Play all audios:
ABSTRACT Trace elements are important for human health but may exert toxic or adverse effects. Mechanisms of uptake, distribution, metabolism, and excretion are partly under genetic control
but have not yet been extensively mapped. Here we report a comprehensive multi-element genome-wide association study of 57 essential and non-essential trace elements. We perform genome-wide
association meta-analyses of 14 trace elements in up to 6564 Scandinavian whole blood samples, and genome-wide association studies of 43 trace elements in up to 2819 samples measured only in
the Trøndelag Health Study (HUNT). We identify 11 novel genetic loci associated with blood concentrations of arsenic, cadmium, manganese, selenium, and zinc in genome-wide association
meta-analyses. In HUNT, several genome-wide significant loci are also indicated for other trace elements. Using two-sample Mendelian randomization, we find several indications of weak to
moderate effects on health outcomes, the most precise being a weak harmful effect of increased zinc on prostate cancer. However, independent validation is needed. Our current understanding
of trace element-associated genetic variants may help establish consequences of trace elements on human health. SIMILAR CONTENT BEING VIEWED BY OTHERS GENOME-WIDE META-ANALYSIS OF IRON
STATUS BIOMARKERS AND THE EFFECT OF IRON ON ALL-CAUSE MORTALITY IN HUNT Article Open access 16 June 2022 INVESTIGATION OF THE IMPACT OF EXPOSURE TO TRACE ELEMENTS ON HEALTH AND DISEASE FROM
THE TOXILAUS STUDY Article Open access 29 November 2024 A GENOME-WIDE META-ANALYSIS YIELDS 46 NEW LOCI ASSOCIATING WITH BIOMARKERS OF IRON HOMEOSTASIS Article Open access 03 February 2021
INTRODUCTION Trace elements are present in living organisms at low concentrations, often defined as concentrations below 100 parts per million or less than 100 μg/g1. Essential trace
elements are vital for growth, development and normal physiology and biochemistry, and external sources are necessary as the body is unable to synthesize them. Other trace elements are
classified as non-essential or toxic2. The major sources of exposure to trace elements in the general population are food, water, and air. Trace elements therefore often show considerable
geographical variations3. Essential trace elements may also be toxic at elevated concentrations, and their uptake and metabolism are generally tightly regulated in the body4,5,6. The
mechanisms underlying variation in trace element concentrations between individuals are far from fully understood. Variations in trace element concentrations have previously been considered
to be governed mainly by dietary intakes of food items with elevated concentrations7 and environmental exposure (e.g., geochemical variations, work-related exposure and anthropogenic
pollution)8,9, but genetic variation may also be an important factor10,11,12,13,14,15,16. A well-known example is body iron, where protein-altering mutations in the _HFE_ gene cause a severe
iron overload condition, hemochromatosis17. Knowledge about genetic factors controlling trace element concentrations may shed light on the biological pathways of trace elements in the body.
Few genome-wide association studies (GWASs) have investigated trace element concentrations and no previous studies have examined a comprehensive multielement panel of trace elements. Early
twin studies have shown evidence for heritable variation in humans for copper, selenium, zinc, arsenic, cadmium, lead, and mercury10,11 and recent GWASs have reported genetic associations
with blood concentrations of manganese, copper, selenium, zinc, lead, cadmium, and mercury12,13,14,15,16,18. However, many of the previous studies were limited by sample size or investigated
only one or a few trace elements. Among the trace elements we investigated here, no published GWAS was found for 39 of them as of today. Essential as well as non-essential trace elements
have been linked to several health-related outcomes in humans, including neurodegenerative disorders19,20,21,22,23,24,25,26,27,28,29,30,31, autoimmune diseases32,33,34, endocrinological
diseases35,36,37,38,39, cancers40,41,42 and bone health43,44,45,46. However, there is inconsistent and sometimes conflicting evidence for a protective or harmful effect of the trace elements
on different diseases. Most of these studies have been observational and so the findings may have been influenced by confounding factors. Mendelian randomization (MR) methods have been
developed to obtain an additional level of evidence for causal relationships. The potential causality of increased trace element levels on various diseases may be explored using genetic
instruments for the individual elements. Trace elements have been measured in whole blood in two large Norwegian population-based studies: the Trøndelag Health Study (HUNT)8,47,48,49,50, and
the Norwegian Mother, Father, and Child Cohort Study (MoBa)7,51,52,53. Further, genetic variants have been genotyped genome-wide and imputed using the Haplotype Reference Consortium
reference panel (v1.1) 54 in both cohorts. GWASs of trace elements have also been reported in other cohorts, including the Swedish Prospective Investigation of the Vasculature in Uppsala
Seniors (PIVUS) study15. The main aim of the current study was to identify genetic variants associated with whole blood trace element concentrations, and to explore potential causal
associations between trace elements and cancers, neurodegenerative, autoimmune, endocrinological and bone related health outcomes. We, therefore, performed GWASs in HUNT and MoBa followed by
a meta-analysis with the PIVUS study15. Further, we used the genetic associations for causal inference with Mendelian randomization, thereby estimating effects of circulating trace elements
on health-related outcomes highlighted in previous literature. RESULTS GENETIC LOCI ASSOCIATED WITH TRACE ELEMENTS We identified 20 independent genetic loci (among 21 associations) that
reached the genome-wide significance threshold (_p_ < 5 × 10–8) in the meta-analyses of blood concentrations of 14 trace elements measured in the HUNT (14 trace elements, sample size _N_
= 2819), MoBa (11 trace elements, _N_ = 2812) and PIVUS (11 trace elements, _N_ = 949) studies (Supplementary Data 1). The loci were consistently associated (i.e., same direction of the
effect) with concentrations of essential (copper [number of variants, _n_ = 1], manganese [_n_ = 10], selenium [_n_ = 2], and zinc [_n_ = 3]) and toxic (arsenic [_n_ = 1], cadmium [_n_ = 3],
and lead [_n_ = 1]) trace elements across the meta-analyzed cohorts (Table 1, Supplementary Data 2 and 3, Supplementary Figs. 1–14). To the best of our knowledge, ten of these associations
had previously been reported12,13,14,15,16,55,56 (one locus was associated with both manganese and cadmium levels), and 11 had not. Additionally, in HUNT alone we analyzed 43 trace elements
that we could not meta-analyze because they had not been measured or the associated genetic variants were not tested in any other cohort. Here, we identified 27 genetic loci for 12 trace
elements in the analysis (Supplementary Data 2, Supplementary Figs. 15–38). Among these, we observed a common variant in _MORC4_ with strong evidence of association with strontium
concentrations (minor allele frequency (MAF) = 0.19, _p_ = 2.0 × 10–16), and a common variant in _HK1_ that was associated with magnesium concentrations (MAF = 0.14, _p_ = 2.2 × 10–8). One
locus in _SLC18A2_ was associated with selenium in HUNT (MAF = 0.27, _p_ = 3.8 × 10–8), but the index (lowest _p_-value) variant was not tested in MoBa, and none of the tested proxy variants
(in high linkage disequilibrium (LD) in HUNT, i.e., correlation r2HUNT > 0.8 with the index variant) were associated with selenium concentrations in MoBa. Most of the other variants from
HUNT only were low-frequency (MAF < 5%) or rare (MAF < 0.5%) and given that they were only tested for association in one cohort, they were more likely to be false positive findings.
Sensitivity analyses correcting for fish intake (arsenic, selenium), smoking status (arsenic, cadmium, copper, lead), weekly alcohol intake (iron, manganese, zinc, lead) and diabetes
(magnesium) in HUNT did not substantially change the effect sizes of the index variants (Supplementary Data 4). PROTEIN-ALTERING VARIANTS Among the 20 top association signals, we identified
eight protein-altering single nucleotide polymorphisms (SNPs) associated with trace elements: Four manganese associated index variants (rs13107325 in _SLC39A8_, rs1800562 in _HFE_, rs6099115
in _FAM210B_ and rs855791 in _TMPRSS6_) and one selenium associated index variant (rs1047891 in _CPS1_) were nonsynonymous SNPs. Among these, only rs13107325 had previously been reported
for manganese in a GWAS15. Although the hemochromatosis variant rs1800562 was not associated with manganese at the GWAS significance level in a previous GWAS15, women with _HFE_ variants
were reported to have 12% lower blood manganese concentrations in another study56. Further, three nonsynonymous SNPs were in strong LD (r2 > 0.8) with index variants: rs17279437 in
_SLC6A20_ (_p_ = 9.8 × 10−16, r2HUNT = 0.88 with arsenic index variant rs7306032), rs269868 in _DUOX2_ (_p_ = 5.7 × 10−10, r2HUNT = 0.96 with cadmium index variant rs953733) and rs57659670
in _DUOX2_ (_p_ = 3.2 × 10−10, r2HUNT = 0.99 with cadmium index variant rs953733) (Supplementary Data 5). SNP HERITABILITY ESTIMATES AND GENETIC AND PHENOTYPIC CORRELATIONS OF TRACE ELEMENTS
For the concentrations of 10 trace elements that had sample sizes above 5000 (cadmium, cobalt, copper, lead, manganese, mercury, molybdenum, selenium, thallium, and zinc), the estimated
narrow-sense SNP heritability h2 ranged from h2 = 0.01 ± 0.09 (thallium) to h2 = 0.29 ± 0.10 (manganese). The estimates were higher for essential trace elements (h2 between 0.16 and 0.29)
than for non-essential trace elements (h2 between 0.01 and 0.11) (Supplementary Data 6). We estimated the genetic correlation between all pairs of these 10 trace elements and found absolute
values of the genetic correlations to range from 0.01 (cobalt against lead and cobalt against mercury) to 0.97 (molybdenum against cadmium), although the estimates were imprecise and
therefore mostly uninformative (Supplementary Fig. 39a, Supplementary Table 1). For comparison, we also estimated the phenotypic correlation (Supplementary Fig. 39b, Supplementary Table 1),
where the absolute values ranged from around 0 (manganese against lead and manganese against mercury) to 0.45 (mercury against selenium). We did not observe patterns among the genetic or the
phenotypic correlations related to the known ion binding preferences of the trace elements57, which we chose to group as class A (oxygen-seeking) (molybdenum, manganese, zinc), intermediate
(cobalt, cadmium, copper), and class B (sulfur/nitrogen-seeking) (lead, mercury, selenium). PHENOME-WIDE ASSOCIATIONS OF TRACE ELEMENT LOCI We investigated the genetic relationship between
trace element concentrations and other complex traits by examining associations of the index variants identified by the meta-analyses with 1326 phenotype codes (‘phecodes’58), 30 blood
biomarkers and 167 other continuous traits and measurements of the UK Biobank. In total, 17 index variants from trace element GWA meta-analyses were associated (_p_ < 9.7 × 10−7,
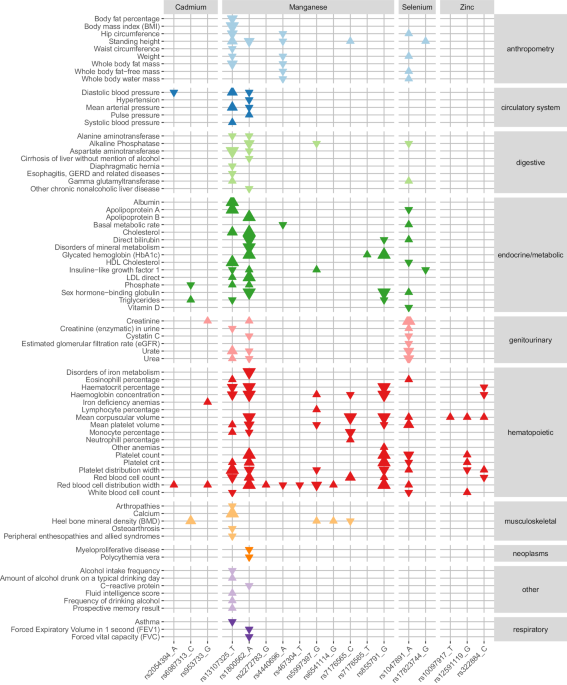
threshold Bonferroni corrected for 51,782 tests) with additional phenotypes (Fig. 1, Supplementary Fig. 40, Supplementary Data 7–9): Three nonsynonymous index variants for manganese
(rs13107325 [_SLC39A8_], rs1800562 [_HFE_], rs855791 [_TMPRSS6_]) and one index variant for cadmium in high LD with nonsynonymous SNPs (rs953733 [_DUOX2_]) were associated with phecodes
(Supplementary Data 7, Fig. 1): The manganese decreasing variant in the metal ion transporter gene _SLC39A8_ (rs13107325) was positively associated with diseases of the esophagus and with
musculoskeletal conditions. The manganese decreasing, but iron increasing, hemochromatosis variant (rs1800562) was positively associated with disorders of mineral and iron metabolism, but
also with several diseases in other biological domains. The manganese decreasing and iron increasing59 variant rs855791 was positively associated with other anemias. The cadmium increasing
variant, rs953733, found in a locus that has also been associated with iron status biomarkers60 (rs73060324), was positively associated with iron deficiency anemias. Further, eight index
variants, representing three trace elements (cadmium, manganese, and selenium), were associated with blood biomarkers in the UK Biobank (Supplementary Data 8, Fig. 1, Supplementary Fig. 40):
Five manganese variants (rs13107325 [_SLC39A8_], rs1800562 [_HFE_], rs7176565 [_DENND4A_], rs5997397 [_HSCB_] and rs855791 [_TMPRSS6_]) were associated primarily with endocrine/metabolic
biomarkers, but also with digestive, genitourinary, musculoskeletal, and inflammatory biomarkers. The cadmium increasing variant rs953733 (_DUOX2_) was positively associated with levels of
creatinine, a marker for kidney function61, while the cadmium decreasing rs6987313 (_LACTB2-AS1_) was associated with phosphate and triglyceride levels. The selenium increasing nonsynonymous
SNP rs1047891 (_CPS1_) was associated with endocrine/metabolic, genitourinary, and digestive biomarkers. Finally, the selenium increasing rs17823744 (_DMGDH_) was negatively associated with
insulin-like growth factor 1 (IGF-1). Among the GWAS results for continuous traits in the UK Biobank, we observed associations for 17 meta-analysis index variants (for cadmium, manganese,
selenium, and zinc) (Supplementary Data 9, Fig. 1, Supplementary Fig. 40). The majority of the associations were with different blood cell indices, but cadmium, manganese and selenium
variants were also associated with continuous traits from several other biological domains, including musculoskeletal and anthropometric measures (cadmium, manganese, selenium), blood
pressure traits (cadmium and manganese), biomarkers in urine and variables derived from endocrine/metabolic blood biomarkers (manganese and selenium), measures of cognitive ability
(manganese) and alcohol habits (manganese). In addition to the associations with the meta-analysis index variants, we also assessed the phenome-wide associations with common and
low-frequency (MAF > 0.5%) variants identified only in HUNT. Here, four common variants (strontium [rs17326228], cesium [rs7785293], magnesium [rs16926246] and silicon [rs62228297]) were
associated (_p_ < 9.7 × 10−7) with blood biomarkers and/or continuous traits in the UK Biobank (Supplementary Data 10–11, Supplementary Fig. 41): The cesium and magnesium variants were
associated with a range of blood cell related traits, the cesium and strontium variants with genitourinary biomarkers and measures, the magnesium variant with endocrine/metabolic biomarkers
and measures, and the silicon variant with standing height. MENDELIAN RANDOMIZATION We used two-sample MR to perform an exploratory examination of potential causal effects of trace elements
on health outcomes within neurodegenerative, autoimmune, and endocrinological diseases, cancers, and bone related domains as highlighted in the
literature19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46. Here, we used the index SNPs from the meta-analyses (manganese, selenium, zinc, copper, lead,
arsenic, and cadmium) or from the GWASs in HUNT (strontium) as instruments for the selected trace elements (F-statistics > 10 for all trace elements except cadmium [F-statistic = 9]:
Supplementary Data 12). For the SNP-outcome associations, we used summary-level data from large GWASs of selected neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, and
multiple sclerosis), autoimmune diseases (rheumatoid arthritis and autoimmune thyroid disorder), endocrinological diseases (hypothyroidism and type 2 diabetes), bone related traits and
disorders (bone mineral density, bone fractures, osteoporosis) and cancers (prostate cancer and colorectal cancer). The most precise association indicated a weak causal effect of zinc on
prostate cancer (odds ratio (OR) = 1.06, 95% CI = 1.01–1.12) (Supplementary Data 12). There were also stronger but less precise associations for arsenic and copper on Parkinson’s disease (OR
= 1.24, 95% CI = 0.98–1.57 [arsenic], OR = 1.17, 95% CI = 0.89–1.54 [copper]), selenium on colorectal cancer (OR = 0.89, 95% CI = 0.75–1.07) and selenium, cadmium and lead on multiple
sclerosis (OR = 0.86, 95% CI = 0.64–1.15 [selenium], OR = 0.86, 95% CI = 0.72–1.01 [cadmium], OR = 0.70, 95% CI = 0.52–0.95 [lead]) which warrant follow-up (Supplementary Data 12). The
results should, however, be interpreted with caution due to limitations such as few instrument SNPs per trace element and known correlations and common biological pathways of trace elements.
Otherwise, the MR estimates were generally imprecise, and generally they did not give convincing evidence for causal effects of trace elements in the remaining outcomes (Supplementary Data
12). DISCUSSION In the present study, we investigated genetic variants associated with whole blood trace element concentrations, combining data from up to 6564 individuals in HUNT, MoBa and
PIVUS. Our GWA meta-analyses identified genetic contributions to whole blood concentrations of essential (copper, manganese, selenium, and zinc) and non-essential or toxic (arsenic, cadmium,
and lead) trace elements. In total, 20 loci were associated with trace element concentrations across multiple cohorts, confirming 9 loci from previous studies and identifying 11 novel loci.
Seven of the novel loci were associated with manganese concentrations, including two nonsynonymous index variants. Four novel genetic loci were associated with selenium, zinc, arsenic, and
cadmium, among which we also identified nonsynonymous variants in high LD (r2 > 0.8) with the index variants. The genetic variants that we identified in the present study had small to
moderate effect sizes. They could, however, still contribute to individual differences in trace element concentrations in combination with many other factors. Identification of these loci
expands our knowledge about the genetic contribution to trace element concentrations and indicates proteins that could aid in establishing mechanisms for absorption, distribution,
metabolism, and excretion. Because essential trace elements are not produced in the body, but are necessary for normal physiology, we would expect genes associated with essential trace
elements to encode proteins that include these elements or are involved in their respective regulatory processes. This is in line with our observations in the meta-analyses of copper, zinc,
and manganese, where we replicated previously reported loci with index variants near or in genes encoding the copper-binding metalloprotein ceruloplasmin (_CP_)62, the metalloenzyme carbonic
anhydrase 1 (_CA1_) involved in the zinc balance63, and two divalent metal ion transporters _SLC30A10_64 and _SLC39A8_65 (nonsynonymous variant), where deficiency in the latter is known to
cause severe manganese deficiency66. Other nonsynonymous variants we observed had, to our knowledge, little or no known underlying biology related to the associated trace elements. For
example, we observed a novel association between selenium and a nonsynonymous variant in the urea cycle gene _CPS1_. Although the variant is associated with vitamin D67, which has in turn
been hypothesized to interact with selenium68, and the gene has been observed to be upregulated in mice fed excessive amounts of selenium69, the underlying biology of the association with
selenium in humans is not established. Because some trace elements interact with each other, or have common uptake mechanisms and co-transport, an imbalance in the concentration of one trace
element might also change the concentration of others7,49,70,71: For example, manganese is partly transported by both zinc and iron transporters72, and low iron stores have been associated
with higher blood concentrations of other trace elements7,49. Further, functional variants in iron metabolism genes have been associated with lower blood manganese concentrations56,
including rs1800562 in _HFE_ that we replicated here. The novel loci we observed to be associated with manganese, as well as the _CPS1_ locus for selenium, are in line with this pattern: One
manganese associated nonsynonymous SNP is known in iron deficiency anemia (rs855791 in _TMPRSS6_)73. Further, the gene that is closest to the index variant in another locus is the
interleukin-6 regulator gene _TUT7_74, which stimulates the iron regulator hepcidin75. The index variants in other novel loci are close to genes involved in mitochondrial iron uptake
(_FAM210B_76, where we identified the nonsynonymous manganese index variant rs6099115) or genes associated with hematological traits or involved in the synthesis of iron containing compounds
(_LYRM4_, _FAM210B_, _DENND4A_, _FECH_, _HSCB_)76,77,78,79. The _CPS1_ index variant (rs1047891) was in high LD (r2HUNT = 0.92) with an index variant (rs715) for total iron binding capacity
in our previous study60. These loci were not associated (_p_ > 5 × 10−8) with iron in the current study; however, the analysis of iron had limited statistical power with less than half
the sample size of manganese. Non-essential trace elements typically do not have their own transport proteins or specific mechanisms for metabolism and are generally taken up into the body
using the routes of macronutrients80 or essential trace elements with similar chemical properties70. This is illustrated by one of the known loci we replicate for the toxic metal cadmium,
where the index variant is nearest to the zinc/manganese transporter gene _SLC39A8_, which was also associated with manganese. For cadmium, we also report a novel locus with two
nonsynonymous variants in _DUOX2_, both in high LD with the index variant. The gene codes for a thyroid hormone synthesis related protein81 with a heme binding site82 and was associated with
iron status in previous GWASs60,83. Similarly, the nonsynonymous variant rs17279437 in the glycine transporter gene _SLC6A20_ has been associated with excessive glycine excretion84. In this
study, the variant was in high LD with the index variant for arsenic, which could potentially indicate that the protein can also transport arsenic. Glycine levels have also been associated
with arsenic exposure in mouse models85. The genetic associations observed only in HUNT warrant further replication. One interesting result identified in HUNT was a selenium locus within a
solute carrier gene (_SLC18A2_). Although the index variant was not tested in MoBa, variants in high LD (r2 > 0.8) with the index variant were tested, but not associated with selenium in
that data. The non-association in MoBa could however potentially be a result of sex-specific or pregnancy-specific exposure patterns. Further, index variants in gold and magnesium loci were
in or nearest to genes associated with hemoglobin and iron regulation (_CD163_ and _HK1_)86,87. The strong association between hypomagnesemia and type 2 diabetes88, combined with a
previously reported association between the magnesium _HK1_ index variant with levels of the type 2 diabetes biomarker glycated hemoglobin (HbA1c)89 could support this finding. However,
associations between _HK1_ and HbA1c could also reflect the erythrocyte lifetime regardless of diabetes status90. We also observed a strong association between strontium concentrations and a
locus in _MORC4_. Strontium is chemically very similar to calcium. It has effects on bone balance91, and could potentially induce skeletal abnormalities in very high doses91. A rare genetic
variant in _MORC4_ has been associated with a 3.4 times increased risk of osteoarthritis (Open Targets Platform92, accessed 25.05.2022), and another _MORC_ family member, _MORC3_, is
involved in calcium homeostasis and maintenance of bone remodeling93. We therefore speculate that _MORC4_ could have similar functions or be involved in similar pathways. In the PheWAS
analyses, we observed the well-known association of functional variants in iron metabolism genes (the hemochromatosis _HFE_ variant, rs1800562, and the _TMPRSS6_ variant, rs855791) with
disorders of iron and mineral metabolism and anemias. As expected, and previously demonstrated in the UK Biobank60, rs1800562 was also associated with several known clinical manifestations
of _HFE_ hemochromatosis94,95. Likewise, the index variant in the _DUOX2_ (cadmium) locus known from GWASs of iron status biomarkers60,83,96, was associated with iron deficiency anemia. The
many associations between trace element index variants and blood cell indices could also potentially reflect their correlation with iron status. The associations between digestive and
musculoskeletal disorders and rs13107325 (manganese), which alters the metal ion transporter ZIP8 (encoded by _SLC39A8_), could potentially highlight the role of manganese or other divalent
trace metals in these conditions. Associations with basal metabolic rate and metabolic biomarkers could possibly be related to the processing of trace elements in the body, and associations
with biomarkers for liver and kidney function could be related to the clearance of trace elements that are toxic or in high abundance. However, population stratification could also have
caused false associations with some of the variants. We estimated the heritability of concentrations of 10 trace elements with meta-analysis sample size above 5000, ranging from low to
moderately high heritability (0.01 ± 0.09 [thallium] to 0.29 ± 0.10 [manganese]). The lower heritability for non-essential trace elements compared to essential trace elements could reflect
that humans have not evolved genes and biological pathways to handle non-essential trace elements. The very low heritability of thallium could also be because most of the samples from MoBa
were below the detection limit. Further, many of the heritability estimates could be low because LD Score regression underestimates the heritability of traits that are not highly
polygenic97. The genetic and phenotypic correlations between nine trace elements did generally not correspond well to each other, however, the genetic correlations were highly imprecise and
therefore mostly uninformative. Mercury and selenium had the strongest phenotypic correlation, which is in line with the known mercury-selenium antagonistic relationship98. In general, the
genetic correlations were higher than the phenotypic correlations, although the range of the different phenotypic correlation estimates were similar to those previously reported in MoBa7.
This could potentially reflect a relatively low polygenicity of trace element concentrations, where few genetic loci might influence many different trace elements, while there are a variety
of different factors influencing the phenotypic variations. Using two-sample MR, we observed indications of a weak harmful effect of circulating zinc on prostate cancer. The effect of zinc
on prostate cancer is debated, but a harmful effect has been found in some previous studies99. There was also a weak positive association for arsenic and copper on Parkinson’s disease, and
weak negative associations for selenium on colorectal cancer and selenium, cadmium, and lead on multiple sclerosis. Otherwise, we observed little evidence for causal roles of trace elements
in the remaining selected health outcomes. The apparently protective effect of lead on multiple sclerosis is not in line with the known inhibitory effect of lead on a heme biosynthesis
catalyst, aminolevulinate dehydratase (ALAD)100, and (lead induced) impairment of heme synthesis as a suggested potential trigger for multiple sclerosis101. Generally, true direct protective
effects of non-essential or toxic trace elements seem implausible, although their effect could potentially be indirect, for example, if the toxic trace elements influence essential trace
elements in the body. Based on the PheWAS results and the known correlations and common biological pathways between several trace elements, the estimates were particularly vulnerable to
misspecification of the primary phenotype and/or horizontal pleiotropy. Further, blood concentrations might not indicate other tissue or organ specific trace element concentrations. MR
analyses can also be influenced by selection bias and competing risks, which might also explain apparent protective effects of toxic trace elements on late-life health outcomes. Few
instrument SNPs per trace element provided limited opportunities for reliable sensitivity analyses. Some estimates were precise around the null, but in general, the confidence intervals were
wide and compatible with both moderate protection and harmful effects and were therefore uninformative. The low precision could be due to low case sample sizes in the outcome data and/or
genetic instruments that had few SNPs with small to moderate effects on the exposures, potentially introducing weak instrument bias102. This was quantified by the low variance explained and
low F-statistics of some of the instruments. We chose not to include any correction for multiple testing, since this was a first exploratory analysis with low power. The findings were based
on the analyses of variation in trace element concentrations in a general population, and we were therefore unable to estimate any effects of extreme trace element concentrations. The
current results should be interpreted with caution, and future well-powered multivariable MR analyses might be helpful to discriminate between the effects of different trace elements.
Further, as we only analyzed genetic associations with trace element concentrations measured in adults, it was not possible to evaluate any effects of trace elements specific to growth and
development stages in newborns to adolescents103. Low sample sizes and lack of cohorts with both trace elements and health outcomes available also prevented us from exploring non-linear
associations with MR. This study has several limitations. Neither the HUNT nor the MoBa populations have generally been exposed to high levels of toxic trace elements, which makes these
excellent populations for detecting genetic factors, but on the other hand many trace elements had a high proportion of measurements below the detection limit, thereby lowering the effective
sample size. Accordingly, expanding the analyses to additional populations and ancestry groups could have been highly informative, both due to differences in LD structure and variation in
exposure to trace elements from the diet and the environment. Further, trace elements are distributed differently in different organs, tissues, and body fluids104,105,106, and whole blood is
therefore not the preferred tissue for monitoring the status of all trace elements. Some trace elements could also be influenced by evaporation, contamination from syringes or leakage of
trace elements from glass vials. Differences between studies and populations also created known and potential limitations. Without access to individual level data in PIVUS, we were unable to
harmonize all covariates. For example, the PIVUS association model included triglycerides and cholesterol as covariates, while the models used in HUNT and MoBa did not. Although HUNT, PIVUS
and MoBa are all Scandinavian cohorts, the populations from which they have been sampled have clear differences, particularly in terms of birth year, age, and sex, and although the
directions of effect were consistent across all three studies, the differences observed in effect size across these studies for a few SNPs could potentially be related to population-specific
exposure patterns or factors related to pregnancy (MoBa). For example, fish intake might influence Swedes more than Norwegians, because there are indications that some toxic trace elements
have accumulated more in fish caught in the shallow Bothnian Bay than along the deep Norwegian coastline107. Further, pregnant women might change their diet and different generations could
have been exposed to different levels of environmental pollution throughout their lives. The main limitation was that most of the trace elements were only measured in HUNT, and both low
sample sizes and the lack of replication cohorts were general limitations. Further, trace element concentrations are associated with many different factors, and while we have controlled for
some of these, there might still be unmeasured confounding by population stratification related to factors we have not controlled for, especially for rare variants. A particular limitation
was the lack of detailed data on dietary habits. The heritability and genetic correlation estimates found using LD Score regression were limited by the potentially low polygenicity of the
trace elements and genomic control (GC) correction of summary statistics, both which may contribute to heritability being underestimated. Additionally, using summary statistics from mixed
models may contribute to overestimation. Finally, small sample sizes (and for some trace elements many measurements below the detection limit) resulted in highly imprecise estimates. The
imprecision was especially large for the genetic correlations, which were further limited by heritability z-scores that were all below 4108. In summary, we have identified novel genetic loci
and replicated previously indicated loci for essential and non-essential trace elements. These highlighted interesting genes that may help establish biological pathways and mechanisms for
uptake, distribution, metabolism, and excretion of trace elements in humans. MR analyses provided several indications of weak to moderate associations between trace elements and health
outcomes, the most precise being a weak harmful effect of genetically determined circulating zinc on prostate cancer. However, generally imprecise MR estimates gave no convincing evidence
for causal roles of genetically determined levels of other trace elements on health outcomes. Studies of populations with higher trace element exposure burdens or with larger samples are
needed to investigate moderate and weak effects. METHODS COHORT DESCRIPTIONS HUNT The HUNT Study47,48 is a longitudinal population-based health study conducted in the county of Trøndelag,
Norway. Data and samples have been collected through four cross-sectional surveys: HUNT1 (1984–86), HUNT2 (1995–97), HUNT3 (2006–08) and HUNT4 (2017–2019). Approximately 123,000 individuals
(aged ≥ 20 years) have participated in one or more HUNT surveys. Approximately 88,000 individuals have been genotyped using one of three Illumina HumanCoreExome arrays: 12 v.1.0, 12 v.1.1
and 24 with custom content (UM HUNT Biobank v1.0). Sample and variant quality control (QC) was performed using standard practices as described elsewhere109: Samples were excluded if they had
a call rate < 99%, estimated contamination > 2.5%, large chromosomal copy number variants, lower call rate of a technical duplicate pair or twin, gonosomal constellations other than
XX or XY, or a discrepancy between the inferred sex and reported gender. After genotyping, variants were excluded if they had a call rate < 99%, deviation from the Hardy–Weinberg
equilibrium (_p_ < 10−4 in unrelated samples of European ancestry), Gentrain score < 0.15, cluster separation < 0.3, probe sequences that could not be mapped perfectly to the
reference genome, or if another assay with higher call rate had genotyped the same variant. All variants were imputed from the HRC v1.1 reference panel110 merged with 2201 sequenced samples
from HUNT, using Minimac3111. Trace elements have been measured in whole blood samples collected in HUNT2 and HUNT3 using high-resolution inductively coupled plasma mass spectrometry
(HR-ICP-MS) (Thermo Finnigan Element 2, Thermo Finnigan, Bremen, Germany) at three laboratories as part of previous studies. Here, we combined measurements of nine trace elements (As, Cd,
Co, Cu, Pb, Mn, Hg, Se, Zn) in 930 samples (HUNT2) that have been analyzed by the National Institute of Occupational Health in Norway (STAMI), 53 trace elements (Al, Sb, As, Ba, Be, B, Br,
Cd, Ca, Ce, Cs, Cl, Cr, Co, Cu, Ga, Ge, Au, Ho, In, Fe, La, Pb, Li, Mg, Mn, Hg, Mo, Nd, Ni, Nb, Pa, P, Pt, Pr, Re, Rh, Rb, Sm, Se, Si, Sr, S, Ta, Tb, Tl, Sn, W, U, V, Y, Zn, Zr) that have
been measured in 757 samples (HUNT3) by one laboratory at NTNU (hereafter named NTNU1), and 30 trace elements (As, Be, Bi, B, Br, Cd, Ca, Cs, Cr, Cu, Ga, Au, In, Ir, Fe, Pb, Mg, Mn, Hg, Mo,
Ni, Rb, Se, Ag, Sr, Tl, Th, Sn, W, Zn) that have been measured in 1539 samples (HUNT3) by a second laboratory at NTNU (hereafter named NTNU2). For 23 trace elements in HUNT3, some samples
(maximum 30) had missing information on lab assignment, and we assigned these to NTNU2. There were no individuals with multiple measurements. Trace element measurements were returned to the
HUNT Databank after sample and trace element QC at the respective laboratories. The samples analyzed by the STAMI laboratory were collected as part of a sub-study of iron status in women
(selection criteria: female, age between 20 and 55 years old, non-pregnant, not blood donor in the past 2 years), the samples analyzed by HUNT1 were collected as part of a neuroimaging
sub-study (selection criteria: age between 50 and 65, participation in previous HUNT surveys, exclusion criteria: MRI contraindications (pacemaker of the heart, clipped cerebral aneurysm,
cochlear implants, severe claustrophobia, or body weight above 150 kg)), and 267 of the 1539 samples analyzed by NTNU2 were selected among type 2 diabetes cases as part of a diabetes related
sub-study. Samples from the NTNU1 laboratory that were either below the detection limit or more than 10 times the median value had been removed and were not included in the current
analysis. The specific reason for removal of these measurements was not available, therefore we excluded these measurements for our analysis. The proportion of unavailable samples per trace
element from NTNU1 ranged from 0 to 69% (average 17%, median 2%). For samples from the NTNU2 laboratory, we replaced measurements below the detection limit with randomly generated numbers
between 0 and the element-specific detection limit, because the true measurements were unavailable. The proportion of measurements below the detection limit from NTNU2 ranged from 0% to 97%
(average 11%, median 0%). Details of sample collection, storage of samples, sample processing, quality control and ICP-MS analyses have been reported in detail elsewhere8,49,50. MOBA The
Norwegian Mother, Father and Child Cohort Study (MoBa) is a population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health. Participants were recruited from
all over Norway from 1999–2008. The women consented to participation in 41% of the pregnancies. The cohort includes approximately 114,500 children, 95,200 mothers and 75,200 fathers53. The
current study is based on version 12 of the quality-assured data files released for research in January 2019. The establishment of MoBa and initial data collection was based on a license
from the Norwegian Data Protection Agency and approval from The Regional Committees for Medical and Health Research Ethics. In short, pregnant women were recruited in their first trimester
and invited to fill in three questionnaires during pregnancy, and to donate blood and urine samples at the time of ultrasound screening around gestational weeks 17–19 (mean 18.5). Blood
samples were obtained from both parents during pregnancy and from mothers and children (umbilical cord) at birth. Follow-up is conducted through questionnaires and linkage to national health
registries53. A total of 98,000 MoBa participants have been genotyped using one of three arrays: Illumina HumanCoreExome, Illumina Global Screening Array, or Illumina OmniExpress. In the
current study, we used genetic data collected as part of the Better Health by Harvesting Biobanks (HARVEST) study released by MoBa Genetics v.1.0
(https://github.com/folkehelseinstituttet/mobagen/wiki/MoBaGenetics1.0). The sample and variant quality control in MoBa has been described elsewhere112. All variants were imputed from the
HRC reference panel v1.1 at the Sanger Imputation Service. The Norwegian Environmental Biobank is a sub-study within MoBa established with the aim of biomonitoring nutrients and
environmental contaminants in MoBa participants. The study included 2999 pregnant women with available genetic data who had donated blood and urine samples and had responded to
questionnaires 1–6 in MoBa7,52. A total of 11 trace elements (As, Cd, Co, Cu, Mn, Mo, Pb, Se, Tl, Zn, Hg), were measured in whole blood donated by the women in gestational week 18 at the
department of laboratory medicine at Lund University (Sweden). All trace elements except Hg were analyzed using an ICP-MS (iCAP Q, Thermo Fisher Scientific, Bremen, Germany). Total Hg was
determined in acid-digested samples by cold vapor atomic fluorescence spectrophotometry (Sandborgh-Englund 1998). During the analysis campaign, the laboratory participated in the German
External Quality Assessment Scheme (G-EQAS), with good agreement between obtained element concentrations in quality control samples used and expected values. Details of sample collection,
storage of samples, sample processing and ICP-MS analyses have been reported in detail elsewhere7. ASSOCIATION ANALYSES We performed genome-wide association analyses of 59 trace elements
measured in up to 2819 individuals in HUNT and 11 trace elements measured in 2812 individuals in MoBa. For 48 trace elements in HUNT (Al, Sb, As, Ba, Be, Bi, B, Br, Cd, Ca, Ce, Cs, Cl, Co,
Cu, Ga, Ge, Au, Ho, In, Ir, Fe, La, Pb, Li, Mg, Mn, Hg, Mo, Nd, Nb, Pa, P, Rh, Rb, Sm, Se, Ag, Sr, S, Ta, Tb, Tl, Sn, W, U, Y, Zn) and 9 trace elements in MoBa (Cd, Co, Cu, Hg, Mn, Mo, Pb,
Se, Tl, Zn), we used a linear mixed model regression under an additive genetic model for each variant as implemented in BOLT-LMM v.2.3.4113, thereby controlling for relatedness between study
participants. We performed association analyses of 9 trace elements in HUNT (Cr, Ni, Pt, Pr, Re, Si, Th, V, Zr) and 2 trace elements in MoBa (As, Mo) in unrelated individuals using PLINK
2.0114, because BOLT-LMM was unable to estimate the trace element heritability. In total, human GWASs had not been previously published for 39 trace elements (Sb, Ba, Be, B, Br, Bi, Ce, Cs,
Ga, Ge, Au, Ho, In, Ir, La, Li, Nd, Nb, Pd, Pt, Pr, Re, Rh, Rb, Sm, Si, Ag, Sr, S, Ta, Tb, Tl, Th, Sn, W, U, V, Y, Zr). Distributions, sample size and proportion of measurements below the
detection limit per study are given for each trace element in Supplementary Data 1. Prior to analysis, we applied rank-based inverse normal transformation of the trace element concentrations
after adjusting for age and sex (HUNT) using linear regression. Age, and the first ten genetic principal components (PCs) of ancestry were included as covariates in all the analyses. Sex,
genotyping batch, geographical region (coast, town/fjord, or inland/mountain) and analysis laboratory (NTNU1, NTNU2, STAMI) were included as additional covariates in HUNT where appropriate.
We performed genomic control correction of all GWAS results with an inflation factor λ > 1 (calculated from variants with MAF ≥ 0.01). Variants with a minor allele count <10 or an
imputation R2 < 0.3 were excluded from the analyses. After visual inspection of the Manhattan and quantile-quantile plots, we excluded the full set of results for Bi and Th due to
excessive inflation of the p-values (Supplementary Figs. 42–45). We used METAL v.2011-03-25115 to perform fixed-effect inverse variance weighted GWA meta-analysis of 14 trace elements (Al,
As, Cd, Cr, Co, Cu, Pb, Mn, Hg, Mo, Se, Tl, Zn) analyzed in at least two of three studies: the HUNT, MoBa and/or publicly available summary statistics from the PIVUS study15, where 11 trace
elements (Al, Cd, Co, Cu, Cr, Hg, Mn, Mo, Ni, Pb, Zn) have been measured in 949 seniors from Uppsala, Sweden, and rank inverse normalized trace element concentrations had been tested for
association using a linear regression under an additive model, adjusting for triglycerides, cholesterol, gender and two principal components of ancestry. Age was not included as covariate
because the PIVUS participants were of the same age. DEFINITION OF ASSOCIATED VARIANTS, ASSOCIATED LOCI, AND LOCUS NOVELTY We considered genetic variants with _p_ < 5 × 10−8 to be
statistically significant at a genome-wide significance level and defined these as associated with the given trace element. Genetic loci were defined as 500 kilobase pairs to each side of
genome-wide significant variants in the same region. A locus was classified as novel for a given trace element if it had not been reported for the respective trace element before. Previously
published loci were identified with a literature search and a look-up in the NHGRI-EBI GWAS catalog116. Index SNPs were identified as the genetic variant with the lowest p-value in each
locus. We used PLINK v.1.9114 to identify variants in high LD (correlation r2 > 0.8) with the index variants, based on a reference panel of 5000 unrelated individuals in HUNT. The index
variants and variants in high LD with the index variants were annotated using ANNOVAR v.2019Oct24117. SENSITIVITY ANALYSES For trace elements where tobacco smoke (Cd, As, Pb, Cu)118, wine
(Pb)119 or fish (As, Se)120 is a major source of human intake, or where alcohol is thought to regulate uptake or metabolism (Fe, Mn, Zn)121,122,123, we repeated the association analyses of
the associated loci in HUNT, including smoking status (self-reported, never versus ever smokers (including ex-smokers, occasionally and daily smokers)), frequency of fat fish intake
(self-reported) and units of alcohol per week (self-reported) as covariates, respectively (Supplementary Data 4). Further, since some of the analyzed samples were selected from type 2
diabetes cases, and type 2 diabetes is associated with hypomagnesemia88, we repeated the association analyses of magnesium including diabetes status (excluding type 1 diabetes) as covariate.
Diabetes cases were defined as either any non-type 1 diabetes (self-reported) and/or fasting serum glucose ≥7.0 mmol/liter and/or serum glucose ≥ 11.0 mmol/liter 2 hours after first having
fasted overnight and then consumed 75 g of glucose dissolved in ~3 dl water. HERITABILITY ESTIMATION AND GENETIC AND PHENOTYPIC CORRELATION BETWEEN TRACE ELEMENTS We used LD Score
regression108,124 to estimate the narrow-sense SNP heritability (Vg/Vp ± 1SE, where Vg is the variance explained by the SNPs and Vp is the total phenotypic variance) of 11 trace elements
from the GWA meta-analysis summary level SNP results, using LD scores estimated from individuals of European ancestry in the HUNT population. The analysis was performed in trace elements
with a meta-analysis sample size > 5000, as recommended for the LD Score regression software. Each set of summary statistics were restricted to well imputed SNPs in HapMap3. Further, we
used LD Score regression to estimate the genetic correlation between all pairs of 10 trace elements with an estimated SNP heritability > 0 and a sample size > 5000 in the GWA
meta-analysis. The cadmium-mercury and molybdenum-cobalt correlations were excluded because the genetic estimates were out of bounds (higher than 1.0 or lower than −1.0). Additionally, we
estimated the phenotypic correlation between the same pairs of trace elements in HUNT using Spearman rank correlation. Prior to the phenotypic correlations, the trace elements were corrected
for median concentration per lab and log2 transformed. PHENOME-WIDE ASSOCIATION TESTS (PHEWAS) We tested for associations of 34 common and low-frequency (MAF > 0.5%) trace element index
variants (21 from meta-analysis and 13 from GWAS in HUNT) with 1326 phecodes, 167 continuous traits and 30 biomarkers in participants of white British ancestry in the UK Biobank, using
publicly available summary statistics (https://pan.ukbb.broadinstitute.org/)125. Four variants (rs146233512 [gold], rs763064690 [indium], rs78394934 [iron], rs927502065 [tungsten]) were
excluded because they were not tested in the UK Biobank. To correct for the total number of tests (_n_ = 51,782), we used a Bonferroni corrected _p_-value significance threshold of 9.7 ×
10−7. MENDELIAN RANDOMIZATION (MR) OF INDIVIDUAL TRACE ELEMENTS ON SELECTED HEALTH RELATED OUTCOMES To explore potential causal associations of trace elements on selected outcomes, we used
two-sample MR: We applied the inverse-variance weighted (IVW) method for trace elements with multiple index SNPs, and the Wald ratio method for trace elements with only one index SNP, as
implemented in the TwoSampleMR126 and MRInstruments127 packages in R v3.6.3 and R v4.0.5. The exposures were selected based on robust genetic associations with trace elements in the current
study, i.e., meta-analyzed index variants and the common index variant in the locus strongly associated with Sr in HUNT. The outcomes were selected based the availability of instrument
summary statistics for a priori outcomes of interest highlighted in previous literature: Alzheimer’s disease (Mn, Pb, As, Cd, Cu, Se and Zn)19,20,24,25,26,27,128, Parkinson’s disease (Pb,
As, Cd, Cu, Mn, Se, and Zn)19,28,29,30, multiple sclerosis (Zn, Mn, Se, Pb, As and Cd)21,22,23, autoimmune thyroid disease (Se)32, hypothyroidism (Se)32, osteoporosis (Cu)43,45, bone mineral
density (Cu, Cd, Mn, Zn, and Sr)44,45,46, bone fracture (Sr)46, rheumatoid arthritis (Cd and Zn)33,34, type 2 diabetes (Cu, Mn, Se and Zn)37,38,39, colorectal cancer (Zn and Se)41,42 and
prostate cancer (Se, Zn)40,42. We used SNP-exposure associations from the GWA meta-analysis (Cu, Zn, Mn, Se, Pb, As, Cd) or GWAS in HUNT (Sr) of each trace element (Supplementary Data 2),
and SNP-outcome associations were collected from independent genome-wide summary level data129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146 (Supplementary Data 12).
For the SNP associations with multiple sclerosis, we calculated the beta coefficient as the natural logarithm of the OR, and the standard error as \({SE}={|beta}/Q(\frac{p}{2})|\), where | |
denotes absolute values, _p_ denotes the _p_-value and \(Q\) denotes the inverse standard normal distribution. Outcomes obtained from https://pan.ukbb.broadinstitute.org125 were defined as:
hypothyroidism (phecode: 244), and osteoporosis (phecode: 743.1). We estimated the variance explained and F-statistic for the instruments from a linear regression of the trace element
concentrations versus the sum of estimated allele counts (dosages) for the trace element increasing alleles in HUNT (Supplementary Data 12). Except for manganese, the trace element
instruments consisted of fewer than 4 SNPs, which provided limited opportunities for reliable sensitivity analyses. STATISTICS AND REPRODUCIBILITY Unless otherwise specified, the statistical
analyses were performed in R v.3.6.4. The sample size per trace element, proportion of measurements below the detection limit, and the distributions of trace element concentrations, age,
and sex in HUNT, MoBa and PIVUS are reported in Supplementary Data 1. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked
to this article. DATA AVAILABILITY Summary level data supporting the findings are available in the Supplementary materials and from https://dataverse.no/dataverse/ntnu/
(https://doi.org/10.18710/UYPCL0). Numerical source data underlying Fig. 1 can be found in Supplementary Data 7–9. The consent given by the participants in HUNT and MoBa does not open for
storage of individual level data in repositories or journals. Researchers associated with Norwegian research institutes can apply for the use of HUNT data. Researchers from other countries
may apply if collaborating with a Norwegian Principal Investigator. Information for data access can be found at https://www.ntnu.edu/hunt/data. Researchers who want access to MoBa data sets
for replication should apply to https://helsedata.no. Access to data from either HUNT or MoBa requires approval from The Regional Committees for Medical and Health Research Ethics in Norway.
CODE AVAILABILITY We used the following publicly available software (URLs) to generate and analyze the data: R v3.6.3 and R v.4.0.5, BOLT-LMM v.2.3.4
(https://alkesgroup.broadinstitute.org/BOLT-LMM/downloads), PLINK 2.0 (https://www.cog-genomics.org/plink/2.0/), METAL v.2011-03-25 (https://genome.sph.umich.edu/wiki/METAL), ANNOVAR
v.2019Oct24 (https://annovar.openbioinformatics.org) and LD Score Regression (https://github.com/bulic/ldsc). REFERENCES * Morrison, G. H., Cheng, K. L. & Grasserbauer, M. General
aspects of trace analytical methods-IV. Recommendations for nomenclature, standard procedures and reporting of experimental data for surface analysis techniques. _Pure. Appl. Chem._ 51,
2243–2250 (1979). Article Google Scholar * Nielsen, F. H. Should bioactive trace elements not recognized as essential, but with beneficial health effects, have intake recommendations. _J.
Trace Elem. Med. Biol._ 28, 406–408 (2014). Article CAS PubMed Google Scholar * Elder, A., Nordberg, G. F. & Kleinman, M. Chapter 3—Routes of exposure, dose, and toxicokinetics of
metals. in _Handbook on the Toxicology of Metals_ 4th edn (eds Nordberg, G. F., Fowler, B. A. & Nordberg, M.) 45–74 (Academic Press, 2015). * Dev, S. & Babitt, J. L. Overview of iron
metabolism in health and disease. _Hemodial. Int._ 21, S6–S20 (2017). Article PubMed PubMed Central Google Scholar * Li, Y. Copper homeostasis: emerging target for cancer treatment.
_IUBMB Life_ 72, 1900–1908 (2020). Article CAS PubMed Google Scholar * Chen, P., Bornhorst, J. & Aschner, M. Manganese metabolism in humans. _Front Biosci. (Landmark Ed.)_ 23,
1655–1679 (2018). Article CAS PubMed Google Scholar * Caspersen, I. H. et al. Patterns and dietary determinants of essential and toxic elements in blood measured in mid-pregnancy: The
Norwegian Environmental Biobank. _Sci. Total Environ._ 671, 299–308 (2019). Article CAS PubMed Google Scholar * Simić, A. et al. Trace elements in whole blood in the general population
in Trøndelag County, Norway: The HUNT3 Survey. _Sci. Total Environ._ 806, 150875 (2022). Article PubMed Google Scholar * Birgisdottir, B. E. et al. Essential and toxic element
concentrations in blood and urine and their associations with diet: Results from a Norwegian population study including high-consumers of seafood and game. _Sci. Total Environ._ 463–464,
836–844 (2013). Article PubMed Google Scholar * Whitfield, J. B. et al. Genetic effects on toxic and essential elements in humans: arsenic, cadmium, copper, lead, mercury, selenium, and
zinc in erythrocytes. _Environ. Health Perspect._ 118, 776–782 (2010). Article CAS PubMed PubMed Central Google Scholar * Whitfield, J. B. et al. Evidence of genetic effects on blood
lead concentration. _Environ. Health Perspect._ 115, 1224–1230 (2007). Article CAS PubMed PubMed Central Google Scholar * Evans, D. M. et al. Genome-wide association study identifies
loci affecting blood copper, selenium and zinc. _Hum. Mol. Genet_ 22, 3998–4006 (2013). Article CAS PubMed PubMed Central Google Scholar * Warrington, N. M. et al. Genome-wide
association study of blood lead shows multiple associations near ALAD. _Hum. Mol. Genet_ 24, 3871–3879 (2015). Article CAS PubMed PubMed Central Google Scholar * Jäger, S. et al. Blood
copper and risk of cardiometabolic diseases: a Mendelian randomization study. _Hum. Mol. Genet._ 31, 783–791 (2022). Article PubMed Google Scholar * Ng, E. et al. Genome-wide association
study of toxic metals and trace elements reveals novel associations. _Hum. Mol. Genet._ 24, 4739–4745 (2015). Article CAS PubMed PubMed Central Google Scholar * Borné, Y. et al. Genome
wide association study identifies two loci associated with cadmium in erythrocytes among never-smokers. _Hum. Mol. Genet._ 25, 2342–2348 (2016). Article PubMed Google Scholar * Girelli,
D. et al. Hemochromatosis classification: update and recommendations by the BIOIRON Society. _Blood_ 139, 4–7 (2022). Article Google Scholar * Yang, W. et al. Genome-wide association and
Mendelian randomization study of blood copper levels and 213 deep phenotypes in humans. _Commun. Biol._ 5, 405 (2022). Article CAS PubMed PubMed Central Google Scholar * Chin-Chan, M.,
Navarro-Yepes, J. & Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. _Front. Cell Neurosci._ 9, 124
(2015). Article PubMed PubMed Central Google Scholar * Rahman, M. A. et al. Exposure to environmental arsenic and emerging risk of Alzheimer’s disease: perspective mechanisms, management
strategy, and future directions. _Toxics_ 9, 188 (2021). Article CAS PubMed PubMed Central Google Scholar * Sheykhansari, S. et al. Redox metals homeostasis in multiple sclerosis and
amyotrophic lateral sclerosis: a review. _Cell Death Dis._ 9, 348 (2018). Article PubMed PubMed Central Google Scholar * Nirooei, E. et al. Blood trace element status in multiple
sclerosis: a systematic review and meta-analysis. _Biol. Trace Elem. Res._ 200, 13–26 (2022). Article CAS PubMed Google Scholar * Tsai, C. P. & Lee, C. T. C. Multiple sclerosis
incidence associated with the soil lead and arsenic concentrations in Taiwan. _PLoS One_ 8, e65911 (2013). Article CAS PubMed PubMed Central Google Scholar * Branca, J. J. V., Morucci,
G. & Pacini, A. Cadmium-induced neurotoxicity: Still much ado. _Neural Regen. Res._ 13, 1879–1882 (2018). Article CAS PubMed PubMed Central Google Scholar * Lopes de Andrade, V.,
Marreilha dos Santos, A. P. & Aschner, M. Chapter Eleven—Neurotoxicity of metal mixtures. in _Advances in Neurotoxicology_ 5 (eds. Aschner, M. & Costa, L. G.) 329–364 (Academic
Press, 2021). * Lei, P., Ayton, S. & Bush, A. I. The essential elements of Alzheimer’s disease. _J. Biol. Chem._ 296, 100105 (2021). Article CAS PubMed Google Scholar * Xie, Z., Wu,
H. & Zhao, J. Multifunctional roles of zinc in Alzheimer’s disease. _Neurotoxicology_ 80, 112–123 (2020). Article CAS PubMed Google Scholar * Lee, C.-P., Zhu, C.-H. & Su, C.-C.
Increased prevalence of Parkinson’s disease in soils with high arsenic levels. _Parkinsonism Relat. Disord._ 88, 19–23 (2021). Article PubMed Google Scholar * Pamphlett, R. et al.
Concentrations of toxic metals and essential trace elements vary among individual neurons in the human locus ceruleus. _PLoS One_ 15, e0233300 (2020). Article CAS PubMed PubMed Central
Google Scholar * Adani, G., Filippini, T., Michalke, B. & Vinceti, M. Selenium and other trace elements in the etiology of Parkinson’s disease: a systematic review and meta-analysis of
case-control studies. _Neuroepidemiology_ 54, 1–23 (2020). Article PubMed Google Scholar * Parmalee, N. L. & Aschner, M. Manganese and aging. _Neurotoxicology_ 56, 262–268 (2016).
Article CAS PubMed Google Scholar * Rayman, M. P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. _Proc. Nutr. Soc._ 78, 34–44
(2019). Article CAS PubMed Google Scholar * Reyes-Hinojosa, D. et al. Toxicity of cadmium in musculoskeletal diseases. _Environ. Toxicol. Pharmacol._ 72, 103219 (2019). Article CAS
PubMed Google Scholar * Bonaventura, P., Benedetti, G., Albarède, F. & Miossec, P. Zinc and its role in immunity and inflammation. _Autoimmun. Rev._ 14, 277–285 (2015). Article CAS
PubMed Google Scholar * Marín Martínez, L., Molino Pagán, D. & López Jornet, P. Trace elements in saliva as markers of type 2 diabetes mellitus. _Biol. Trace Elem. Res._ 186, 354–360
(2018). Article PubMed Google Scholar * Fernández-Cao, J. C. et al. Zinc intake and status and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. _Nutrients_ 11,
1027 (2019). Article PubMed PubMed Central Google Scholar * Sanjeevi, N., Freeland-Graves, J., Beretvas, N. S. & Sachdev, P. K. Trace element status in type 2 diabetes: a
meta-analysis. _J. Clin. Diagn. Res._ 12, OE01–OE08 (2018). CAS PubMed Google Scholar * Eshak, E. S. et al. Manganese intake from foods and beverages is associated with a reduced risk of
type 2 diabetes. _Maturitas_ 143, 127–131 (2021). Article CAS PubMed Google Scholar * Vinceti, M., Filippini, T. & Rothman, K. J. Selenium exposure and the risk of type 2 diabetes: a
systematic review and meta-analysis. _Eur. J. Epidemiol._ 33, 789–810 (2018). Article PubMed Google Scholar * Costello, L. C. & Franklin, R. B. Zinc is decreased in prostate cancer:
an established relationship of prostate cancer! _J. Biol. Inorg. Chem._ 16, 3–8 (2011). Article CAS PubMed Google Scholar * Prasad, A. S., Beck, F. W. J., Snell, D. C. & Kucuk, O.
Zinc in cancer prevention. _Nutr. Cancer_ 61, 879–887 (2009). Article CAS PubMed Google Scholar * Vinceti, M. et al. Selenium for preventing cancer. _Cochrane Database Syst. Rev._ 1,
CD005195 (2018). PubMed Google Scholar * Zheng, J., Mao, X., Ling, J., He, Q. & Quan, J. Low serum levels of zinc, copper, and iron as risk factors for osteoporosis: a meta-analysis.
_Biol. Trace Elem. Res._ 160, 15–23 (2014). Article CAS PubMed Google Scholar * Alghadir, A. H., Gabr, S. A., Al-Eisa, E. S. & Alghadir, M. H. Correlation between bone mineral
density and serum trace elements in response to supervised aerobic training in older adults. _Clin. Interv. Aging_ 11, 265–273 (2016). CAS PubMed PubMed Central Google Scholar * Aaseth,
J., Boivin, G. & Andersen, O. Osteoporosis and trace elements—an overview. _J. Trace Elem. Med. Biol._ 26, 149–152 (2012). Article CAS PubMed Google Scholar * Kołodziejska, B.,
Stępień, N. & Kolmas, J. The influence of strontium on bone tissue metabolism and its application in osteoporosis treatment. _Int. J. Mol. Sci._ 22, 6564 (2021). Article PubMed PubMed
Central Google Scholar * Krokstad, S. et al. Cohort profile: the HUNT study, Norway. _Int. J. Epidemiol._ 42, 968–977 (2013). Article CAS PubMed Google Scholar * Åsvold, B. O. et al.
Cohort profile update: the HUNT study, Norway. _Int. J. Epidemiol._ 52, e80–e91 (2022). Article PubMed Central Google Scholar * Meltzer, H. M. et al. Low iron stores are related to higher
blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. _Environ. Res._ 110, 497–504 (2010). Article CAS PubMed Google Scholar *
Syversen, T. et al. Trace elements in the large population-based HUNT3 survey. _Biol. Trace Elem. Res._ 199, 2467–2474 (2021). Article CAS PubMed Google Scholar * Rønningen, K. S. et al.
The biobank of the Norwegian mother and child cohort study: a resource for the next 100 years. _Eur. J. Epidemiol._ 21, 619–625 (2006). Article PubMed PubMed Central Google Scholar *
Paltiel, L. et al. The biobank in the Norwegian Mother and Child Cohort Study—present status. _Nor. Epidemiol._ 24, 29–35 (2014). Google Scholar * Magnus, P. et al. Cohort Profile Update:
The Norwegian Mother and Child Cohort Study (MoBa). _Int. J. Epidemiol._ 45, 382–388 (2016). Article PubMed Google Scholar * McCarthy, S. et al. A reference panel of 64,976 haplotypes for
genotype imputation. _Nat. Genet_. 48, 1279–1283 (2016). Article CAS PubMed PubMed Central Google Scholar * Rentschler, G. et al. Cadmium concentrations in human blood and urine are
associated with polymorphisms in zinc transporter genes. _Metallomics_ 6, 885–891 (2014). Article CAS PubMed Google Scholar * Claus Henn, B. et al. Associations of iron metabolism genes
with blood manganese levels: a population-based study with validation data from animal models. _Environ. Health_ 10, 97 (2011). Article PubMed PubMed Central Google Scholar * Nieboer, E.
& Richardson, D. H. S. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. _Environ. Pollut. B_ 1, 3–26
(1980). Article CAS Google Scholar * Wei, W. Q. et al. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic
health record. _PLoS One_ 12, e0175508 (2017). Article PubMed PubMed Central Google Scholar * Wang, C.-Y., Meynard, D. & Lin, H. Y. The role of TMPRSS6/matriptase-2 in iron
regulation and anemia. _Front Pharm._ 5, 114 (2014). Article Google Scholar * Moksnes, M. R. et al. Genome-wide meta-analysis of iron status biomarkers and the effect of iron on all-cause
mortality in HUNT. _Commun. Biol._ 5, 591 (2022). Article CAS PubMed PubMed Central Google Scholar * Waikar, S. S. & Bonventre, J. V. Creatinine kinetics and the definition of acute
kidney injury. _J. Am. Soc. Nephrol._ 20, 672–679 (2009). Article CAS PubMed PubMed Central Google Scholar * Yang, F. et al. Characterization, mapping, and expression of the human
ceruloplasmin gene. _Proc. Natl. Acad. Sci. USA_ 83, 3257–3261 (1986). Article CAS PubMed PubMed Central Google Scholar * Pinter, T. B. J. & Stillman, M. J. The zinc balance:
competitive zinc metalation of carbonic anhydrase and metallothionein 1A. _Biochemistry_ 53, 6276–6285 (2014). Article CAS PubMed Google Scholar * Chen, P., Bowman, A. B., Mukhopadhyay,
S. & Aschner, M. SLC30A10: a novel manganese transporter. _Worm_ 4, e1042648 (2015). Article PubMed PubMed Central Google Scholar * Nebert, D. W. & Liu, Z. SLC39A8 gene encoding
a metal ion transporter: discovery and bench to bedside. _Hum. Genom._ 13, 51 (2019). Article Google Scholar * Park, J. H. et al. SLC39A8 deficiency: biochemical correction and major
clinical improvement by manganese therapy. _Genet. Med._ 20, 259–268 (2018). Article CAS PubMed Google Scholar * Manousaki, D. et al. Genome-wide association study for vitamin D levels
reveals 69 independent loci. _Am. J. Hum. Genet._ 106, 327–337 (2020). Article CAS PubMed PubMed Central Google Scholar * Mathew, A., Bashir, S., de Roos, B. & Sneddon, A. A.
Interaction of selenium and vitamin D and its relevance to atherosclerosis. _Proc. Nutr. Soc._ 78, E41 (2019). Article Google Scholar * Hu, L. et al. Mitochondrial protein profile in mice
with low or excessive selenium diets. _Int. J. Mol. Sci._ 17, 1137 (2016). Article PubMed PubMed Central Google Scholar * Bárány, E. et al. Iron status influences trace element levels in
human blood and serum. _Environ. Res._ 98, 215–223 (2005). Article PubMed Google Scholar * Tapiero, H., Townsend, D. M. & Tew, K. D. Trace elements in human physiology and pathology.
Copper. _Biomed. Pharmacother._ 57, 386–398 (2003). Article CAS PubMed PubMed Central Google Scholar * Ye, Q. et al. Influence of iron metabolism on manganese transport and toxicity.
_Metallomics_ 9, 1028–1046 (2017). Article CAS PubMed Google Scholar * An, P. et al. TMPRSS6, but not TF, TFR2 or BMP2 variants are associated with increased risk of iron-deficiency
anemia. _Hum. Mol. Genet_. 21, 2124–2131 (2012). Article CAS PubMed Google Scholar * Lin, C. C. et al. Terminal uridyltransferase 7 regulates TLR4-triggered inflammation by controlling
Regnase-1 mRNA uridylation and degradation. _Nat. Commun._ 12, 3878 (2021). Article CAS PubMed PubMed Central Google Scholar * Camaschella, C., Nai, A. & Silvestri, L. Iron
metabolism and iron disorders revisited in the hepcidin era. _Haematologica_ 105, 260–272 (2020). Article PubMed PubMed Central Google Scholar * Yien, Y. Y. et al. FAM210B is an
erythropoietin target and regulates erythroid heme synthesis by controlling mitochondrial iron import and ferrochelatase activity. _J. Biol. Chem._ 293, 19797–19811 (2018). Article CAS
PubMed PubMed Central Google Scholar * Tong, W. H. et al. TLR-activated repression of Fe-S cluster biogenesis drives a metabolic shift and alters histone and tubulin acetylation. _Blood
Adv._ 2, 1146–1156 (2018). Article CAS PubMed PubMed Central Google Scholar * Ast, T. et al. Hypoxia rescues frataxin loss by restoring iron sulfur cluster biogenesis. _Cell_ 177,
1507–1521.e16 (2019). Article CAS PubMed PubMed Central Google Scholar * Chen, M. H. et al. Trans-ethnic and ancestry-specific blood-cell genetics in 746,667 individuals from 5 global
populations. _Cell_ 182, 1198–1213.e14 (2020). Article CAS PubMed PubMed Central Google Scholar * Roggenbeck, B. A., Banerjee, M. & Leslie, E. M. Cellular arsenic transport pathways
in mammals. _J. Environ. Sci._ 49, 38–58 (2016). Article CAS Google Scholar * Rigutto, S. et al. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by
cAMP-dependent protein kinase and protein kinase C-dependent phosphorylation. _J. Biol. Chem._ 284, 6725–6734 (2009). Article CAS PubMed PubMed Central Google Scholar * Meitzler, J. L.
& Ortiz De Montellano, P. R. Structural stability and heme binding potential of the truncated human dual oxidase 2 (DUOX2) peroxidase domain. _Arch. Biochem. Biophys._ 512, 197–203
(2011). Article CAS PubMed PubMed Central Google Scholar * Bell, S. et al. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. _Commun.
Biol._ 4, 156 (2021). Article CAS PubMed PubMed Central Google Scholar * Suhre, K. et al. A genome-wide association study of metabolic traits in human urine. _Nat. Genet._ 43, 565–569
(2011). Article CAS PubMed Google Scholar * Shi, X. et al. Metabolomic analysis of the effects of chronic arsenic exposure in a mouse model of diet-induced fatty liver disease. _J.
Proteome Res._ 13, 547–554 (2014). Article CAS PubMed Google Scholar * Madsen, M., Graversen, J. H. & Moestrup, S. K. Haptoglobin and CD163: Captor and receptor gating hemoglobin to
macrophage lysosomes. _Redox Rep._ 6, 386–388 (2001). Article CAS PubMed Google Scholar * Soranzo, N. et al. Common variants at 10 genomic loci influence hemoglobin A1C levels via
glycemic and nonglycemic pathways. _Diabetes_ 59, 3229–3239 (2010). Article CAS PubMed PubMed Central Google Scholar * Gommers, L. M. M., Hoenderop, J. G. J., Bindels, R. J. M. & De
Baaij, J. H. F. Hypomagnesemia in type 2 diabetes: a vicious circle? _Diabetes_ 65, 3–13 (2016). Article CAS PubMed Google Scholar * Mohlke, K. L. & Boehnke, M. Recent advances in
understanding the genetic architecture of type 2 diabetes. _Hum. Mol. Genet._ 24, R85–R92 (2015). Article CAS PubMed PubMed Central Google Scholar * Wang, D., Wang, Y., Madhu, S.,
Liang, H. & Bray, C. L. Total hemoglobin count has significant impact on A1C—Data from National Health and Nutrition Examination Survey 1999–2014. _Prim. Care Diabetes_ 13, 316–323
(2019). Article PubMed Google Scholar * Marie, P. J., Ammann, P., Boivin, G. & Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. _Calcif. Tissue Int._ 69,
121–129 (2001). Article CAS PubMed Google Scholar * Ochoa, D. et al. Open targets platform: supporting systematic drug-target identification and prioritisation. _Nucleic Acids Res._ 49,
D1302–D1310 (2021). Article CAS PubMed Google Scholar * Hong, G. et al. The emerging role of MORC family proteins in cancer development and bone homeostasis. _J. Cell Physiol._ 232,
928–934 (2017). Article CAS PubMed Google Scholar * Brissot, P. et al. Haemochromatosis. _Nat. Rev. Dis. Prim._ 4, 18016 (2018). Article PubMed Google Scholar * Pietrangelo, A.
Hereditary hemochromatosis—a new look at an old disease. _N. Engl. J. Med._ 350, 2383–2397 (2004). Article CAS PubMed Google Scholar * Benyamin, B. et al. Novel loci affecting iron
homeostasis and their effects in individuals at risk for hemochromatosis. _Nat. Commun._ 5, 4926 (2014). Article CAS PubMed Google Scholar * Evans, L. M. et al. Comparison of methods
that use whole genome data to estimate the heritability and genetic architecture of complex traits. _Nat. Genet._ 50, 737–745 (2018). Article CAS PubMed PubMed Central Google Scholar *
Khan, M. A. K. & Wang, F. Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. _Environ. Toxicol. Chem._
28, 1567–1577 (2009). Article CAS PubMed Google Scholar * Oczkowski, M., Dziendzikowska, K., Pasternak-Winiarska, A., Włodarek, D. & Gromadzka-Ostrowska, J. Dietary factors and
prostate cancer development, progression, and reduction. _Nutrients_ 13, 496 (2021). Article CAS PubMed PubMed Central Google Scholar * Bernard, A. & Lauwers, R. Metal‐induced
alterations of δ‐aminolevulinic acid dehydratase. _Ann. N. Y. Acad. Sci._ 514, 41–47 (1987). Article CAS PubMed Google Scholar * Morelli, A., Ravera, S., Calzia, D. & Panfoli, I.
Impairment of heme synthesis in myelin as potential trigger of multiple sclerosis. _Med. Hypotheses_ 78, 707–710 (2012). Article CAS PubMed Google Scholar * Burgess, S. & Thompson,
S. G. Avoiding bias from weak instruments in Mendelian randomization studies. _Int. J. Epidemiol._ 40, 755–764 (2011). Article PubMed Google Scholar * Sanderson, E., Richardson, T. G.,
Morris, T. T., Tilling, K. & Smith, G. D. Estimation of causal effects of a time-varying exposure at multiple time points through Multivariable Mendelian randomization. _PLoS Genet_. 18,
e1010290 (2022). Article CAS PubMed PubMed Central Google Scholar * Longnecker, M. P. et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate
measure of selenium intake. _Epidemiology_ 7, 384–390 (1996). Article CAS PubMed Google Scholar * Długaszek, M. Studies on relationships between essential and toxic elements in selected
body fluids, cells and tissues. _Chem. Biol. Interact._ 297, 57–66 (2019). Article PubMed Google Scholar * Rahil-Khazen, R., Botann, B. J., Myking, A. & Ulvik, R. Multi-element
analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES). _J. Trace Elem. Med. Biol._ 16, 15–25 (2002). Article
CAS PubMed Google Scholar * Leivuori, M. & Niemistö, L. Sedimentation of trace metals in the Gulf of Bothnia. _Chemosphere_ 31, 3839–3856 (1995). Article CAS Google Scholar *
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. _Nat. Genet._ 47, 1236–1241 (2015). Article CAS PubMed PubMed Central Google Scholar *
Ferreira, M. A. et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. _Nat. Genet._ 49, 1752–1757 (2017). Article CAS PubMed PubMed Central
Google Scholar * Iglesias, A. I. et al. Haplotype reference consortium panel: Practical implications of imputations with large reference panels. _Hum. Mutat._ 38, 1025–1032 (2017). Article
CAS PubMed Google Scholar * Das, S. et al. Next-generation genotype imputation service and methods. _Nat. Genet._ 48, 1284–1287 (2016). Article CAS PubMed PubMed Central Google
Scholar * Helgeland, Ø. et al. Characterization of the genetic architecture of infant and early childhood body mass index. _Nat. Metab._ 4, 344–358 (2022). Article CAS PubMed Google
Scholar * Loh, P. R. et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. _Nat. Genet._ 47, 284–290 (2015). Article CAS PubMed PubMed Central
Google Scholar * Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. _Gigascience_ 4, 7 (2015). Article PubMed PubMed Central Google
Scholar * Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. _Bioinformatics_ 26, 2190–2191 (2010). Article CAS PubMed
PubMed Central Google Scholar * Buniello, A. et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. _Nucleic Acids
Res._ 47, D1005–D1012 (2019). Article CAS PubMed Google Scholar * Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing
data. _Nucleic Acids Res._ 38, e164 (2010). Article PubMed PubMed Central Google Scholar * U.S. Department of Health and Human Services. Chapter 3–Chemistry and Toxicology of Cigarette
Smoke and Biomarkers of Exposure and Harm. in _How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General_ 40–41
(Centers for Disease Control and Prevention (US), 2010). * Towle, K. M., Garnick, L. C. & Monnot, A. D. A human health risk assessment of lead (Pb) ingestion among adult wine consumers.
_Int. J. Food Contam_ 4, 7 (2017). Article Google Scholar * Næss, S. et al. Mercury, lead, arsenic, and cadmium in Norwegian seafood products and consumer exposure. _Food Addit. Contam.
Part B Surveil_ 13, 99–106 (2020). Article Google Scholar * Skalny, A. V., Skalnaya, M. G., Grabeklis, A. R., Skalnaya, A. A. & Tinkov, A. A. Zinc deficiency as a mediator of toxic
effects of alcohol abuse. _Eur. J. Nutr._ 57, 2313–2322 (2018). Article CAS PubMed Google Scholar * Han, M., Böhlke, M., Maher, T. & Kim, J. Alcohol exposure increases manganese
accumulation in the brain and exacerbates manganese-induced neurotoxicity in mice. _Arch. Toxicol._ 95, 3665–3679 (2021). Article CAS PubMed Google Scholar * Dostalikova-Cimburova, M. et
al. Role of duodenal iron transporters and hepcidin in patients with alcoholic liver disease. _J. Cell Mol. Med._ 18, 1840–1850 (2014). Article CAS PubMed PubMed Central Google Scholar
* Bulik-Sullivan, B. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. _Nat. Genet._ 47, 291–295 (2015). Article CAS PubMed
PubMed Central Google Scholar * Pan-UKBB team [Data set] https://pan.ukbb.broadinstitute.org (2020). * Hemani, G. et al. The MR-Base platform supports systematic causal inference across
the human phenome. _eLife_ 7, e34408 (2018). Article PubMed PubMed Central Google Scholar * Hemani, G. MRInstruments: data sources for genetic instruments to be used in MR. R package
version 0.3.2. https://github.com/MRCIEU/MRInstruments/ (2020). * Balachandran, R. C. et al. Brain manganese and the balance between essential roles and neurotoxicity. _J. Biol. Chem._ 295,
6312–6329 (2020). Article CAS PubMed PubMed Central Google Scholar * Saevarsdottir, S. et al. Multiomics analysis of rheumatoid arthritis yields sequence variants that have large
effects on risk of the seropositive subset. _Ann. Rheum. Dis._ 81, 1085–1095 (2022). Article CAS PubMed Google Scholar * Patsopoulos, N. A. et al. Multiple sclerosis genomic map
implicates peripheral immune cells and microglia in susceptibility. _Science (2019)_ 365, eaav7188 (2019). CAS Google Scholar * Huyghe, J. R. et al. Discovery of common and rare genetic
risk variants for colorectal cancer. _Nat. Genet._ 51, 76–87 (2019). Article CAS PubMed Google Scholar * Schumacher, F. R. et al. Association analyses of more than 140,000 men identify
63 new prostate cancer susceptibility loci. _Nat. Genet._ 50, 928–936 (2018). Article CAS PubMed PubMed Central Google Scholar * Nalls, M. A. et al. Identification of novel risk loci,
causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. _Lancet Neurol._ 18, 1091–1102 (2019). Article CAS PubMed PubMed Central
Google Scholar * Morris, J. A. et al. An atlas of genetic influences on osteoporosis in humans and mice. _Nat. Genet._ 51, 258–266 (2019). Article CAS PubMed Google Scholar *
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. _Nat. Genet._ 54, 412–436 (2022). Article CAS PubMed PubMed Central Google
Scholar * Saevarsdottir, S. et al. FLT3 stop mutation increases FLT3 ligand level and risk of autoimmune thyroid disease. _Nature_ 584, 619–623 (2020). Article CAS PubMed Google Scholar
* Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. _Nat. Genet._ 50, 1505–1513 (2018).
Article CAS PubMed PubMed Central Google Scholar * Saevarsdottir, S. et al. Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the
seropositive subset. [Data set] https://download.decode.is/form/2022/RA_All.txt.gz (2022). * Patsopoulos, N. A. et al. Multiple sclerosis genomic map implicates peripheral immune cells and
microglia in susceptibility. [Data set] https://imsgc.net/ (2019). * Huyghe, J. R. et al. Discovery of common and rare genetic risk variants for colorectal cancer. [Data set] _NHGRI-EBI GWAS
Catalog_ https://www.ebi.ac.uk/gwas/studies/GCST012879 (2019). * Schumacher, F. R. et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci.
[Data set] http://practical.icr.ac.uk/blog/?page_id=8164 (2018). * Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a
meta-analysis of genome-wide association studies. [Data set] https://drive.google.com/drive/folders/10bGj6HfAXgl-JslpI9ZJIL_JIgZyktxn (2019). * Morris, J. A. et al. An atlas of genetic
influences on osteoporosis in humans and mice. [Data set] http://www.gefos.org/sites/default/files/Morrisetal2018.NatGen.SumStats.tar_0.gz (2019). * Bellenguez, C. et al. New insights into
the genetic etiology of Alzheimer’s disease and related dementias. [Data set] _NHGRI-EBI GWAS Catalog_ https://www.ebi.ac.uk/gwas/studies/GCST90027158/ (2022). * Saevarsdottir, S. et al.
FLT3 stop mutation increases FLT3 ligand level and risk of autoimmune thyroid disease. [Data set] https://download.decode.is/form/2020/AITD2020.gz (2020). * Mahajan, A. et al. Fine-mapping
type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. [Data set] http://diagram-consortium.org/downloads.html (2018). Download
references ACKNOWLEDGEMENTS The Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Center (Faculty of Medicine and Health Sciences, NTNU, Norwegian University
of Science and Technology), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. The genotyping in HUNT was financed by the
National Institutes of Health (NIH R35 HL135824-03); University of Michigan; the Research Council of Norway; the Liaison Committee for Education, Research and Innovation in Central Norway;
and the Joint Research Committee between St Olav’s hospital and the Faculty of Medicine and Health Sciences, NTNU. The genetic investigations of the HUNT Study are a collaboration between
researchers from the K.G. Jebsen Center for Genetic Epidemiology, NTNU, and the University of Michigan Medical School and the University of Michigan School of Public Health. The K.G. Jebsen
Center for Genetic Epidemiology was financed by Stiftelsen Kristian Gerhard Jebsen (SKGJ-MED-015); Faculty of Medicine and Health Sciences, NTNU, Norway. We thank HUNT participants for
donating their time, samples, and information to help others. The Norwegian Mother, Father and Child Cohort Study (MoBa) is supported by the Norwegian Ministry of Health and Care Services
and the Ministry of Education and Research. We are grateful to all the participating families in Norway who take part in this on-going cohort study. We thank the Norwegian Institute of
Public Health (NIPH) for generating high-quality genomic data. This research is part of the HARVEST collaboration, supported by the Research Council of Norway (#229624). We also thank the
NORMENT Center for providing genotype data, funded by the Research Council of Norway (#223273), South East Norway Health Authorities and Stiftelsen Kristian Gerhard Jebsen. We further thank
the Center for Diabetes Research, the University of Bergen for providing genotype data and performing quality control and imputation of the data funded by the ERC AdG project
SELECTionPREDISPOSED, Stiftelsen Kristian Gerhard Jebsen, Trond Mohn Foundation, the Research Council of Norway, the Novo Nordisk Foundation, the University of Bergen, and the Western Norway
Health Authorities. The NIPH has contributed to funding of the Norwegian Environmental Biobank (NEB). The laboratory measurements have partly been funded by the Research Council of Norway
through research projects (#275903 and #268465), and the human biomonitoring project HBM4EU, funded by the European Union’s Horizon 2020 research and innovation program under grant agreement
No 733032. FUNDING Open access funding provided by Norwegian University of Science and Technology. AUTHOR INFORMATION Author notes * These authors jointly supervised this work: Cristen J.
Willer, Kristian Hveem, Ben M. Brumpton. AUTHORS AND AFFILIATIONS * HUNT Center for Molecular and Clinical Epidemiology, Department of Public Health and Nursing, NTNU—Norwegian University of
Science and Technology, Trondheim, Norway Marta R. Moksnes, Ailin F. Hansen, Brooke N. Wolford, Laurent F. Thomas, Humaira Rasheed, Laxmi Bhatta, Nicole M. Warrington, Therese H. Nøst,
Bjørn Olav Åsvold, Cristen J. Willer, Kristian Hveem & Ben M. Brumpton * Department of Clinical and Molecular Medicine, NTNU—Norwegian University of Science and Technology, Trondheim,
Norway Laurent F. Thomas * BioCore—Bioinformatics Core Facility, NTNU—Norwegian University of Science and Technology, Trondheim, Norway Laurent F. Thomas * Clinic of Laboratory Medicine, St.
Olav’s Hospital, Trondheim University Hospital, Trondheim, Norway Laurent F. Thomas * MRC Integrative Epidemiology Unit, Population Health Sciences, Bristol Medical School, University of
Bristol, Bristol, UK Humaira Rasheed * Division of Medicine and Laboratory Sciences, University of Oslo, Oslo, Norway Humaira Rasheed * Department of Chemistry, NTNU—Norwegian University of
Science and Technology, Trondheim, Norway Anica Simić & Trond Peder Flaten * Department of Food Safety, Division of Climate and Environmental Health, Norwegian Institute of Public
Health, Oslo, Norway Anne Lise Brantsæter * Department of Internal Medicine, Division of Cardiology, University of Michigan, Ann Arbor, MI, USA Ida Surakka * Department of Computational
Medicine and Bioinformatics, University of Michigan, Ann Arbor, MI, USA Wei Zhou & Cristen J. Willer * Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston,
MA, USA Wei Zhou * Program in Medical and Population Genetics, Broad Institute of Harvard and MIT, Cambridge, MA, USA Wei Zhou * Centre for Fertility and Health, Norwegian Institute of
Public Health, Oslo, Norway Per Magnus * Mohn Center for Diabetes Precision Medicine, Department of Clinical Science, University of Bergen, Bergen, Norway Pål R. Njølstad * Children and
Youth Clinic, Haukeland University Hospital, Bergen, Norway Pål R. Njølstad * NORMENT Centre, University of Oslo, Oslo, Norway Ole A. Andreassen * Division of Mental Health and Addiction,
Oslo University Hospital, Oslo, Norway Ole A. Andreassen * Department of Neuroscience, NTNU—Norwegian University of Science and Technology, Trondheim, Norway Tore Syversen * Department of
Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China Jie Zheng *
Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key
Laboratory for Endocrine Tumor, Shanghai Digital Medicine Innovation Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China Jie Zheng * MRC Integrative
Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Bristol, UK Jie Zheng * Department of Biostatistics, University of Michigan School of Public Health, Ann Arbor, MI,
USA Lars G. Fritsche * Center for Statistical Genetics, University of Michigan School of Public Health, Ann Arbor, MI, USA Lars G. Fritsche * Institute for Molecular Biosciences, The
University of Queensland, Brisbane, QLD, Australia David M. Evans & Nicole M. Warrington * Frazer Institute, The University of Queensland, Woolloongabba, QLD, Australia David M. Evans
& Nicole M. Warrington * MRC Integrative Epidemiology Unit (IEU), University of Bristol, Bristol, UK David M. Evans & Nicole M. Warrington * Department of Community Medicine, UiT The
Arctic University of Norway, Tromsø, Norway Therese H. Nøst * HUNT Research Centre, Department of Public Health and Nursing, NTNU—Norwegian University of Science and Technology, Levanger,
Norway Bjørn Olav Åsvold, Kristian Hveem & Ben M. Brumpton * Department of Endocrinology, Clinic of Medicine, St. Olav’s Hospital, Trondheim University Hospital, Trondheim, Norway Bjørn
Olav Åsvold * Division of Cardiovascular Medicine, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA Cristen J. Willer * Department of Human Genetics, University of
Michigan, Ann Arbor, MI, USA Cristen J. Willer * Clinic of Medicine, St. Olav’s Hospital, Trondheim University Hospital, Trondheim, Norway Ben M. Brumpton Authors * Marta R. Moksnes View
author publications You can also search for this author inPubMed Google Scholar * Ailin F. Hansen View author publications You can also search for this author inPubMed Google Scholar *
Brooke N. Wolford View author publications You can also search for this author inPubMed Google Scholar * Laurent F. Thomas View author publications You can also search for this author
inPubMed Google Scholar * Humaira Rasheed View author publications You can also search for this author inPubMed Google Scholar * Anica Simić View author publications You can also search for
this author inPubMed Google Scholar * Laxmi Bhatta View author publications You can also search for this author inPubMed Google Scholar * Anne Lise Brantsæter View author publications You
can also search for this author inPubMed Google Scholar * Ida Surakka View author publications You can also search for this author inPubMed Google Scholar * Wei Zhou View author publications
You can also search for this author inPubMed Google Scholar * Per Magnus View author publications You can also search for this author inPubMed Google Scholar * Pål R. Njølstad View author
publications You can also search for this author inPubMed Google Scholar * Ole A. Andreassen View author publications You can also search for this author inPubMed Google Scholar * Tore
Syversen View author publications You can also search for this author inPubMed Google Scholar * Jie Zheng View author publications You can also search for this author inPubMed Google Scholar
* Lars G. Fritsche View author publications You can also search for this author inPubMed Google Scholar * David M. Evans View author publications You can also search for this author
inPubMed Google Scholar * Nicole M. Warrington View author publications You can also search for this author inPubMed Google Scholar * Therese H. Nøst View author publications You can also
search for this author inPubMed Google Scholar * Bjørn Olav Åsvold View author publications You can also search for this author inPubMed Google Scholar * Trond Peder Flaten View author
publications You can also search for this author inPubMed Google Scholar * Cristen J. Willer View author publications You can also search for this author inPubMed Google Scholar * Kristian
Hveem View author publications You can also search for this author inPubMed Google Scholar * Ben M. Brumpton View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS M.R.M. analyzed the data and wrote the first draft of the manuscript. B.M.B., K.H., and C.J.W. conceived and designed the study. A.F.H., B.N.W., L.F.T., H.R., and L.B.
contributed to analyses. A.S., T.S., T.H.N., and T.P.F. gave advice on phenotypes and phenotype definitions. K.H., C.J.W., P.M., P.R.N., O.A.A., A.L.B., T.S., A.S., and T.P.F. contributed to
data acquisition. M.R.M., A.F.H., I.S., W.Z., J.Z., L.F., D.M.E., N.M.W., T.H.N., B.O.Å., T.P.F., C.J.W., K.H. and B.M.B. interpreted the results. All authors revised the final version of
the paper. CORRESPONDING AUTHORS Correspondence to Marta R. Moksnes or Ben M. Brumpton. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS APPROVAL
The current study has been approved by the Norwegian Data Protection Authority and approved by the Regional Ethics Committee for Medical and Health Research Ethics in Northern Norway (REK
Reference Number: 51604). All MoBa and HUNT participants have signed informed written consent. The MoBa cohort is currently regulated by the Norwegian Health Registry Act. PEER REVIEW PEER
REVIEW INFORMATION _Communications Biology_ thanks Kaixiong Ye and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Kaoru
Ito and George Inglis. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1-12 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Moksnes, M.R., Hansen, A.F., Wolford, B.N. _et al._ A
genome-wide association study provides insights into the genetic etiology of 57 essential and non-essential trace elements in humans. _Commun Biol_ 7, 432 (2024).
https://doi.org/10.1038/s42003-024-06101-z Download citation * Received: 09 May 2023 * Accepted: 22 March 2024 * Published: 09 April 2024 * DOI: https://doi.org/10.1038/s42003-024-06101-z
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative