Play all audios:
ABSTRACT STUDY DESIGN: Prospective clinical study of two treatments. OBJECTIVE: To compare mechanical ventilation (MV) with phrenic nerve stimulation (PNS) for treatment of respiratory
device-dependent (RDD) spinal cord-injured (SCI) patients. SETTING: Department for spinal cord-injured patients of an insurance-company-run trauma hospital in Hamburg, Germany. METHODS:
Prospective data collection of treatment-related data over 20 years. RESULTS: In total, 64 SCI-RDD patients were treated during the study period. Of these, 32 of the patients with
functioning phrenic nerves and diaphragm muscles were treated with PNS and 32 patients with destroyed phrenic nerves were mechanically ventilated. Incidence of respiratory infections (RIs
per 100 days) prior to use of final respiratory device was equal in both groups, that is (median (interquartile range)) 1.43 (0.05–3.92) with PNS and 1.33 (0.89–2.21) with MV (_P_=0.888);
with final device in our institution it was 0 (0–0.92) with PNS and 2.07 (1.49–4.19) with MV (_P_<0.001); at final location it was 0 (0–0.02) with PNS and 0.14 (0–0.31) with MV
(_P_<0.001). Thus, compared to MV, respiratory treatment with PNS significantly reduces frequency of RI. Quality of speech is significantly better with PNS. Nine patients with PNS, but
only two with MV, were employed or learned after rehabilitation (_P_=0.093). The primary investment in the respiratory device is higher with PNS, but it can be paid off in our setting within
1 year because of the reduced amount of single use equipment, easier nursing and fewer RIs compared to MV. CONCLUSIONS: PNS instead of MV for treatment of SCI-RDD reduces RIs, running costs
of respiratory treatment and obviously improves patients' quality of life. SIMILAR CONTENT BEING VIEWED BY OTHERS PULMONARY REHABILITATION IN HIGH CERVICAL SPINAL CORD INJURY: A SERIES
OF 133 CONSECUTIVE CASES Article 28 May 2022 TRANSCUTANEOUS ELECTRICAL DIAPHRAGMATIC STIMULATION REDUCES THE DURATION OF INVASIVE MECHANICAL VENTILATION IN PATIENTS WITH CERVICAL SPINAL
CORD INJURY: RETROSPECTIVE CASE SERIES Article 09 April 2021 RESPIRATORY COMPLICATIONS DURING INITIAL REHABILITATION AND SURVIVAL FOLLOWING SPINAL CORD INJURY IN SWEDEN: A RETROSPECTIVE
STUDY Article 22 September 2020 INTRODUCTION Permanent respiratory device dependency (RDD) due to cervical spinal cord injury (SCI) traditionally is treated with different kinds of
mechanical ventilation (MV).1, 2 However, electroventilation3 has become a choice again through its modern versions such as diaphragm pacing (DP),4, 5 carousel stimulation (CS)6 and
four-pole-sequential phrenic nerve stimulation (PNS).7 In electroventilation, an electrical system rhythmically stimulating the phrenic nerves takes over for the malfunctioning or
inaccessible respiratory centre; a normal phrenic nerve and normal diaphragm muscle are prerequisites.8 The reason to develop the Diaphragm Pacer4 and similar devices6, 7 was to ‘free the
patient from the mechanical ventilator’.4 By using the mechanical energy of the patient's diaphragm, the patient is freed from the ventilator tube, the tracheostoma and with his helpers
from the bulky energy supply of mechanical ventilators (MVs). However, when deciding on which device to use, more weight was frequently put on the higher price for the device, the surgical
risk of implantation, improvement of MVs and absence of indications than was put on freedom from MV, the improved quality of speech and nursing facilitation.1 Previous publications comparing
MV and electroventilation for SCI patients did not present evidence in favour of one or the other solution, but reported the opinions of authors and patients.9, 10, 11, 12, 13 We,
therefore, when starting to use PNS in Hamburg, decided to collect prospectively clinically meaningful data. Our main outcomes were survival, frequency of respiratory tract infection (RI)
and resocialization, that is living at home, learning or earning one's livelihood. The aim of the study thus was to provide data for the clinician to decide, whether to use MV or PNS in
respiratory device-dependent tetraplegic patients. PATIENTS AND METHODS We included all patients treated in the special unit for RDD, patients of the department for SCI, patients of the
insurance company-run (BG) Trauma Hospital in Hamburg, Germany, from 1987 through 2006. All RDD-patients were primarily mechanically ventilated through tracheostoma. The tracheostomy tube
was plugged or the tracheostoma was lost in patients on PNS. Most patients were referred from hospitals that provide primary care and rehabilitation for SCI, but have no possibility to check
the function of phrenic nerves and diaphragm muscles, cannot implant a PNS and cannot condition5 the muscle. The function of phrenic nerves and diaphragm muscles was ascertained by
neurophysiologic and fluoroscopic/sonographic studies.14 Whenever possible with patients who agreed, we implanted a phrenic nerve stimulator (Atrotech Ltd, Tampere, Finland).7 All patients
with non-functioning phrenic nerves received a mobile MV. Ventilator settings: pressure controlled through tracheostoma, positive end-expired pressure 2–8 cm H2O, _P_-in 8–20 cm H2O, _T_-in
1.5–2.5 s; when speaking, _T_-in is increased to 2.5 s and _P_-in to 20 cm H2O. For PNS and MV, respiratory rate is 8–14 times per minute and I:E 1–1.5 or 2. No special adjustment is used
for speaking with PNS. With both modes of ventilation we aimed at low respiratory frequencies, reasonable tidal volumes and normal oxygen saturation and end tidal carbon dioxide tension when
using room air. During their stay in our department, we registered the patients' time from trauma to their arrival in our department, their length of stay in our department, the
pre-trauma social conditions and any possible chronic diseases or handicaps, the frequency of RIs and the quality of speech. Data on RI were also collected for the interim time outside of
our department. The agreed definition of RI was the patient presented with fever, leucocytosis, increased production of secretions and the doctor in charge diagnosed the reason to be RI,
with antimicrobic therapy being necessary. Three periods concerning RI appear in the course after SCI: the period before use of final respiratory device (1), of which we analysed the final
120 days only; the period using the final respiratory device inside the institution (2) and the period after institution at the final location (3). The incidence of RI in each period was
calculated and is presented as RI per 100 days. All patients were seen for a check-up once a year. Data were collected for each stay in our department separately. We registered the frequency
of RI and social conditions. If necessary, data were completed from hospital files and from the special file kept in our department. SH personally collected all data for this study and also
evaluated the quality of speech on a scale from 0 to 6 (0: no voice; 1: whispering, intermittently; 2: whispering; 3: low voice, intermittently; 4: low voice; 5: normal voice,
intermittently; 6: normal voice). The ethical committee of our institution approved the study. All patients gave their informed consent. STATISTICS Due to the skew distributions, values of
continuous variables are expressed as median with quartile range or range. Differences between devices were tested by the Mann–Whitney test. Differences between time periods were tested by
the Wilcoxon signed-rank test. Categorical variables were tested by the Pearson's _χ_2-test or Fisher's exact test. Other tests are presented with the appropriate results.
Statistical analyses were performed using SPSS 14.0.2 for Windows. A _P_-value less than 0.05 was considered statistically significant. RESULTS In total, 40 patients stayed in our department
for full rehabilitation, 24 stayed in our special unit for ‘respiratory rehabilitation’ only, that is from the implantation/purchase of the respiratory device until its control was mastered
by patients and caregivers; of the latter 24, 3 visited us for only evaluation of phrenic nerves and diaphragm muscles (Table 1). Duration of rehabilitation was equal for patients on PNS
(249 (7–1303) days) and patients on MV (290 (4–582) days). Recovery from implantation of the PNS and accustoming the diaphragm muscle to its continuous use (conditioning5) lasted only 51.06
(30–196) days and did not prolong rehabilitation. However, after beginning the continuous use of the final respiratory device with patients not primarily treated in other institutions, those
on PNS (_n_=20) remained 483.5 (212–1303) days, those on MV (_n_=17) 348 (257–582) days in our institution. No patient with normal phrenic nerves and diaphragm muscles wanted MV. In total,
32 patients were treated with PNS; 32 patients remained on MV. Patients on MV were significantly older than those on PNS; there were no other significant differences between groups (Table
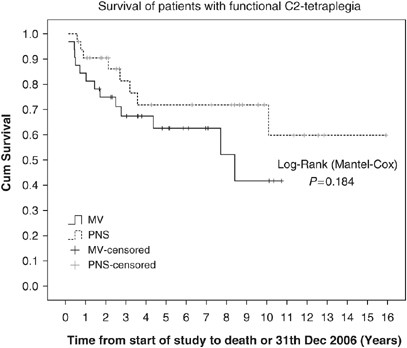
2). Median participation time in the study until death or 31 Dec 2006 was 3.4 years (range, 0.6–15.9) with PNS and 3.6 (0.1–10.7) years with MV. In Figure 1, a trend is obvious in favour of
PNS, but the difference compared to MV is not statistically significant (log-rank _P_=0.184). Total 12 patients on PNS and 14 on MV died during the observation period (_P_=0.1023); of these,
3 with PNS and 10 with MV died of RI (_P_=0.0472). Regarding RI (Table 3), there is no significant difference between groups in period I,. However, during both ‘post-implantation’ periods,
2 and 3, there are significantly fewer RIs with PNS than with MV. There is no difference between PNS and MV for the ability to talk. The quality of speech is significantly better with PNS,
where the lowest score was 3 (6 (5.25–6)), than with MV, where speech scores were frequently 1 and 2 (3.5 (2–5.75)) (_P_<0.001). Total 2 patients on PNS and 4 on MV died in our
institution; 29 on PNS and 25 on MV left home, 1 on PNS and 3 on MV to a nursing home. Today (31 Dec 2006) 20 on PNS and 18 on MV live at home. Seven patients on PNS and two on MV returned
to School or High School, two patients on PNS but none on MV returned to work and all others retired (Table 4). DISCUSSION We aimed at presenting prospectively registered clinically
meaningful data on the fate of patients treated with PNS or MV for treatment of SCI-RDD. Survival, RI and resocialization were the main outcomes. Whereas survival and RI are well-defined
entities, no validated score was available for our patients on their quality of life15 and for their quality of speech when our study started. DURATION OF REHABILITATION Of the first five
patients using PNS, three remained exceptionally long in our department (1303, 1085, 1170 days) because their referring institutions refused to accept patients using such a strange mode of
ventilation (Table 1). The problem was solved after a change of the regulations on home ventilation in 1996. PATIENTS' ALLOCATION Patients were not randomized to PNS or MV. Instead,
fate (peripheral nerve damage or not) and patient choice determined a patient's group. This drawback is present in all reports on the respiratory treatment of SCI-RDD and also in the
rare studies that compared DP, CS or PNS with MV.9, 10, 11, 12, 13 In our department we permanently treat five to eight SCI-RDD patients in all stages of rehabilitation, and about 60
patients a year come for their annual check-up. Thus, every new patient meets many patients using PNS or MV. We did not try to persuade patients suitable for PNS to choose MV in order to
serve as a perfect control. Neither did we try to persuade patients on MV to choose ventilation through nose/face mask, ‘non-invasive ventilation’,16 because of the uncertainty of the
connection, difficulties with bronchial toilet and the impairment of communication. SURVIVAL There is no significant difference in our study in the duration of life (Figure 1). Carter _et
al._9 found longer survival on MV, but estimate that ‘the longevity in this group (MV) may be artificially inflated’ due to the long study period that includes different ways of patient
selection and different versions of devices. Esclarin _et al._10 report on longer life with DP than with MV, but without significant difference. DeVivo and Ivie17 surveyed 435 SCI-RDD
patients from 1973 through 1992; the main reason for premature death was pulmonary complications. RI contributes to death in 13 of 23 of our patients (_P_=0.1023); however, this occurred in
10 of 14 of our MV patients (_P_=0.0472). RESPIRATORY TRACT INFECTION We think the striking difference in RI between patients on PNS and MV (Table 3) is due to the different use of the
tracheostoma. With MV, coughing is impossible, and the tube is frequently opened for suctioning. With PNS the tracheostoma is omitted or the tube is plugged,18, 19 which makes active, though
weak coughing possible and suctioning unnecessary. Esclarin _et al._10 and Soni13 also reported a significant difference of RI in favour of DP/PNS. Wolf _et al._11 found RIs in eight
patients on CS and in six patients on MV, whereas Carter _et al._9 did not report on this detail. PART-TIME USE We encourage our patients to use frog breathing or to use their accessory
respiratory muscles in the neck intermittently, which ability facilitates nursing and improves the chance to survive respiratory device failure. All of our patients need their respiratory
device during sleep. Total 10 patients on PNS and 4 on MV use their device part-time; the latter do so because of our recommendation. With PNS, additional reasons for part-time use are an
intermittently working respiratory centre, one-side implantation or being younger than 10 years of age; for the latter, 12 h per day is recommended maximum stimulation time. The relation of
full-time to part-time use of PNS/DP is also about the same in the patient populations of Carter _et al._,9 Wolf _et al._,11 Soni13 and of Similowski and Derenne12; the latter stressed the
fact that irrational beliefs frequently prevent the patients from using PNS full-time.12 Full-time use of DP for all patients is reported only by Esclarin _et al._10 QUALITY OF SPEECH With
pressure-controlled MV, patients talk during inspiration; with PNS, they talk during expiration. There is no difference between PNS and MV for the ability to talk in our study and that of
Esclarin _et al._10 We additionally assessed the quality of speech, for which no validated tool was available when starting our study. Thus, our findings suffer from being incomparable and
from an unblinded assessor. We found the quality of speech significantly better with PNS than with MV (_P_=0.0005); the same fact is also stated by Wolf _et al._11 QUALITY OF LIFE At the
begin of our study no validated score measured treatment-induced changes in the quality of life of our patients.15 Therefore, we can give only the opinions of patients and doctors, like
previous authors did.5, 9, 10, 11, 12, 13, 19 Patients and their doctors found the quality of life better with PNS than with MV: patients on PNS showed more self-confidence and no one wanted
to return to MV. Patients on MV frequently regretted not to be suitable for PNS. On the Spinal Cord Independence Measure,20 a SCI-RDD patient on MV receives 3 of 100 possible points; when
using PNS, he receives 11 points. That is just 10% of the function of a non-SCI patient, but almost four times more than being on MV. RESOCIALIZATION Table 4 may give the impression that
using PNS leads more frequently to an active life after rehabilitation than when using MV. However, our PNS-treated patients were significantly younger than those treated with MV; therefore,
already before trauma, there are more active persons in group PNS than in group MV. Seven of the nine active PNS users were students/pupils before trauma and went on studying; two had
modern professions, where manual skills are unnecessary. The trend in favour of PNS in Table 4 thus obviously is due to age, not to the type of respiratory treatment. COSTS OF RESPIRATORY
TREATMENT A patient on PNS with a plugged or omitted tracheostoma does not need respiratory tubes, filters and no suction catheters; obviously less money is needed for equipment and for
nursing time. In our setting, we need 1 hour per day more of respiratory nursing with MV than with PNS, which means €10 950 per year; respiratory single use equipment with MV is about €6000
per year. Similowski and Derenne12 reported the costs per patient for respiratory equipment in their stimulated group (_n_=9) to be 66% of that in the group on MV (_n_=13). However, Wolf _et
al._11 saw five paid nurses per patient when using CS, but 3.5 when using MV, which may be due to their more complicated stimulator. In our study and those of Esclarin _et al._10 and
Similowski and Derenne,12 the higher first year investment for PNS is paid off after about 3 years because of the savings in single use equipment and nursing time. Treatment of one
respiratory infection in our institution means 12 additional days of intensive care, each day costing €1610; thus one treatment costs €19 320. The costs of RI per 100 days after the
introduction of the final respiratory device were thus (Table 3) €9080 with PNS and €74 962 with MV. If we add the costs of RI to those of single use equipment and additional nursing with
MV, a PNS/DP would be paid off within 1 year after start of use. CONCLUSION Treatment of respiratory insufficiency after cervical SCI with a PNS instead of MV * 1) significantly reduces
upper airway infections, * 2) reduces costs for single use airway equipment, * 3) improves the quality of speech, * 4) obviously improves patients' quality of life, * 5) probably
reduces mortality and prolongs life, * 6) 1 and 2 together pay off the higher primary investment with PNS during the first year after start of use of PNS. REFERENCES * Fromm B, Hundt G,
Gerner HJ, Baer GA, Exner G, Botel U _et al_. Management of respiratory problems unique to high tetraplegia. _Spinal Cord_ 1999; 37: 239–244. Article CAS PubMed Google Scholar *
Shneerson J . Methods of mechanical ventilation instead of implanted phrenic nerve stimulators, Implanted phrenic nerve stimulators for respiratory insufficiency. In: Baer GA, Frey H,
Talonen PP (eds). _Implanted Phrenic Nerve Stimulators for Respiratory Insufficiency_. University of Tampere: Tampere, 1989, pp 77–84. Google Scholar * Duchenne GB . _De l′electrisation
localisee et de son application a la pathologie et la therapeutique par courants induits at par courants galavaniques interrompus et continus_, 3rd edn. J.B.Bailliere et fils: Paris, 1872,
pp 907–918. Google Scholar * Judson JP, Glenn WWL . Radio-frequency electrophrenic respiration. _JAMA_ 1968; 203: 1033–1037. Article CAS PubMed Google Scholar * Glenn WWL, Hogan JF,
Loke JSO, Ciesielski TE, Phelps ML, Rowedder R . Ventilatory support by pacing of the conditioned diaphragm in quadriplegia. _N Engl J Med_ 1984; 310: 1150–1155. Article CAS PubMed Google
Scholar * Thoma H, Gerner H, Holle J, Kluger P, Mayr W, Meister B _et al_. The phrenic pacemaker. Substitution of paralyzed functions in tetraplegia. _Trans Am Soc Artif intern Organs_
1987; 33: 472–479. CAS Google Scholar * Talonen PP, Baer GA, Hakkinen V, Ojala JK . Neurophysiological and technical considerations for the design of an implantable phrenic nerve
stimulator. _Med Biol Eng Comput_ 1990; 28: 31–37. Article CAS PubMed Google Scholar * Creasey G, Elefteriades J, DiMarco A, Talonen P, Bijak M, Girsch W _et al_. Electrical stimulation
to restore respiration. _J Rehabil Res Dev_ 1996; 33: 123–132. CAS Google Scholar * Carter RE, Donovan WH, Halstead L, Wilkerson MA . Comparative study of electrophrenic nerve stimulation
and mechanical ventilatory support in traumatic spinal cord injury. _Paraplegia_ 1987; 25: 86–91. CAS PubMed Google Scholar * Esclarin A, Bravo P, Arroyo O, Mazaira J, Garrido H, Alcaraz
MA . Tracheostomy versus diaphragmatic pacemaker ventilation in high spinal cord injury. _Paraplegia_ 1994; 32: 687–693. CAS PubMed Google Scholar * Wolf C, Meiners WC, Eisenhuth J .
Personal situation and situation at home of respiratory-dependent patients with tetraplegia: electronic versus mechanical ventilation. In: Baer GA, Exner G (eds). _Functional Electrical
Stimulation in Paralysed Respiratory Muscles_, 1st edn. Tampere University Press: Tampere, 2000, pp 48–58. Google Scholar * Similowski T, Derenne JP . Stimulation phrénique implantée.
_Medicine et Therapeutique_ 2001; 7: 457–469. Google Scholar * Soni BM . Use of Phrenic nerve stimulator in high ventilator dependent spinal cord injury. In: Hunt KJ (ed). Conference
Proceeding 37.2002 Glasgow, University of Strathclyde. * McCauley RGK, Labib KB . Diaphragmatic paralysis evaluated by phrenic nerve stimulation during fluoroscopy or real-time ultrasound.
_Radiology_ 1984; 153: 33–36. Article CAS PubMed Google Scholar * Caine N . Developing a method of measuring quality of life before and after phrenic nerve stimulation. In: Baer GA, Frey
H, Talonen PP (eds). _Implanted Phrenic Nerve Stimulators for Respiratory Insufficiency_. University of Tampere: Tampere, 1989, pp 109–123. Google Scholar * Bach JR, Alba AS . Noninvasive
options for ventilatory support of the traumatic high level quadriplegic patient. _Chest_ 1990; 98: 613–619. Article CAS Google Scholar * DeVivo MJ, Ivie III CS . Life expectancy of
ventilator-dependent persons with spinal cord injuries. _Chest_ 1995; 108: 226–232. Article CAS Google Scholar * Elefteriades JA, Quin JA, Hogan JF, Holcomb WG, Letsou GV, Chlosta WF _et
al_. Long-term follow-up of pacing of the conditioned diaphragm in quadriplegia. _Pacing Clin Electrophysiol_ 2002; 25: 897–906. Article PubMed Google Scholar * Glenn WWL . Diaphragm
pacing: present status. _Pacing Clin Electrophysiol_ 1978; 1: 357–370. Article CAS PubMed Google Scholar * Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT _et al_.
A multicenter international study on the spinal cord independence measure, version III: Rasch psychometric validation. _Spinal Cord_ 2007; 45: 275–291. Article CAS Google Scholar Download
references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * BG-Trauma Hospital, Hamburg, Germany S Hirschfeld & G Exner * Research Unit, Pirkanmaa Hospital District, University of Tampere,
Tampere, Finland T Luukkaala * Tampere School of Public Health, University of Tampere, Tampere, Finland T Luukkaala * Department of Anaesthesiology, Medical School, University of Tampere,
Tampere, Finland G A Baer Authors * S Hirschfeld View author publications You can also search for this author inPubMed Google Scholar * G Exner View author publications You can also search
for this author inPubMed Google Scholar * T Luukkaala View author publications You can also search for this author inPubMed Google Scholar * G A Baer View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to G A Baer. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Hirschfeld, S., Exner, G., Luukkaala, T. _et al._ Mechanical ventilation or phrenic nerve stimulation for treatment of spinal cord injury-induced respiratory insufficiency. _Spinal Cord_ 46,
738–742 (2008). https://doi.org/10.1038/sc.2008.43 Download citation * Received: 30 August 2007 * Revised: 10 April 2008 * Accepted: 10 April 2008 * Published: 13 May 2008 * Issue Date:
November 2008 * DOI: https://doi.org/10.1038/sc.2008.43 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * spinal cord injuries * respiratory
insufficiency * ventilators, mechanical * electric stimulation * respiratory tract infection