate PCA, identifiable as: Na, P1, N1a, N1b, N1c, P2,
P3a, P3b, nP3, SW1 and SW2. Figure 4A shows that, in the long indifferent PCA, these 11 components carried 84.4% of the variance. In the long significant PCA (Fig. 4B), Na and SW1 emerged
only when the threshold was reduced from 2.0% to 1.9%; note variance values in red font. Factor 10 was an unidentifiable prestimulus component at − 18 ms; its omission left 85.8% variance.
The short indifferent PCA (Fig. 4C) produced 11 comparable components, carrying 83.7% of the variance. The PCA of the short significant data set (Fig. 4D) produced 11 similar components,
carrying 80.7% of the variance. There were also three unidentifiable components, at − 82 ms (i.e., prestimulus), 102, and 436 ms; these were omitted from further consideration. The
topographies of the accepted components from the four PCAs are shown in Fig. 5. The four sets of peak latencies of these corresponding components were closely related. Averaged across
significance, Fig. 6A shows that latencies of the long group components correlated with those of the short group components (_r_ = 0.999, _p_ < 0.0001), and demonstrated a non-significant
mean increase of some 3.3 ms (matched samples t-test: _t_ = 1.34, two-way _p_ = 0.210). Averaged across ISI, Fig. 6B shows that the latencies of the significant condition components
correlated with those of the indifferent condition components (_r_ = 0.996, _p_ < 0.0001), with a significant mean increase of some 10.7 ms (matched samples t-test: _t_ = − 1.51, two-way
_p_ = 0.031). The components contributing to this increase in latency with significance (i.e., those clearly above the line of equality in Fig. 5B) were N1b, N1c, P2, P3a, P3b, and nP3.
Usually, ERP amplitudes are calculated from a region of interest (ROI) reflecting the topography of the component. Here the four sets of topographies (shown in Fig. 5) differed somewhat with
significance and ISI. To avoid the complication of different ROIs, we used the mean of the across-scalp amplitudes for each component. The mean of this over all trials was examined as a
function of significance and ISI. Na and P1 showed no significant difference between significant and indifferent conditions, no difference between long and short ISI, and no interaction
effects. N1a was somewhat larger at the long ISI (_F_ = 3.05, _p_ = 0.089, ηp2 = 0.07), but showed no other effects. In N1b there were no main effects of significance or ISI, but these
interacted significantly, with an increase in the significant _cf._ indifferent condition that was larger for the long ISI group (_F_ = 4.48, _p_ = 0.041, ηp2 = 0.11). N1c showed a reduction
in amplitude with significant _cf._ indifferent stimuli (_F_ = 27.37, _p_ < 0.001, ηp2 = 0.42), and this was somewhat larger at long ISI (_F_ = 3.30, _p_ = 0.077, ηp2 = 0.08). P2 was
larger in significant than indifferent conditions (_F_ = 6.93, _p_ = 0.012, ηp2 = 0.15), and was somewhat larger with long _cf._ short ISIs (_F_ = 4.06, _p_ = 0.051, ηp2 = 0.10); these
effects did not interact. P3a did not vary across significance or ISI. P3b was larger in significant than indifferent conditions (_F_ = 13.35, _p_ = 0.001, ηp2 = 0.26), and larger at the
long than short ISI (_F_ = 4.69, _p_ = 0.037, ηp2 = 0.11); these effects did not interact. nP3 was reduced somewhat in significant compared to the indifferent condition (_F_ = 4.04, _p_ =
0.052, ηp2 = 0.10), and was larger at long than short ISI (_F_ = 5.71, _p_ = 0.022, ηp2 = 0.13); these interacted, with the significant condition reduction larger at the short ISI (_F_ =
6.73, _p_ = 0.013, ηp2 = 0.15). The positive SW1 was larger for the short than long ISI group (_F_ = 21.55, _p_ < 0.001, ηp2 = 0.36), and this effect was larger with significant than
indifferent stimuli (_F_ = 4.54, _p_ = 0.040, ηp2 = 0.11). SW2 was more positive at short _cf._ the long ISI (_F_ = 10.99, _p_ = 0.002, ηp2 = 0.22). TRIALS EFFECTS IN LPC COMPONENTS Trials
effects in the LPC components (P3a, P3b, nP3, SW1, SW2) are reported here as potential OR correlates. Amplitudes are plotted over trials in Fig. 7a for the mean across groups and conditions,
and in Fig. 7b for the individual datasets. P3a showed no overall decrement over trials 1 to 10, but had a faster _initial_ decrement for significant stimuli, apparent in a greater
quadratic trend over trials (_F_ = 5.75, _p_ = 0.021, ηp2 = 0.13), and the linear decrement was largest for significant stimuli at the short ISI (_F_ = 5.35, _p_ = 0.026, ηp2 = 0.12). P3b
showed a significant linear decrement (_F_ = 10.68, _p_ = 0.002, ηp2 = 0.22) that was somewhat larger for significant than indifferent conditions (_F_ = 3.39, _p_ = 0.073, ηp2 = 0.08). nP3
decremented over trials, apparent in significant linear (_F_ = 26.32, _p_ < 0.001, ηp2 = 0.41) and quadratic (_F_ = 6.18, _p_ = 0.017, ηp2 = 0.14) trends; the linear trend was larger for
the indifferent than significant condition (_F_ = 5.33, _p_ = 0.026, ηp2 = 0.12). For the positive SW1, the linear decrement over trials was somewhat greater for significant than indifferent
conditions (_F_ = 3.57, _p_ = 0.066, ηp2 = 0.09). The SW2 positivity showed greater linear decrement over trials at short than long ISI (_F_ = 5.18, _p_ = 0.029, ηp2 = 0.12), particularly
in the significant condition (_F_ = 4.16, _p_ = 0.048, ηp2 = 0.10). As shown in Fig. 7A, P3a showed no evidence of recovery at trial 11 _cf._ trial 10; rather, a reduction reached
statistical significance (_F_ = 5.09, _p_ = 0.030, ηp2 = 0.12). Figure 7B shows how P3b had greater recovery in significant than indifferent conditions (_F_ = 5.51, _p_ = 0.024, ηp2 = 0.13).
nP3 showed strong recovery (_F_ = 12.99, _p_ = 0.001, ηp2 = 0.25) that was independent of significance and ISI. SW1 positivity showed significantly greater response at the change (_F_ =
18.99, _p_ < 0.001, ηp2 = 0.33) that was independent of stimulus significance and ISI. SW2 positivity showed no recovery at the change. Dishabituation at trial 12 _cf._ trial 10 was not
apparent in P3a, P3b, nP3, SW1 or SW2. SIMILARITIES BETWEEN SCR AND P300 COMPONENT TRIALS EFFECTS Over the 12 trials, the Spearman correlation between mean P3a trials effects (Fig. 7A) and
mean SCR trials effects (Fig. 2A) was not significant, _r_(10) = 0.15, one-tailed _p_ = 0.325. However, exploratory analyses indicated that this correlation reached statistical significance
for significant stimuli at the long ISI, _r_(10) = 0.50, one-tailed _p_ = 0.050, but was negative for the indifferent condition at the short ISI, _r_(10) = − 0.69, two-tailed _p_ = 0.014;
compare plots in Figs. 7B and 2B. Illustrative scatterplots are presented in Supplementary Fig. S1. For P3b, the correlation between mean ERP (Fig. 7A) and mean SCR trials effects (Fig. 2A)
approached significance, _r_(10) = 0.45, one-tailed _p_ = 0.069. Exploratory analyses of the individual condition data in Figs. 7B and 2B indicated that this correlation reached statistical
significance in the significant stimulus condition at both short, _r_(10) = 0.64, one-tailed _p_ = 0.012, and long ISIs, _r_(10) = 0.57, one-tailed _p_ = 0.027. See scatterplots in
Supplementary Fig. S2. With nP3, the correlation between the ERP profile (Fig. 7A) and mean SCR trials effects (Fig. 2A) was significant, _r_(10) = 0.79, one-tailed _p_ = 0.001. For the
separate condition-specific data in Figs. 7B and 2b, this correlation reached statistical significance in the indifferent condition at both short and long ISIs; both _r_(10) ≥ 0.60,
one-tailed _p_ ≤ 0.019, and in the significant condition at the short ISI, _r_(10) = 0.63, one-tailed _p_ = 0.014. These data are shown as scatterplots in Supplementary Fig. S3. The Spearman
correlation between mean SW1 trials effects (Fig. 7A) and mean SCR trials effects (Fig. 2A) approached significance, _r_(10) = 0.48, one-tailed _p_ = 0.059. Analyses of the individual
condition data in Figs. 7B and 2B indicated that this correlation reached statistical significance only in the significant condition at the short ISI, _r_(10) = 0.53, one-tailed _p_ = 0.038;
see Supplementary Fig. S4. For the SW2 positivity, the correlation with mean SCR trials effects was not statistically significant, _r_(10) = 0.36, one-tailed _p_ = 0.128. Assessment of the
individual condition data indicated that this correlation in the significant condition was positive at the short ISI, _r_(10) = 0.55, one-tailed _p_ = 0.033, but was non-significant (and
negative) at the long ISI, _r_(10) = − 0.49, two-tailed _p_ = 0.11. See scatterplots in Supplementary Fig. S5. DISCUSSION The novelty of this study was the joint examination of electrodermal
and ERP responses to both indifferent and significant stimuli in auditory dishabituation paradigms presented to two groups at different ISIs. Table 1 presents an overview of the impact of
significance and ISI on SCR and all identified components, together with the OR-relevant trials effects in the P300 components. The main aim was to clarify trials effects in the impact of
stimulus significance and ISI for the different measures in relation to response _decrement_ with stimulus repetition, response _recovery_ to a change stimulus, and _dishabituation_
(response increase) to the repetition of the standard stimulus following the change stimulus. This basic pattern in response to indifferent stimuli was compared with that to significant
stimuli, in a counterbalanced task requiring participants to silently count the stimuli for later reporting to the experimenter. SCRS Averaged over trials, electrodermal responses were
larger to significant than to indifferent stimuli, and to stimuli presented at long than short ISIs, with no interaction. The enhancing effect of stimulus significance in the OR has been
noted since the early work by Sokolov1 and commonly reported in the literature23,38,39. The main effect of the between-group difference in ISI is relatively novel, but it is compatible with
previous findings by Recio and colleagues17. Importantly, there was no interaction between stimulus significance and ISI, suggesting the independence of the two experimental variables. In
the current study, across significance and ISI, the SCR showed response decrement, recovery at the stimulus change, followed by dishabituation, confirming the classic response profile
defining a decremental process as habituation4,5. The habituation profile in the electrodermal response has long been reported1,2. Here, SCR showed the predicted main effects of ISI,
significance, and repetition/change, which define the OR. Importantly, greater decrement over trials was apparent for SCRs to significant stimuli, and this was attributed to the larger
initial responses at the first trial. This faster decrement is contrary to the Sokolovian concept that responses to significant stimuli habituate more slowly than those to indifferent
stimuli1,23. The greater initial OR to significant stimuli across ISI groups is most likely attributable to a heightened arousal state to facilitate the direction of attention towards the
counting task40. This heightened arousal likely played a role in the ISI effects as we observed greater decrement for responses in the short compared to the long ISI group, particularly in
the significant condition. These aspects of the decremental profile need exploration in future studies. Further, recovery at the change stimulus showed the same pattern as the decrement
rate: larger for significant than indifferent stimuli, and for the short ISI group, particularly in the significant condition, suggesting that decrement over trials and recovery at a change
stimulus are driven by similar or comparable mechanisms. However, dishabituation to the following stimulus did _not_ differ significantly with the experimental variables, showing only some
enhancement at the short ISI. The small effect sizes suggest that the experimental variables have little functional effect on dishabituation of SCR amplitudes. This would naturally imply a
difference in the mechanism between the phenomena of _decrement/recovery_ and _dishabituation_ that goes beyond novelty processing13,14,22. We will return to this later. ERPS The four sets
of ERPs, across all trials for both conditions and ISIs had similar components in our separate PCAs: Na, P1, N1a, N1b, N1c, P2, P3a, P3b, nP3, SW1, and SW2. Components from P1 to SW1 closely
matched the latency and topography of those reported in habituation paradigms by MacDonald and Barry38, and Study 1 of Barry and colleagues39. A similar Na at 29 ms was reported in two
dishabituation studies with very long ISIs (50–70 s)36,37. The SW2 seen here had not been extracted previously using shorter epochs as input to tPCA. The latencies of the components found
here were highly correlated across the four datasets. Latencies of components from significant stimuli were significantly longer than for indifferent stimuli; this was most apparent in N1b,
N1c, P2, P3a, P3b, and nP3. This might reflect the engagement of numerical cognitive processes due to the counting of stimuli (either via direct retrieval or an automated counting
process)41. Note also the somewhat condition-specific topographies for some components (e.g., N1c, P3a, P3b, nP3, SW1, SW2) evident in Fig. 4, supporting this notion of differential
neurocognitive processing that has previously been linked with the left inferior parietal cortex42. These possibilities are beyond the scope of the present study, but deserve future
exploration. There was no significant latency difference between matched components in the different ISI groups. Across trials, amplitudes of Na and P1 were independent of the experimental
variables. In significant compared to indifferent conditions, P2 and P3b were larger, while N1c and nP3 were smaller. At very long ISIs (50–70 s) MacDonald and Barry38 had reported
enhancements to significant stimuli in N1a, P2, and SW1; the differences here are probably due to the much shorter ISIs. N1a was somewhat enhanced at long ISI. Main effects of longer ISI
were apparent in significant increases in P3b and nP3, replicating the P300-ISI effect25,26,27,28,29,30,31, but reductions in SW1 and SW2 were observed. There were some interactions for ERP
component amplitudes between significance and ISI. Although N1b showed no main effects, its amplitude was significantly larger to significant stimuli at the long ISI. The N1c reduction to
significant stimuli was somewhat larger for long ISIs, but the nP3 reduction to significant stimuli was significantly larger at short ISIs. The SW1 enhancement at short ISIs was larger for
significant stimuli. While such interactive effects are important in adding to our general knowledge about the determinants of different ERP components, they are not our major focus here and
will not be discussed further. P300 COMPONENT TRIALS EFFECTS Trials effects in the components forming the P300 complex9 were examined and compared to those in the SCR OR model, building on
our earlier work34. Across significance and ISI, P3a did not decrease linearly over trials, but significant stimuli produced a faster initial decrement (quadratic trend) and the linear
decrement was larger for significant stimuli at short ISIs. In P3b, the linear decrement was significant across conditions and ISI, and was somewhat larger for significant than indifferent
stimuli. With nP3, a linear decrement was significant across all data sets, and larger for indifferent than significant conditions, compatible with Sokolovian predictions for the OR1,23, but
contrary to the SCR finding. For SW1 positivity, the linear decrement over trials was somewhat larger for significant than indifferent stimuli. The linear decrement for the SW2 positivity
was greater at the short ISI, particularly in the significant condition. Recovery at the change stimulus was not apparent in P3a; indeed, a significant reduction was apparent instead. P3b
showed recovery only with significant stimuli, indicating its sensitivity to stimulus relevance43. With nP3 and SW1, similar enhanced responding was apparent for all conditions and ISIs; SW2
showed no recovery. No LPC component showed evidence of dishabituation. In summary, across significance and ISI, P3a, SW1 and SW2 did not decrement significantly, while P3b and nP3 showed
significant linear decrement. An increase at the change trial was apparent in nP3 and SW1; the latter effect strictly should not be termed “recovery” as it did not follow decrement over
repeated standards. That is, only nP3 showed a true sensitivity to stimulus novelty with decrement and recovery across all experimental conditions, matching the response profile of SCR. This
replicates the prior findings of Barry and colleagues9. Variations in this mean profile in response to significance (e.g., enhanced decrement in P3b [like SCR], and reduced decrement in
nP3) suggests their differential sensitivity to other mechanisms. There was no evidence of dishabituation in any P300 subcomponent. This finding confirms the ERP results of the three
parametric dishabituation studies summarised in MacDonald and Barry44. Those data led to the suggestion that dishabituation should “be abandoned as an aspect of habituation” (P. 125), and
this is supported here for our ERP components. Our findings in SCR suggested above that a different mechanism is involved in decrement/recovery versus dishabituation, and together with the
P300 component results, argue for a wider re-examination of the validity of this habituation criterion in humans (_cf_. Rankin and collegues5, and Thompson and Spencer4). SCR/P300 COMPONENT
COMPARISONS The correlation analyses indicated that the mean LPC component pattern over all trials compared with that of the SCR did not approach significance for P3a, approached
significance for P3b, was significant and strong for nP3, approached significance for SW1, but was not significant for SW2. In addition, the exploratory analyses showed trials effects linked
to SCR for P3a in the significant condition at the long ISI, P3b in the significant condition at both ISIs, and with SW1 and SW2 in the significant condition at the short ISI. For nP3,
these links were for the indifferent condition at both ISIs and in the significant condition at the short ISI. Overall, these results indicate that the various P300 components have complex
relations with SCR as regards their response over trials to stimulus repetition and change, stimulus significance, and ISI. These novel findings need exploration in future studies varying
stimulus presentation parameters such as intensity and ISI jointly. The SCR OR profile and its relationships to the stimulus parameters tested here provided a model for comparison with the
P300, long touted as the likely ERP correlate of the OR. In numbers of studies8,10,34,35,36,37,38,44 we have repeatedly found that temporal PCA extracted P3a, P3b, nP3, and SW1 components
from the P300, and we focussed here on these as potential OR measures. Using a longer ERP analysis epoch here, we also examined a later SW2 than previously discussed. Across trials,
increased stimulus significance did not affect P3a, but increased P3b, reduced nP3, and had no main effect on SW1 or SW2; longer ISI had no effect on P3a, but increased P3b and nP3, and
decreased SW1 and SW2. Across significance and ISI, P3a, SW1 and SW2 did not decrement, while P3b and nP3 did; recovery to a change following decrement was shown by nP3 only. No components
showed dishabituation here. These results confirm the suggestion of MacDonald and Barry44 that none of the P300 components mirror SCR, but leave open the possibility that their totality in
the P300 may serve as the central analogue of the SCR OR measure, as previously suggested13,18. METHODS PARTICIPANTS Forty undergraduate students participated in this study in return for
course credit (mean age = 19.3, _SD_ = 1.6 years; 36 right-handed, 24 males). All provided informed consent prior to participating and were free to withdraw at any time without penalty.
Participants self-reported no use of psychotropic medication, no neurological or psychiatric illnesses, refrained from psychoactive substances for at least 12 h, and from tea, coffee,
alcohol, and cigarettes for at least 2 h prior to testing. All participants had normal or corrected-to-normal vision and self-reported normal hearing. ETHICS STATEMENT All experimental
procedures were carried out in accordance with relevant guidelines and regulations in a protocol approved by the joint South Eastern Sydney/Illawarra Area Health Service and University of
Wollongong Health and Medical Human Research Ethics Committee. Participants were informed about the experiment and told that they were free to withdraw at any time without penalty; all
provided written informed consent. PROCEDURE Participants completed a demographic and screening questionnaire before they were fitted with EEG and skin conductance recording apparatus. They
were seated in a darkened air-conditioned room 60–80 cm in front of a 48.3 cm (19 inch) Dell LCD monitor. Prior to the experiment, participants completed an electrooculogram (EOG)/EEG
calibration task45. Participants were then instructed to fixate on a 10 × 10 mm grey cross centred on a black background during the task. Stimuli consisted of 1000 and 1500 Hz tones
(counterbalanced between subjects), each of 50 ms duration (15 ms rise/fall time) at 60 dB SPL, delivered binaurally through Sony MDR V700 circumaural stereo headphones. The stimulus
sequence was 10 standards of one frequency, a deviant tone of a different frequency, followed by 2–4 standards of the original frequency. Half the participants (_N_ = 20) received a
short-ISI version of the task (5–7 s SOA), and half (_N_ = 20) received a long-ISI version (13–15 s SOA). All participants completed two counterbalanced conditions presented approximately 3
min apart: indifferent, in which there was no task in relation to the auditory stimuli; and significant, where participants were directed to silently count the tones and report the number to
the researcher at the end of the condition. MATERIAL AND APPARATUS Electrodermal data were recorded from the distal volar surface of digits II and III of the non-dominant hand using
sintered silver/silver-chloride (Ag/AgCl) electrodes filled with isotonic electrode paste of 0.05 M NaCl in an inert ointment base. Skin conductance was sampled using a constant voltage
device (UFI Bioderm model 2701) at 0.5 V. Continuous EEG data and DC-coupled skin conductance output were recorded DC–30 Hz at 1000 Hz with a Neuroscan Synamps 2 digital signal-processing
system and Neuroscan 4.3.1 Acquire software using the default gain setting, and stored on a Dell Optiplex 755 computer. EEG data were acquired from 19 scalp sites (Fp1, Fp2, F7, F3, Fz, F4,
F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2) with an electrode cap using tin electrodes placed in accordance with the International 10/20 System46, plus A1 and A2. Over the duration
of this two-group study, some aspects of the protocol were modified to keep up with changing recommendations. In particular, physically-linked ears (with carefully-balanced impedances) were
used as a reference for the short-ISI group, and A1 was used for the long-ISI group (later re-referenced to digitally-linked A1/A2); it is not believed that this difference would impact the
present findings. The cap was grounded by an electrode located midway between Fp1, Fp2 and Fz. Display and stimulus markers were controlled by a linked stimulus computer using
Neurobehavioral Systems Inc. Presentation V 13.0 Build 01.23.09 software. EOG was recorded using tin cup electrodes placed 2 cm above and below the left eye for vertical movements, and on
the outer canthus of each eye for horizontal movements. Impedance was less than 5 kΩ for cap, EOG, and reference electrodes. DATA EXTRACTION SKIN CONDUCTANCE RESPONSE Raw data were band-pass
filtered (0.1–3.0 Hz, zero-phase shift, 24 dB/Octave) and epoched offline 1 s pre- to 5 s post-stimulus using Neuroscan 4.3.1 Edit Software. For each trial, pre-response levels (1 s pre- to
1 s post-stimulus onset) were linearly extrapolated to compensate for falling baselines47, using the linear detrend function in Neuroscan. Each response with onset latency 1–3 s
post-stimulus48 was quantified for each subject and each trial, as the difference between the extrapolated baseline and the maximum value of the subsequent peak13,14,22,47. SCRs were
square-root transformed to reduce skew7,23. Trials that contained non-stimulus related responses were removed and replaced by an average of the available trials immediately before and/or
after the aberrant trial. ERPS EEG data were EOG corrected using the RAAA EOG Correction Program45. Where necessary, data were re-referenced to digitally linked ears and extracted offline
using the Neuroscan Edit software, low pass filtered (0.1–30 Hz, zero-phase shift, 24 dB/Octave), epoched from 100 ms pre- to 550 ms post-stimulus; single trials were baselined to the
pre-stimulus period. Data were manually inspected for any additional artefact, but there were no contaminated trials that required exclusion from analysis. PRINCIPAL COMPONENTS ANALYSES The
single trial data from − 100 to 550 ms for each of the conditions (indifferent vs. significant) and ISIs (long vs. short) from 19 scalp locations were down-sampled to 500 Hz and submitted to
four separate temporal PCAs to reduce misallocation of variance49. PCAs were derived in MATLAB (The Mathworks, R2012b Version 8.0.0.783) using Kayser and Tenke's50 erpPCA and Varimax4M
functions, with a heuristic modification as reported in Barry and colleagues49 based on Dien’s51 approach of _not_ removing the grand mean ERP waveform from each case before calculating the
final component waveforms. Factors were quantified separately for each group (4,560 observations: 20 participants × 12 trials × 19 sites). The PCAs used the unstandardised covariance matrix
with Kaiser normalisation, and all 325 unrestricted factors underwent Varimax rotation, following Kayser and Tenke50. We considered the components ranked in variance order, expecting
components in the existing ERP literature. We initially examined all components carrying more than 2.0% variance, to avoid omitting any substantive components, and this threshold was lowered
to seek corresponding components if a component was identified in one group or condition only. Component amplitudes were formed by multiplying the factor loading × factor score × standard
deviations of the variables. STATISTICAL ANALYSES For each variable, _z_ scores (over condition, ISI, and trial) were used to detect univariate outliers (|_z_|≥ 2.5, _p_ < 0.01) following
Ho's52 recommendations for small sample sizes (_N_ ≤ 80). Data transformations increased the number of outliers and given the limited sample size (_N_ = 20 per ISI group), nature of
the data (12 trials within-subjects/conditions), and low percentage of outliers (1.38% of all datapoints), outlier scores were _rescaled_ (rather than _omitted_) to avoid participant loss
and maintain statistical power53. Each identified outlier was individually proportionately rescaled to meet the criterion for retention (|_z_|< 2.5). Across the 11,520 data points (20
participants × 2 groups × 2 conditions × 12 trials × 12 variables), 159 adjustments were required; within each variable, no more than 1.8% (17 of 960) individual datapoints were rescaled,
and the mean scaling factors ranged from 0.68 to 0.87 (_M_ = 0.79, _SD_ = 0.05). Mixed univariate MANOVAs assessed the between-subjects effect of ISI (long vs. short), and the
within-subjects effect of significance (indifferent vs. significant) across all 12 trials, separately for SCRs and ERP components. Separate mixed univariate MANOVAs were used to examine
within-subjects response decrement (trial factor) for SCRs and selected ERP components (linear and quadratic trends over trials 1–10), with factors of ISI and condition. Separate mixed
univariate MANOVAs were carried out to assess response recovery (trial 11 vs. 10) and dishabituation (trial 12 vs. 10), again with factors of ISI and significance. All _F_ tests had _df_ =
(1, 38). Correlations were used to compare the grand mean SCR pattern over trials with corresponding patterns in the P300 components. The SCR trials data violated the assumption of
normality, so Spearman’s Rank Order correlations were used. Positive correlations were expected to indicate potential OR measures and were tested with one-way significance levels with alpha
set to 0.05; all other correlations are reported as two-tailed tests. It should also be noted that, as this paper details results for a number of dependent measures, the frequency of Type I
errors increases. However, Howell54 argues that this increase in frequency of Type I errors cannot be controlled by adjusting α-levels because the probability of Type I error remains
unchanged. DATA AVAILABILITY All data used in this study are available from the corresponding author on reasonable request. REFERENCES * Sokolov, E. N. _Perception and the Conditioned
Reflex_ (Pergamon Press, 1963). Google Scholar * Sokolov, E. N. Higher nervous functions: The orienting reflex. _Annu. Rev. Physiol._ 25, 545–580 (1963). CAS PubMed Google Scholar *
Sokolov, E. N. The orienting reflex, its structure and mechanisms. In _Orienting Reflex and Exploratory Behavior_ (Eds. Voronin, L. G., Leontiev, A. N., Luria, A. R., Sokolov, E. N. &
Vinogradova. O. S.). (1965). * Thompson, R. F. & Spencer, W. A. Habituation: A model phenomenon for the study of neuronal substrates of behavior. _Psychol. Rev._ 73, 16–43 (1966). CAS
PubMed Google Scholar * Rankin, C. H. _et al._ Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. _Neurobiol. Learn. Mem._ 92,
135–138 (2009). PubMed Google Scholar * Macefield, V. G. & Wallin, B. G. The discharge behaviour of single sympathetic neurones supplying human sweat glands. _J. Auton. Nerv. Syst._
61, 277–286 (1996). CAS PubMed Google Scholar * Barry, R. J. & Sokolov, E. N. Habituation of phasic and tonic components of the orienting reflex. _Int. J. Psychophysiol._ 15, 39–42
(1993). CAS PubMed Google Scholar * Barry, R. J., MacDonald, B. & Rushby, J. A. Single-trial event-related potentials and the orienting reflex to monaural tones. _Int. J.
Psychophysiol._ 79, 127–136 (2011). PubMed Google Scholar * Barry, R. J. _et al._ Components in the P300: Don’t forget the Novelty P3!. _Psychophysiology_ 57, e13371 (2020). PubMed Google
Scholar * Rushby, J. A. & Barry, R. J. Single-trial event-related potentials to significant stimuli. _Int. J. Psychophysiol._ 74, 120–131 (2009). PubMed Google Scholar * Alexander,
D. M. _et al._ Separating individual skin conductance responses in a short interstimulus-interval paradigm. _J. Neurosci. Meth._ 146, 116–123 (2005). CAS Google Scholar * Breska, A., Maoz,
K. & Ben-Shakhar, G. Interstimulus intervals for skin conductance response measurement. _Psychophysiology_ 48, 437–440 (2011). PubMed Google Scholar * Steiner, G. Z. & Barry, R.
J. Pupillary responses and event-related potentials as indices of the orienting reflex. _Psychophysiology_ 48, 1648–1655 (2011). PubMed Google Scholar * Steiner, G. Z. & Barry, R. J.
The mechanism of dishabituation. _Front. Integr. Neurosci._ 8, 14 (2014). PubMed PubMed Central Google Scholar * Barry, R. J. & Rushby, J. A. An orienting reflex perspective on
anteriorisation of the P3 of the event-related potential. _Exp Brain Res._ 173, 539–545 (2006). PubMed Google Scholar * Lim, C. L. _et al._ Dynamics of SCR, EEG, and ERP activity in an
oddball paradigm with short interstimulus intervals. _Psychophysiology_ 36, 543–551 (1999). CAS PubMed Google Scholar * Recio, G., Schacht, A. & Sommer, W. Effects of inter-stimulus
interval on skin conductance responses and event-related potentials in a Go/NoGo task. _Biol. Psychol._ 80, 246–250 (2009). PubMed Google Scholar * Rushby, J. A., Barry, R. J. &
Doherty, R. J. Separation of the components of the late positive complex in an ERP dishabituation paradigm. _Clin. Neurophysiol._ 116, 2363–2380 (2005). PubMed Google Scholar * Donchin, E.
_et al._ Cognition and event-related potentials II. The orienting reflex and P300. _Ann. NY Acad. Sci._ 425, 39–57 (1984). ADS CAS PubMed Google Scholar * Gatchel, R. J. & Lang, P.
J. Effects of interstimulus interval length and variability on habituation of autonomic components of the orienting response. _J. Exp. Psychol._ 103, 802–804 (1974). CAS PubMed Google
Scholar * Berti, S., Vossel, G. & Gamer, M. The orienting response in healthy aging: Novelty P3 indicates no general decline but reduced efficacy for fast stimulation rates. _Front.
Psychol._ 8, 1780 (2017). PubMed PubMed Central Google Scholar * Steiner, G. Z. & Barry, R. J. Exploring the mechanism of dishabituation. _Neurobiol. Learn. Mem._ 95, 461–466 (2011).
PubMed Google Scholar * Barry, R. J. Stimulus significance effects in habituation of the phasic and tonic orienting reflex. _Integr. Phys. Beh. Sci._ 39, 166–179 (2004). Google Scholar *
Barry, R. J. Habituation of the orienting reflex and the development of preliminary process theory. _Neurobiol. Learn. Mem._ 92, 235–242 (2009). PubMed Google Scholar * Fitzgerald, P. G.
& Picton, T. W. Temporal and sequential probability in evoked potential studies. _Can. J. Psychol._ 35, 188–200 (1981). CAS PubMed Google Scholar * Gonsalvez, C. J. & Polich, J.
P300 amplitude is determined by target-to-target interval. _Psychophysiology_ 39, 388–396 (2002). PubMed Google Scholar * Miltner, W., Johnson, R. & Braun, C. Auditory and
somatosensory event-related potentials: II. Effects of inter-stimulus interval. _J. Psychophysiol._ 5, 27–42 (1991). Google Scholar * Nakajima, Y. & Imamura, N. Probability and
interstimulus interval effects on the N140 and the P300 components of somatosensory erps. _Int. J. Neurosci._ 104, 75–91 (2000). CAS PubMed Google Scholar * Polich, J. P300, probability,
and interstimulus interval. _Psychophysiology_ 27, 396–403 (1990). CAS PubMed Google Scholar * Polich, J. Probability and inter-stimulus interval effects on the P300 from auditory
stimuli. _Int. J. Psychophysiol._ 10, 163–170 (1990). CAS PubMed Google Scholar * Polich, J., Brock, T. & Geisler, M. W. P300 from auditory and somatosensory stimuli: Probability and
inter-stimulus interval. _Int. J. Psychophysiol._ 11, 219–223 (1991). CAS PubMed Google Scholar * Ritter, W., Vaughan, H. G. & Costa, L. D. Orienting and habituation to auditory
stimuli: A study of short term changes in average evoked responses. _Electroencephalogr. Clin. Neuro._ 25, 550–556 (1968). CAS Google Scholar * Nieuwenhuis, S., De Geus, E. J. &
Aston-Jones, G. The anatomical and functional relationship between the P3 and autonomic components of the orienting response: P3 and orienting response. _Psychophysiology_ 48, 162–175
(2011). PubMed PubMed Central Google Scholar * Barry, R. J., MacDonald, B., De Blasio, F. M. & Steiner, G. Z. Linking components of event-related potentials and autonomic measures of
the orienting reflex. _Int. J. Psychophysiol._ 89, 366–373 (2013). PubMed Google Scholar * Barry, R. J., Steiner, G. Z. & De Blasio, F. M. Reinstating the novelty P3. _Sci. Rep._ 6,
31200 (2016). ADS CAS PubMed PubMed Central Google Scholar * MacDonald, B., Barry, R. J. & Bonfield, R. C. Trials and intensity effects in single-trial ERP components and autonomic
responses in a dishabituation paradigm with very long ISIs. _Int. J. Psychophysiol._ 98, 394–412 (2015). PubMed Google Scholar * MacDonald, B. & Barry, R. J. Trial effects in
single-trial ERP components and autonomic responses at very long ISIs. _Int. J. Psychophysiol._ 92, 99–112 (2014). PubMed Google Scholar * MacDonald, B. & Barry, R. J. Significance and
novelty effects in single-trial ERP components and autonomic responses. _Int. J. Psychophysiol._ 117, 48–64 (2017). PubMed Google Scholar * Barry, R. J. _et al._ Stimulus intensity
effects and sequential processing in the passive auditory ERP. _Int. J. Psychophysiol._ 176, 149–163 (2022). PubMed Google Scholar * Coull, J. T. Neural correlates of attention and
arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. _Prog. Neurobiol._ 55, 343–361 (1998). CAS PubMed Google Scholar * Thevenot, C. &
Barrouillet, P. Are small additions solved by direct retrieval from memory or automated counting procedures? A rejoinder to Chen and Campbell (2018). _Psychon. Bull. Rev._ 27, 1416–1418
(2020). PubMed PubMed Central Google Scholar * Rivera, S. M., Reiss, A. L., Eckert, M. A. & Menon, V. Developmental changes in mental arithmetic: Evidence for increased functional
specialization in the left inferior parietal cortex. _Cereb. Cortex._ 15, 1779–1790 (2005). CAS PubMed Google Scholar * Verleger, R. Effects of relevance and response frequency on P3b
amplitudes: Review of findings and comparison of hypotheses about the process reflected by P3b. _Psychophysiol._ 57, e13542 (2020). Google Scholar * MacDonald, B. & Barry, R. J.
Integration of three investigations of novelty, intensity, and significance in dishabituation paradigms: A study of the phasic orienting reflex. _Int. J. Psychophysiol._ 147, 113–127 (2020).
PubMed Google Scholar * Croft, R. J. & Barry, R. J. EOG correction of blinks with saccade coeffcients: A test and revision of the aligned-artefact average solution. _Clin.
Neurophysiol._ 111, 444–451 (2000). CAS PubMed Google Scholar * Jasper, H. H. Report of the committee on methods of clinical examination in electroencephalography. _Electroen. Clin.
Neuro._ 10, 370–375 (1958). Google Scholar * Barry, R. J., Feldmann, S., Gordon, E., Cocker, K. I. & Rennie, C. Elicitation and habituation of the electrodermal orienting response in a
short interstimulus interval paradigm. _Int. J. Psychophysiol._ 15, 247–253 (1993). CAS PubMed Google Scholar * Barry, R. J. Scoring criteria for response latency and habituation in
electrodermal research: A study in the context of the orienting response. _Psychophysiol._ 27, 94–100 (1990). CAS Google Scholar * Barry, R. J., De Blasio, F. M., Fogarty, J. S. &
Karamacoska, D. ERP Go/NoGo condition effects are better detected with separate PCAs. _Int. J. Psychophysiol._ 106, 50–64 (2016). PubMed Google Scholar * Kayser, J. & Tenke, C. E.
Optimizing PCA methodology for ERP component identification and measurement: Theoretical rationale and empirical evaluation. _Clin. Neurophysiol._ 114, 2307–2325 (2003). PubMed Google
Scholar * Dien, J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. _J. Neurosci. Methods_ 187, 138–145 (2010). PubMed Google
Scholar * Ho, R. _Handbook of Univariate and Multivariate Data Analysis with IBM SPSS_ (Chapman and Hall/CRC, 2013). MATH Google Scholar * Tabachnick, B. & Fidell, L. _Using
Multivariate Statistics_ (Pearson Education, 2018). Google Scholar * Howell, D. _Statistical Methods for Psychology_ (Thompson Wadsworth, 1997). Google Scholar Download references AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Brain and Behaviour Research Institute and School of Psychology, University of Wollongong, Wollongong, Australia Robert J. Barry, Genevieve Z.
Steiner-Lim, Adele E. Cave, Frances M. De Blasio & Brett MacDonald * NICM Health Research Institute, Western Sydney University, Penrith, Australia Genevieve Z. Steiner-Lim Authors *
Robert J. Barry View author publications You can also search for this author inPubMed Google Scholar * Genevieve Z. Steiner-Lim View author publications You can also search for this author
inPubMed Google Scholar * Adele E. Cave View author publications You can also search for this author inPubMed Google Scholar * Frances M. De Blasio View author publications You can also
search for this author inPubMed Google Scholar * Brett MacDonald View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.B. and G.S.
conceptualised the study. G.S. collected the data, A.C. and F.D. processed data and prepared the figures; R.B. wrote the first manuscript draft. All authors contributed to revisions and
reviewed the final manuscript. CORRESPONDING AUTHOR Correspondence to Robert J. Barry. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit
line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,
you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Barry, R.J., Steiner-Lim, G.Z., Cave, A.E. _et al._ Effects of interstimulus interval and significance on electrodermal and central measures of the phasic orienting
reflex (OR) in a dishabituation task. _Sci Rep_ 13, 13546 (2023). https://doi.org/10.1038/s41598-023-40428-7 Download citation * Received: 01 April 2023 * Accepted: 10 August 2023 *
Published: 19 August 2023 * DOI: https://doi.org/10.1038/s41598-023-40428-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative