Play all audios:
ABSTRACT The study aimed to evaluate the lower limb skin temperature (Tsk) and blood concentrations of lactate (LA) and ammonia (NH3) during exercise and recovery. Eleven elite sprint
athletes (25 ± 3.4 yrs) and 11 elite endurance athletes (24.45 ± 5.4 yrs) performed an incremental running test until exhaustion. Body composition was estimated using the DXA method.
Thermograms of the anterior and posterior surfaces of the lower limbs were recorded at rest, before each test stage (every 3 min, starting from 10 km h−1 and increasing by 2 km h−1), and in
the 5th, 10th, 15th, 20th, and 30th minute of recovery. Endurance athletes had a higher maximum oxygen uptake than sprint athletes (5.0 ± 0.7 vs 4.3 ± 0.4 l·kg−1, _p_ = 0.018), lower
percentage of lean content (79 ± 2 vs 83 ± 2%, _p_ < 0.001), and a higher percentage of fat content in the lower limbs (17 ± 2 vs 12 ± 2%, _p_ < 0.001). In both groups, a significant
decrease in Tsk was observed compared to resting value (endurance athletes—31.5 ± 0.6 °C; sprint athletes—32.3 ± 0.6 °C), during exercise (_p_ < 0.001) and rewarming during recovery (_p_
< 0.001). However, endurance athletes had a lower Tsk than sprint athletes at the exhaustion point (30.0 ± 1.1 vs 31.6 ± 0.8 °C, _p_ < 0.05) and the pattern of change in Tsk differed
between groups (_p_ < 0.001). Tsk in the endurance athletes group decreased throughout the exercise protocol and returned more rapidly to initial values during recovery, while Tsk in the
sprint group stabilised between moderate intensity and exhaustion, recovering more slowly after exercise. Both LA (endurance athletes—max 10.2 ± 1.5; sprint athletes—max 10.1 ± 1.4 mmol⋅L−1,
_p_ < 0.001) and NH3 (endurance athletes—max 75.6 ± 11.5; sprint athletes—max 76.7 ± 9.0 mmol⋅L−1, _p_ < 0.001) increased during exercise and decreased during recovery (_p_ <
0.001). During exercise, lower levels and slower increases in LA were observed during exercise in the endurance athletes’ group (_p_ < 0.05). A negative correlation was revealed between
Tsk and fat percentage (r = −0.43 to −0.71, _p_ < 0.05). Tsk was positively correlated with LA during recovery (r = 0.43 to 0.48, _p_ < 0.05), and negatively during recovery (r = −0.45
to −0.54, _p_ < 0.05). Differences between groups in maximum aerobic capacity, the pattern of change in Tsk, and the correlation between Tsk and LA suggest that individuals who decrease
less Tsk during exercise and higher Tsk during recovery are those with better aerobic capacity. In addition, athletes with less body fat dissipate heat from their tissues more efficiently.
SIMILAR CONTENT BEING VIEWED BY OTHERS EFFECTS OF DIFFERENT INSPIRATORY MUSCLE WARM-UP LOADS ON MECHANICAL, PHYSIOLOGICAL AND MUSCLE OXYGENATION RESPONSES DURING HIGH-INTENSITY RUNNING AND
RECOVERY Article Open access 02 July 2022 THE LACTATE RESPONSE TO A SECOND BOUT OF EXERCISE IS NOT REDUCED IN A CONCURRENT LOWER-LIMB EXERCISE PROGRAM Article Open access 04 December 2023
ASSOCIATION BETWEEN PHYSICAL DEMANDS, SKIN TEMPERATURE AND WELLBEING STATUS IN ELITE FOOTBALL PLAYERS Article Open access 23 August 2023 INTRODUCTION Knowing the optimal physiological
parameters related to exercise intensity and recovery efficiency in highly trained athletes is of great interest to scientists and sports coaches. Lactate (LA) and ammonia (NH3) are commonly
used biomarkers that have been extensively studied during various types of exercise1,2,3,4. Lactate is formed when adenosine triphosphate (ATP) is generated under oxygen deficiency
conditions5. Increased LA concentration is an indicator of anaerobic muscle metabolism and impaired muscle function during exercise5. NH3 in the blood is a direct product of adenosine
monophosphate (AMP) degradation6 and can be used as an extracellular marker of ATP stores in skeletal muscle7. Therefore, the increases and the maximum concentrations of LA and NH3 in the
blood are considered good indicators to monitor the training process4,8,9. The levels of LA and NH3 during exercise and the speed of their use indicate the training status of untrained
individuals, and in the case of trained athletes, they can indicate the type of exercise performed8,9,10. One of the mechanisms strongly activated during exercise is thermoregulation
manifested by changes in skin temperature (Tsk). Depending on the type of physical effort, changes in Tsk may have different patterns11. For constant intensity endurance exercise, there is
an initial decrease in Tsk followed by a plateau phase12 or an immediate return to baseline or higher Tsk levels until the exercise is complete11. In the case of resistance exercise,
researchers observed an increase in Tsk in exercise areas11. Most research groups observed that during exercise of increasing intensity, Tsk gradually decreases and returns to baseline
values during the initial post-exercise recovery period13,14,15. In other cases, such as strength exercise, Tsk decreases or remains constant after exercise to increase during long-term
recovery16,17. It can be assumed that the Tsk response varies depending on the type of effort, but at the same time. Body composition is among the many factors that affect Tsk at rest and
during exercise. So far, a relationship between body mass index (BMI) and Tsk has been observed, that is, a lower BMI is associated with a higher Tsk and a higher BMI with a lower Tsk18. In
particular, the effects of body fat and fat-free mass have been studied, and it is now known that the lower the content of adipose tissue, the higher the Tsk19,20,21. Only a few studies have
been devoted to the relationship between fat tissue and Tsk during exercise22,23. It was shown that Tsk during exercise depends on body components, the proportions of which vary depending
on the sports discipline. To date, there has been a lack of studies to assess the relationship between body composition and Tsk during increased intensity exercise and post-exercise recovery
in athletes of different sports. Because exercise is accompanied by changes in blood lactate24, ammonia levels8, and Tsk16, these parameters could be related to each other. Previously,
several studies have been published on the correlation of Tsk with LA3,25,26, but not with NH3. Apart from the obvious co-occurrence of decreasing Tsk and increasing LA blood levels during
exercise and the opposite trend during recovery, there are no data on the relationship between these indicators during the increasing intensity of exercise. The aim of this study was to
determine the change in Tsk and blood concentrations of two exercise biomarkers (LA, NH3) and the correlations between them during graded exercise and recovery in athletes of different
sports. To our knowledge, this is the first study to monitor variations in Tsk and exercise biomarkers (LA, NH3) levels in blood at baseline, during graded exercise, and after exercise with
multiple samples in elite athletes who have different training backgrounds and metabolic responses. We hypothesise that (i) there are differences between sports in Tsk patterns and in the
blood concentration of exercise biomarkers, (ii) there is a correlation between Tsk and exercise biomarkers, and (iii) the level of Tsk depends on body composition. MATERIAL AND METHODS
EXPERIMENTAL APPROACH TO THE PROBLEM Elite athletes representing two distinct sports disciplines and related physiological adaptations (speed-power vs endurance) participated in the study.
The main procedures included repeated blood sampling and surface temperature measurements at rest, during the progressive exercise test, and during the 30-min recovery period. PARTICIPANTS
The study included 22 highly trained male athletes, all members of the Polish national teams. They were sprint athletes (n = 11, age range 21 to 31 years), practising competitive sport for
9.5 ± 2.6 years, and endurance athletes (n = 11, age range 15 to 34 years) represented by triathletes and long-distance runners, practising sport for 10.2 ± 2.9 years. Detailed
characteristics are shown in Table 1. The inclusion criteria was training professionally in a given discipline for at least the last 5 years and a current call-up to the national team.
Exclusion criteria included any lower extremity injury in the last month, the use of creams and ointments on the skin of the lower limbs, any vigorous physical exertion on the day of the
examination, the undergoing physical therapy, and any signs of health indisposition, especially affecting body temperature above 37.5 °C (measured before tests with a Geratherm
non-Contact—Germany pyrometer). The study was carried out according to relevant guidelines and regulations of the Declaration of Helsinki and was approved by the Ethical Committee of the
Poznan University of Medical Sciences. All participants received a detailed explanation about the objective, testing procedures, risks and benefits of the research, and gave their written
consent before the examination. PROCEDURE All tests were carried out at the Human Movement Laboratory of Poznan University of Physical Education. The laboratory room was air-conditioned and
temperature and humidity (21.0 ± 1.0 °C and 60 ± 5%, respectively) were monitored using a thermohygrometer (Onset HOBO Temp/RH 2.5% Data Logger, UX100-011—USA). Athletes arrived at a
laboratory in the postabsorptive state, fasting for at least 12 h. The first measurement was a whole-body scan to assess body composition. Subsequently, the athletes could have a light meal
consisting of a sandwich and up to 0.5 L of water, without coffee, tea, energy drinks, or ergogenic supplements. Then, the participants performed an incremental treadmill test until
exhaustion accompanied by blood sampling and measurement of Tsk of the lower limbs. ANTHROPOMETRIC AND PHYSIOLOGICAL VARIABLES On the day of the experiment, weight and height were measured
using a digital stadiometer (SECA 285, SECA, Hamburg, Germany). Dual X-ray absorptiometry method, using the Lunar Prodigy Pro DXA device (GE Healthcare, Madison, WI, USA) and enCORE v. 16
SP1 software was used for body composition analysis. The device was calibrated using a phantom according to the manufacturer's guidelines. The athletes were tested in their underwear,
without any metal objects or jewellery. All DXA scans were performed and analysed by the same trained technician according to the manufacturer's protocols. Skeletal muscle mass (SMM)
was calculated on regression models according to Kim et al.27. EXERCISE TEST AND RESPIRATORY PARAMETERS A progressive exercise treadmill test (H/P Cosmos Pulsar, Sports & Medical,
Nussdorf-Traunstein, Germany) was used to track a wide range of intensity of exercise28. After 3 min of standing on the treadmill, the participants walked at a speed of 4 km h−1 for the
first 3 min, then it was increased to 8 km/h−1. After that point, the treadmill speed increased by 2 km h−1 every 3 min until volitional exhaustion. Blood samples were taken at the end of
each 3-min stage, starting from a speed of 10 km h−1. Respiratory parameters were measured with an ergospirometer (Cortex Metamax 3B R2, Leipzig, Germany) and analysed using Metasoft Studio
v. 5.1.0 Software (Cortex-Metamax 3B R2; Cortex Biophysik, Leipzig, Germany). The Polar Bluetooth Smart HR monitor H6 (Polar Electro Oy, Kempele, Finland) was used to monitor heart rate
(HR). Maximal oxygen uptake (VO2max) was considered achieved if at least three of the following criteria were met: (i) a plateau in V̇O2 despite an increase in speed and minute ventilation;
(ii) blood lactate concentration ≥ 9 mmol⋅L−1; (iii) respiratory exchange ratio ≥ 1.10; and (iv) heart rate above 95% maximum heart rate predicted by age29. All participants were familiar
with the exercise protocol as they had previously done. THERMOGRAPHIC MEASUREMENT The athletes were wearing only light sportswear that exposed their lower limbs. It was forbidden to use
ointments, creams, or lotions 24 h before the test. The uncooled FLIR SC640 IR camera (FLIR Systems Inc., SC640 model, Sweden) with noise-equivalent temperature difference (NETD) < 30 mK,
640 × 480 pixels of resolution and a temperature accuracy of ± 2% was used to record the thermograms. The camera was positioned on a tripod 1 m above the ground and 2 m from the
participant. The TISEM checklist has been used to ensure the reliability of thermal imaging research and analysis30. Approximately 20 min before the start of the test, the participants were
acclimatised in the laboratory room in a sitting position so that the lower limbs did not touch each other and had minimal contact with the seat. Infrared images of the anterior and
posterior surfaces of the lower limbs were taken at rest, at the end of each 3-min stage above the speed of 10 km·h−1, immediately after exercise and 5, 10, 15, 20, and 30 min into the
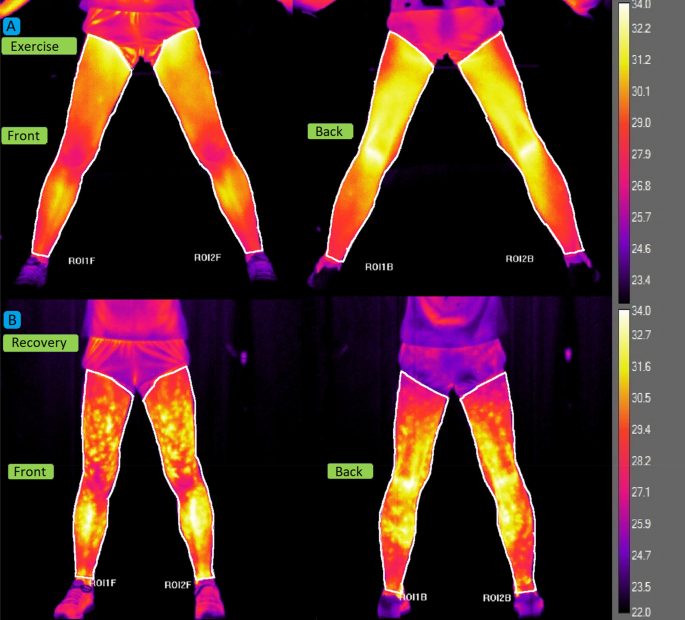
recovery period. For the temperature analysis, regions of interest (ROI) were established (Fig. 1). The mean and standard deviation of the surface Tsk of the front and back sides of the
lower limbs were calculated using dedicated software (Thermacam Researcher Pro 2.10 software, FLIR, Wilsonville, Oregon, USA). The front ROI covered the area between the inguinal ligament
and the talocrural region. The ROI on the back of the lower limbs covered the area between the gluteus sulcus and the talocrural region. BLOOD SAMPLING A catheter (BD Venflon Pro 1.3 3 32
mm; Becton Dickinson, Helsingborg, Sweden) was inserted into the antecubital vein with isotonic saline (0.9% NaCl). Blood samples were collected at the same time as the infrared images were
taken. Later, a 2.7 ml blood sample was taken in 2 monovettes (S-Monovette 2.7 ml KE; Sarstedt, Nümbrecht, Germany), one with a lithium anticoagulant (heparin) and another containing an
anticoagulant (EDTA). After each sampling, the catheter was flushed with isotonic saline (0.9% NaCl) after each sampling to maintain its patency. LACTATE AND AMMONIA The Biosen C-line device
(EKF diagnostic GmbH, Barleben, Germany) was used to measure the LA concentration. For this procedure, 20 ml of whole blood was placed in a capillary. Measurement precision (CV) was 1.5%
for a concentration of 12 mmol·l−1. The PocketChem BA PA-4140 device (Arkay, Kyoto, Japan) with a measuring range of 8‒285 mmol l−1 and CV of 2.3.% was used to determine the level of NH3.
Using a pipette, 20 ml of blood was applied to the test strip (Ammonia Test Kit II; Arkay), which was then placed in the device. STATISTICAL ANALYSIS A one-way analysis of variance (ANOVA)
was used to calculate the differences in descriptive variables. Two-way repeated measures ANOVA was performed to test the main effects of the test stage and group and their interactions for
the response of Tsk, LA, and NH3 to exercise. The sample size was estimated based on the assumption that the effect size will be at least medium. Using an α-level of 0.05, a power (1-β) of
0.8, and _η_2 = 0.14 (large), it was calculated that in total at least 16 participants are required to detect a significant change or differences in temperature, LA, and NH3 concentration
(G*Power; Heinrich-Heine-Universitat Dusseldorf, Dusseldorf, Germany). The Mauchly’s sphericity test was used and the Greenhouse–Geisser correction was applied if the assumption of
sphericity was violated. The effect size for the ANOVA was interpreted as small (0.01), medium (0.06), or large (0.14). If a significant main effect or interaction was found, post hoc
Bonferroni tests were performed. Pearson’s correlation coefficients (r) were used to describe the relationship between biomarker concentrations and Tsk in the combined group of athletes at
each stage of exercise and in recovery, and were defined as small (0.1), medium (0.3), or large (0.5). The significance level for all statistical analyses was established at _p_ < 0.05.
All values were presented as mean ± standard deviation. The calculations were made using the STATISTICA 12.0 software package (Stat-Soft, Tulsa, OK, USA). ETHICAL APPROVAL Research was
approved by Ethical Committee at the Poznan University of Medical Sciences. CONSENT TO PARTICIPATE Written informed consent was obtained from all participants. RESULTS SUBJECTS DESCRIPTION
The study groups differed from each other in several parameters. Sprint athletes had a significantly higher SMM and less leg fat mass compared to the endurance athletes group. Differences
also included higher V̇O2max in endurance athletes, expressed as both absolute (l·kg−1) and relative (ml·kg−1·min−1 and ml·kg SMM−1·min−1) values. There were no significant differences
between the groups with respect to the remaining parameters. All detailed baseline anthropometric and physical characteristics are presented in Table 1. Figures 2A,B show a full series of
thermograms of an athlete from the endurance athletes’ group to better visualise the Tsk response throughout the test protocol. The course of changes in mean levels of Tsk, LA, and NH3
levels during the incremental exercise test and 30-min recovery is shown in Fig. 1. In the case of Tsk (Fig. 3A), there was a significant main effect for the test stages (_p_ < 0.001, η2
= 0.69). The resting temperature was 31.5 ± 0.6 °C for endurance athletes and 32.3 ± 0.6 °C for sprint athletes. In all participants, a decrease in Tsk was observed at the beginning of the
exercise. The first significant decrease in Tsk occurred at the speed of 12 km h−1 (_p_ < 0.001). When exhausted, the endurance athletes reached 30.0 ± 1.1 °C and the sprint athletes
reached 31.6 ± 0.8 °C. Once the exercise was over (exhaustion), the Tsk began to rise, most rapidly in the first 5 min of recovery, until the end of the observation in the 30th minute after
exercise. A statistically significant group effect was also noted (_p_ < 0.05, η2 = 0.22), i.e. the sprint group was characterised by higher Tsk levels than endurance athletes throughout
the test, with a significant difference at the end of the exercise (_p_ < 0.05). There was a statistically significant effect of the interaction group*test stage (_p_ < 0.001, η2 =
0.13). Although Tsk in the endurance group decreased throughout the whole exercise protocol, Tsk in the sprint group stabilized between 12 km h−1 and exhaustion. All endurance athletes
completed the test at the 20 km h−1 stage, ten sprint athletes completed the 18 km h−1 stage, and one the 20 km h−1 stage. In the case of LA (Fig. 3B), there was a significant main effect
for the test stages (_p_ < 0.001, η2 = 0.91). In both groups, the LA levels continuously increased from the beginning of the exercise. The first significant increase in LA occurred at the
speed of 12 km h−1 in the sprint group and at 16 km h−1 (_p_ < 0.001) in the endurance group. LA increased until the end of the test. From then on, it decreased significantly until the
end of the observation in both groups. A statistically significant group effect was noted (_p_ < 0.05, η2 = 0.23), that is, sprinters were characterised by a higher LA level than
endurance athletes in the entire range of intensity of exercise, especially at the speed of 16 and 18 km h−1 (_p_ < 0.05). There was also a statistically significant interaction between
groups*test stages interaction (_p_ < 0.001, η2 = 0.35). In the sprint group, the increase in LA during exercise was almost linear, while endurance athletes exhibited a curvilinear
relationship between lactate and exercise intensity with a slower increase at low intensity. In the case of NH3 (Fig. 3C), there was a significant main effect for the test stages (_p_ <
0.001, η2 = 0.94). In both groups, the NH3 level continuously increased from the beginning of exercise. The first significant increase in NH3 occurred at the speed of 10 km h−1 in the sprint
group and at 12 km h−1 in the endurance group. The NH3 concentration increased until the end of the test. From then on, the NH3 level decreased until the end of the observation in both
groups. There was no group effect (_p_ = 0.279, η2 = 0.06). There was a statistically significant group*test stage interaction (_p_ < 0.05, η2 = 0.13): the increase in NH3 concentration
in the last phase of the test was slower in the endurance group. There were positive moderate correlations between Tsk and LA for the speed range of 10‒16 km h−1 (r = 0.43‒0.48, _p_ <
0.05) and negative Tsk-LA correlations between the 10th and 30th minute of recovery (r ranging from −0.54 to −0.45, _p_ < 0.05). There were no significant correlations between Tsk and NH3
and between LA and NH3 at all stages of the test. Significant positive correlations (r = 0.46–0.74, _p_ < 0.05) occurred between Tsk and the percentage content of lean mass in all test
stages. A significant negative correlation (r ranging from −0.71 to −0.43, _p_ < 0.05) also occurred between Tsk and the percentage of fat content at all stages of the test. The
correlation between Tsk and SMM (r = 0.44, _p_ < 0.05) occurred only in the first measurement, before exercise. DISCUSSION To our knowledge, this is the first study to track changes in
Tsk and exercise biomarkers (LA, NH3) concentration in blood at rest, during incremental exercise, and during the recovery period with many sampling points in highly trained athletes
representing opposite training profiles and resulting metabolic adaptations. Our main findings were that the pattern of change in Tsk and LA concentration levels depends on the
sports-related training profile. The endurance athletes had a lower Tsk observed at the end of the exercise and a slower increase in LA levels during exercise than the sprint athletes. It
was also observed that, regardless of the training profile, a higher Tsk corresponded to a higher LA concentration during exercise. On the contrary, during recovery, a higher Tsk
corresponded to a lower LA concentration. No significant relationship of Tsk with NH3 was observed. Furthermore, Tsk was positively correlated with lean mass and inversely correlated with
fat content. In our study, we observed a significant decrease in Tsk during exercise and rapid rewarming during recovery. This pattern was also observed in other studies. Tanda14 indicated
that long-distance runners showed a decrease in Tsk in the initial phase of running effort, regardless of the type of work performed (in the field or on a treadmill) and environmental
conditions (outdoor or indoor). The Tsk drop was more pronounced until the end of the test with graded load (from 6 to 13.5 km h−1) than with constant load (12 km h−1). In the study by
Oliveira et al.31, a decrease in Tsk was also observed in distant areas of the body. Participants performed an incremental test on the upper limb ergometer; however, Tsk decreased during
exercise in the lower limbs and trunk to return to initial values during recovery. This was confirmed by Hillen et al.11 in a review in which they noted that all studies on endurance and
incremental exercise reported a measurable decrease in Tsk. During constant-intensity endurance exercise, Tsk drops initially and stabilises after a few minutes. Additionally, Fernandes Ade
et al.32 observed a decrease in Tsk of distant areas during aerobic exercise at an intensity of 60% V̇O2max, followed by rewarming during recovery. The gradual increase in exercise intensity
causes a further decrease in Tsk. The difference in Tsk change between constant-intensity and incremental exercise reveals the dependence of Tsk on exercise intensity. Other studies based
on high-intensity or incremental exercise, analysing cycling15, strength training17, or rowing33, confirm a similar course of Tsk. The plausible mechanism for the decrease in Tsk during
incremental exercise involves sympathetic noradrenergic nerve activity and its effects on cutaneous arterial vasoconstriction34. The sympathetic noradrenergic vasoconstrictor nerve,
controlled by the rostral medulla oblongata and medulla oblongata preoptic area, initiates the release of norepinephrine and neuropeptide Y, which activate the α-adrenergic receptors of
smooth muscles of the vascular skin35. This leads to contraction of the cutaneous blood vessels and to the consequent redistribution of blood volume to activated organs36, for example
skeletal muscles37. If the temperature of the deep tissue in the exercising or recovering muscle exceeds a certain level, cholinergic nerve transmission activates the nonadrenergic
vasodilator system38. It leads to active vasodilation of skin vessels for thermoregulation34,38. It seems that changes in Tsk during exercise and at rest are more pronounced and faster in
trained people than in untrained individuals and may indicate the training status39. In our study, there was a difference in the evolution of Tsk between the groups with significantly lower
temperature in endurance athletes at the end of the exercise. Study participants were highly trained and, therefore, similar in terms of training status but different in terms of specific
physiological characteristics and adaptations. We suppose that the differences in Tsk levels and the course obtained in our study are due to these adaptations, including the amount of SMM or
body fat, which is explained later. Of course, a better adaptation to exercise in the endurance athletes group is a more likely cause, for example, in terms of sweating. Especially, from 12
km h−1 we did not observe a clear drop in Tsk in the sprint group compared to the endurance athletes group. Smith and Havenith40 indicated that regional sweat rates do not correlate with
Tsk. Therefore, it cannot be ruled out that measurements of the whole-body surface temperature would give a different picture of the relationships. It is also worth paying attention to the
role of perforator vessels whose operation in the thermoregulation process is visible in thermography as hot spots. Brito et al.41 observed that if the hot spots covered more than 70% of the
body area after physical exercise, this was associated with a lower Tsk. The analysis of hot spots was not the aim of this manuscript, but we think that it is worth considering it in future
studies to better understand thermoregulatory processes. The relationship between body composition (especially body fat) and Tsk has been described in previous studies that are consistent
with our results. Chudecka et al.19 used the bioimpedance technique to assess the effect of adipose tissue on Tsk in adult women. They compared 20 obese women (37.8 ± 2.3% of body fat) with
20 non-obese women (25.7 ± 2.4% of body fat). Women with obesity had lower Tsk values (_p_ < 0.05) of Tsk in the arms, thighs, calves, abdomen and lower ribs. Similarly, Reis et al.42
studied 50 obese (31.5 ± 4.3% of body fat in the lower limbs) and 50 non-obese men (12.73 ± 3.4% of body fat in the lower limbs) using the DXA method and showed a significantly higher Tsk
(_p_ < 0.001) in non-obese men. In our study, we did not find any difference in resting Tsk between the groups. This can be explained by the high training status of the participants,
which results, among other things, in a unique body composition characterised by high lean mass and low fat tissue compared to the general population in both groups. However, when analysing
the combined group of our athletes, we showed a positive correlation between Tsk and lean mass (%) and a negative correlation with leg fat mass (%). It is consistent with other studies. In
the study by Neves et al. 20 on Tsk in 47 men and 47 women, they observed that the percentage of body fat was negatively correlated with the Tsk of the anterior (r = 0.57, _p_ < 0.05) and
posterior surface of the lower limbs (r = 0.63, _p_ < 0.05) in men and anterior (r = 0.36, _p_ < 0.05) and posterior surface of the lower limbs (r = 0.40, _p_ < 0.05) in women,
respectively. Similarly, Salamunes et al.21 observed a negative correlation between the amount of fat tissue and Tsk in the anterior and posterior areas of the lower limbs (r = 0.38 and r =
0.49, _p_ < 0.001, respectively), in 123 women. In the case of SMM, apart from the initial measurement, we did not show any correlation with Tsk. This may indicate that, in highly trained
athletes, SMM does not seem to be a useful parameter in the context of exercise thermoregulation. Lean mass and fat mass provide better diagnostic possibilities. Although the relationship
between BMI and Tsk has already been observed18,19, so far we have not found studies that would indicate permanent correlations between Tsk and body composition during exercise and
post-exercise recovery. As expected from previous studies1,2,3, the decrease in Tsk during exercise was accompanied by an increase in LA and vice versa during recovery. Studies showing the
relationship between NH3 and Tsk during exercise are not available. In the context of changes in Tsk and LA levels during exercise, only a few studies can be indicated. Akimov and Son’Kin2
tested 20 athletes performing incremental exercise on the bicycle ergometer and showed a decrease in Tsk with a concomitant increase in LA. However, Tsk was measured on the forehead.
Similarly, Adamczyk et al.3 studied the relationship between lower limbs temperature and LA in sixteen untrained adults. They monitored parameters only at rest and during a 30-min recovery
(9 measurements) after a one-minute bout of vertical jumps. Those authors observed that the mean Tsk of the lower limbs and the blood LA concentration were weakly negatively correlated (r =
−0.29, _p_ < 0.05). Unfortunately, this correlation was achieved only by combining all 160 measurements for analysis. Similarly to our results, Temfemo et al. 25 showed a significant
positive correlation (r = 0.69, _p_ < 0.001) between Tsk and LA during exercise tests. They tested 18 regional-level soccer players who performed repeated 6-s sprints with increasing load
on a cycle ergometer, interspersed with 5-min recovery periods. Their results showed an increase in Tsk with subsequent stages of the test. However, blood sampling for LA took place only 4
min after each sprint, and the highest Tsk value obtained during the 5-min rest between sprints was used. Therefore, it is difficult to determine whether the change in Tsk was a direct
effect of exercise or rather post-exercise recovery. Previous studies indicate a decrease, not an increase, in Tsk during incremental exercise11. In our study, due to parallel measurements
of Tsk and LA, we observed that during incremental exercise, a lower Tsk was associated with lower LA levels. Previous studies39,43 indicated that trained individuals respond to exercise
with a faster and more pronounced change in Tsk than untrained individuals. Therefore, it can be assumed that a more pronounced decrease in Tsk during exercise may indicate a more efficient
redistribution of blood to the working muscles, which coincides with a slower increase in LA. This could be confirmed by the research by Moreira et al.26, in which judokas were assessed
during incremental exercise. The authors revealed that the greater the decrease in Tsk in the fifth (r = 0.66, _p_ = 0.001) and the 10th (r = 0.55, _p_ = 0.001) minutes after exercise, the
lower the level of LA after exercise. However, one should consider the specificity of the judo test with alternating 2-min exercise bouts and 1-min rest intervals. Unfortunately, Moreira et
al.26 did not measure Tsk and LA during exercise, and the first post-exercise measurement was made 5 min after the end of the test. In particular, those authors correlated LA with total body
Tsk, with the latter significantly decreasing compared to pre-exercise measurement. In our research, we observed that from the 10th minute of recovery, higher Tsk levels were correlated
with lower LA levels. This could support the observation that a more efficient post-exercise blood redistribution from muscles to skin is associated with faster decreases in LA levels.
However, it is not known whether these phenomena are directly related to each other by any physiological or metabolic mechanisms. Of course, we cannot exclude the effect of sweating on the
Tsk regulation, which may vary between individuals40. Future studies should take into account the role of sweating when interpreting Tsk changes. To sum up, it can be assumed that people
with greater or specific exercise capacity show better redistribution of blood between the skin and muscles and/or a more efficient sweating mechanism during exercise and post-exercise
recovery. This is manifested by a greater reduction in Tsk during exercise associated with strong blood transfer to the working muscles11 with a co-occurring lower LA concentration. In the
case of post-exercise recovery, apart from the impact of sweating, a higher Tsk may be associated with strong blood transfer from the skeletal muscles to the skin, which may indicate high
blood perfusion in tissues for thermoregulation. The co-occurrence of low LA levels and Tsk in individuals with high exercise capacity suggests that it is possible to estimate training
status based on the course of changes in Tsk. Since the methodology we used does not allow us to explain the cause-and-effect relationship of the co-occurrence of parallel changes in Tsk and
LA levels, further research is needed to explore this phenomenon. LIMITATIONS Among the limitations of the study, we highlight that we have not evaluated the core temperature and the impact
of sweating on thermoregulation processes. Furthermore, the conclusions of our research apply only to highly trained athletes. CONCLUSIONS During incremental exercise to exhaustion, a
significant decrease in Tsk of the lower limbs is observed, followed by rewarming during recovery. The pattern of change in Tsk is related to the training profile. In endurance athletes, Tsk
is lower throughout the range of exercise intensity, especially at exhaustion and during post-exercise recovery, while in sprint athletes, Tsk stabilizes between moderate intensity and
exhaustion, rising more slowly after exercise. There is a positive correlation between Tsk and blood lactate during exercise at different intensities and the relationship is reversed during
recovery. Furthermore, Tsk depends on body composition, i.e., people with higher lean body mass and lower body fat have higher Tsk during both exercise and recovery, suggesting a more
efficient heat dissipation. Our study suggests that lower Tsk during exercise, higher Tsk during recovery, higher lean body mass, and lower body fat are associated with higher aerobic
capacity in highly trained athletes. DATA AVAILABILITY The datasets generated during and/or analysed during the current study are not publicly available due to use in further analysis, but
are available from the corresponding author on reasonable request. REFERENCES * Graham, T. E., Bangsbo, J., Gollnick, P. D., Juel, C. & Saltin, B. Ammonia metabolism during intense
dynamic exercise and recovery in humans. _Am. J. Physiol. Endocrinol. Metab._ 259(2), E170–E176 (1990). Article CAS Google Scholar * Akimov, E.B., Son’kin, V.D. Skin temperature and
lactate threshold during muscle work in athletes. _Hum. Physiol._ 37, 621–628 (2011). * Adamczyk, J. G., Boguszewski, D. & Siewierski, M. Thermographic evaluation of lactate level in
capillary blood during post-exercise recovery. _Kinesiology._ 46(2), 186–193 (2014). Google Scholar * Włodarczyk, M., Kusy, K., Słomińska, E., Krasiński, Z. & Zieliński, J. Change in
Lactate, Ammonia, and Hypoxanthine Concentrations in a 1-Year Training Cycle in Highly Trained Athletes: Applying Biomarkers as Tools to Assess Training Status. _J. Strength Cond. Res._
34(2), 355–364 (2020). Article PubMed Google Scholar * Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. _BMC Musculoskelet. Disord._ 13, 218.
https://doi.org/10.1186/1471-2474-13-218 (2012). * Zieliński, J. & Kusy, K. Hypoxanthine: A Universal Metabolic Indicator of Training Status in Competitive Sports. _Exerc. Sport Sci.
Rev._ 43(4), 214–221 (2015). Article PubMed Google Scholar * Gorostiaga, E. M. _et al._ Vertical jump performance and blood ammonia and lactate levels during typical training sessions in
elite 400-m runners. _J. Strength Cond. Res._ 24(4), 1138–1149 (2010). Article PubMed Google Scholar * Kantanista, A. _et al._ Combined Analysis of Blood Ammonia and Lactate Levels as a
Practical Tool to Assess the Metabolic Response to Training Sessions in Male and Female Sprinters. _J. Strength Cond. Res._ 35(9), 2591–2598 (2021). Article PubMed Google Scholar * Yuan,
Y., So, R., Wong, S. & Chan, K. M. Ammonia threshold–comparison to lactate threshold, correlation to other physiological parameters and response to training. _Scand. J. Med. Sci.
Sports._ 12(6), 358–364 (2002). Article CAS PubMed Google Scholar * Messonnier, L., Freund, H., Bourdin, M., Belli, A. & Lacour, J. R. Lactate exchange and removal abilities in
rowing performance. _Med. Sci. Sports Exerc._ 29(3), 396–401 (1997). Article CAS PubMed Google Scholar * Hillen, B., Pfirrmann, D., Nägele, M. & Simon, P. Infrared Thermography in
Exercise Physiology: The Dawning of Exercise Radiomics. _Sports Med._ 50(2), 263–282 (2020). Article PubMed Google Scholar * Korman, P., Straburzyńska-Lupa, A., Kusy, K., Kantanista, A.
& Zieliński, J. Changes in body surface temperature during speed endurance work-out in highly-trained male sprinters. _Infrared Phys. Technol._ 78, 209–213 (2016). Article ADS Google
Scholar * Balci, G. A., Basaran, T. & Colakoglu, M. Analysing visual pattern of skin temperature during submaximal and maximal exercises. _Infrared Phys. Technol._ 74, 57–62 (2016).
Article ADS Google Scholar * Tanda, G. Skin temperature measurements by infrared thermography during running exercise. _Exp. Therm. Fluid Sci._ 71, 103–113 (2016). Article Google Scholar
* Trecroci, A. et al. Bilateral asymmetry of skin temperature is not related to bilateral asymmetry of crank torque during an incremental cycling exercise to exhaustion. _PeerJ._ 6, e4438
(2018). * Fernández-Cuevas, I. _et al._ Monitoring skin thermal response to training with infrared thermography. _New Stud. Athl._ 29(1), 57–71 (2014). Google Scholar * Fernández-Cuevas,
I., Torres, G., Sillero-Quintana, M. & Navandar, A. Thermographic assessment of skin response to strength training in young participants. _J. Therm. Anal. Calorim._ 148, 3407–3415
(2023). Article Google Scholar * Reis, H.H.T. et al. Can the body mass index influence the skin temperature of adolescents assessed by infrared thermography? _J. Therm. Biol_. 111, 103424
https://doi.org/10.1016/j.jtherbio.2022.103424 (2023). * Chudecka, M., Lubkowska, A., Kempińska-Podhorodecka, A. Body surface temperature distribution in relation to body composition in
obese women. _J. Therm. Biol_.: 43, 1–6 https://doi.org/10.1016/j.jtherbio.2014.03.001 (2014). * Neves, E.B., Salamunes, A.C.C., de Oliveira, R.M., Stadnik, A.M.W. Effect of body fat and
gender on body temperature distribution. _J. Therm. Biol_. 70,1–8 (2017). * Salamunes, A.C.C., Stadnik, A.M.W., Neves, E.B. The effect of body fat percentage and body fat distribution on
skin surface temperature with infrared thermography. _J. Therm. Biol_. 66, 1–9. https://doi.org/10.1016/j.jtherbio.2017.03.006 (2017). * Neves, E. B. _et al._ The influence of subcutaneous
fat in the skin temperature variation rate during exercise. _Res. Biomed. Eng._ 31, 307–312 (2015). Article Google Scholar * Galan-Carracedo, J., Suarez-Segade, A., Guerra-Balic, M.,
Oviedo, G.R. The Dynamic and Correlation of Skin Temperature and Cardiorespiratory Fitness in Male Endurance Runners. _Int. J. Environ. Res. Public Health_. 11, 2869 (2019). * Beneke, R.,
Leithäuser, R. M. & Ochentel, O. Blood lactate diagnostics in exercise testing and training. _Int. J. Sports Physiol. Perform._ 6(1), 8–24 (2011). Article PubMed Google Scholar *
Temfemo, A., Carling, C. & Ahmaidi, S. Relationship between power output, lactate, skin temperature, and muscle activity during brief repeated exercises with increasing intensity. _J.
Strength Cond. Res._ 25(4), 915–921 (2011). Article PubMed Google Scholar * Gomes Moreira, D. et al. Lactate Concentration Is Related to Skin Temperature Variation After a Specific
Incremental Judo Test. _J. Strength Cond. Res_. 35(8), 2213–2221 (2021). * Kim, J., Wang, Z., Heymsfield, S. B., Baumgartner, R. N. & Gallagher, D. Total-body skeletal muscle mass:
estimation by a new dual-energy X-ray absorptiometry method. _Am. J. Clin. Nutr._ 76(2), 378–383 (2002). Article CAS PubMed Google Scholar * Lamb, K. L., Eston, R. G. & Corns, D.
Reliability of ratings of perceived exertion during progressive treadmill exercise. _Br. J. Sports Med._ 33(5), 336–339 (1999). Article CAS PubMed PubMed Central Google Scholar *
Edvardsen, E., Hem, E., Anderssen, S.A. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: a cross-sectional study. _PLoS One_. 9(1), e85276.
https://doi.org/10.1371/journal.pone.0085276 (2014). * Moreira, D.G. et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement
of human skin temperature. _J. Therm. Biol_. 69, 155–162. https://doi.org/10.1016/j.jtherbio.2017.07.006 (2017). * Oliveira, S.Â.F. et al. Measuring of skin temperature via infrared
thermography after an upper body progressive aerobic exercise_. J. Phys. Educ. Sport ._ 18(1) (2018). * Fernandes Ade, A., Amorim, P.R., Brito, C.J., Sillero-Quintana, M., Bouzas Marins,
J.C. Regional Skin Temperature Response to Moderate Aerobic Exercise Measured by Infrared Thermography. _Asian J. Sports Med_. 7(1). e29243 (2016). * Straburzyńska-Lupa, A., Korman, P.,
Śliwicka, E., Kryściak, J., Ogurkowska, M.B. The use of thermal imaging for monitoring the training progress of professional male sweep rowers. _Sci. Rep_. 12(1), 16507.
https://doi.org/10.1038/s41598-022-20848-7 (2022). * Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. _J. Appl. Physiol._
109(4), 1221–1228 (1985). Article Google Scholar * Ootsuka, Y. & Tanaka, M. Control of cutaneous blood flow by central nervous system. _Temperature (Austin)._ 2(3), 392–405 (2015).
Article PubMed PubMed Central Google Scholar * Williamson, J. W. Autonomic responses to exercise: where is central command?. _Auton. Neurosci._ 188, 3–4 (2015). Article CAS PubMed
Google Scholar * Just, T. P., Cooper, I. R. & DeLorey, D. S. Sympathetic Vasoconstriction in Skeletal Muscle: Adaptations to Exercise Training. _Exerc. Sport Sci. Rev._ 44(4), 137–143
(2016). Article PubMed Google Scholar * Demachi, K., Yoshida, T., Kume, M., Tsuji, M. & Tsuneoka, H. The influence of internal and skin temperatures on active cutaneous vasodilation
under different levels of exercise and ambient temperatures in humans. _Int. J. Biometeorol._ 57(4), 589–596 (2013). Article PubMed ADS Google Scholar * Formenti, D. _et al._ Thermal
imaging of exercise-associated skin temperature changes in trained and untrained female subjects. _Ann. Biomed. Eng._ 41(4), 863–871 (2013). Article PubMed Google Scholar * Smith, C. J.
& Havenith, G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia. _Eur. J. Appl. Physiol._ 111(7), 1391–1404 (2011). Article PubMed Google
Scholar * Brito, C. J. _et al._ Immune Response Related With Skin Thermal Pattern in Judokas: A New Application for Infrared Thermography?. _J. Strength. Cond. Res._ 34(10), 2886–2894
(2020). Article PubMed Google Scholar * Reis, H.H.T. et al. Can Adipose Tissue Influence the Evaluation of Thermographic Images in Adolescents? _Int. J. Environ. Res. Public Health._
20(5). 4405. https://doi.org/10.3390/ijerph20054405 (2023). * Abate, M., Di Carlo, L., Di Donato, L., Romani, G. L. & Merla, A. Comparison of cutaneous termic response to a standardised
warm up in trained and untrained individuals. _J. Sports Med. Phys. Fitness._ 53(2), 209–215 (2013). CAS PubMed Google Scholar Download references FUNDING This work was funded by the
Polish Ministry of Science and Higher Education from financial resources of the National Science Center within the OPUS 5 program (application and Grant Number: 2013/09/B/NZ7/02556). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Physical Therapy and Sports Recovery, Faculty of Health Sciences, Poznan University of Physical Education, 61-871, Poznań, Poland Paweł
Korman & Anna Straburzyńska-Lupa * Department of Athletics, Strength and Conditioning, Faculty of Sport Sciences, Poznan University of Physical Education, 61-871, Poznań, Poland
Krzysztof Kusy & Jacek Zieliński * Department of Physical Education and Lifelong Sports, Faculty of Sport Sciences, Poznan University of Physical Education, 61-871, Poznań, Poland Adam
Kantanista * Faculty of Physical Activity and Sports Sciences (INEF), Universidad Politécnica de Madrid, 28040, Madrid, Spain Manuel Sillero Quintana Authors * Paweł Korman View author
publications You can also search for this author inPubMed Google Scholar * Krzysztof Kusy View author publications You can also search for this author inPubMed Google Scholar * Anna
Straburzyńska-Lupa View author publications You can also search for this author inPubMed Google Scholar * Adam Kantanista View author publications You can also search for this author
inPubMed Google Scholar * Manuel Sillero Quintana View author publications You can also search for this author inPubMed Google Scholar * Jacek Zieliński View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization, P.K. and K.K.; methodology P.K, A.S-L. and K.K.; software, not applicable; validation, P.K., J.Z. and K.K.;
formal analysis, P.K.; investigation, P.K., A.S-L. and K.K.; resources, P.K., K.K. and J.Z.; data curation, P.K..; writing—original draft preparation, P.K.; writing—review and editing, K.K.,
A.S-L and M.SQ..; visualization, A.K.; supervision, K.K. and M.SQ; project administration, J.Z..; funding acquisition, J.Z. All authors have read and agreed to the published version of the
manuscript. CORRESPONDING AUTHOR Correspondence to Paweł Korman. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S
NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Korman, P., Kusy, K., Straburzyńska-Lupa, A. _et al._ Response
of skin temperature, blood ammonia and lactate during incremental exercise until exhaustion in elite athletes. _Sci Rep_ 14, 2237 (2024). https://doi.org/10.1038/s41598-024-52374-z Download
citation * Received: 28 August 2023 * Accepted: 17 January 2024 * Published: 26 January 2024 * DOI: https://doi.org/10.1038/s41598-024-52374-z SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative