Play all audios:
ABSTRACT OBJECTIVES: Hereditary spastic paraplegia (HSP) is a degenerative central nervous system disorder characterized by progressive spasticity and hyperreflexia of the lower limbs.
Often, patients with HSP experience symptoms of voiding dysfunction. Urodynamic evaluations of these patients are rarely reported in the literature and the etiology of voiding dysfunction
remains unclear. The present study characterizes lower urinary tract dysfunction in a large series of patients. METHODS: The medical records of 29 HSP patients who underwent urodynamic
evaluation were retrospectively analyzed. The history of lower urinary tract symptoms was noted and the urodynamic findings analyzed. RESULTS: Urgency was the most dominant complaint
(72.4%), followed by frequency (65.5%), urinary incontinence (55.2%) and hesitancy (51.7%). The urodynamic findings showed signs of central neurogenic bladder in 24 patients (82.7%), with
detrusor overactivity (DO) in 15 patients (51.7%) and detrusor sphincter dyssynergia (DSD) in 19 (65.5%). Post-void residual (PVR) of >10% of the voided volume was found in 12 patients
(41.4%). There were significant relationships between detrusor overactivity and PVR (_P_=0.005), frequency (_P_=0.046) and nocturia (_P_=0.045). Ultrasound examination revealed no upper
urinary tract complications. CONCLUSION: Despite the presence of DO and DSD, HSP patients do not seem to have a high risk of developing ultrasonographically-assessed upper urinary tract
complications after a mean follow-up of 22 years, contrary to spinal cord injury population. These results may guide practitioners in their decision-making about the appropriate evaluation
and treatment of bladder disturbances that accompany hereditary spastic paraplegia. SIMILAR CONTENT BEING VIEWED BY OTHERS DETRUSOR SPHINCTER DYSSYNERGIA: CAN A MORE SPECIFIC DEFINITION
DISTINGUISH BETWEEN PATIENTS WITH AND WITHOUT AN UNDERLYING NEUROLOGICAL DISORDER? Article Open access 07 May 2021 DURATION OF DETRUSOR OVERACTIVITY AS AN INDEPENDENT PREDICTIVE FACTOR OF
UPPER URINARY TRACT DETERIORATION IN PATIENTS WITH TRAUMATIC SPINAL CORD INJURY: RESULTS OF A RETROSPECTIVE COHORT STUDY Article 04 April 2024 RISK FACTORS OF VIDEO URODYNAMICS AND BLADDER
MANAGEMENT FOR LONG-TERM COMPLICATIONS IN PATIENTS WITH CHRONIC SPINAL CORD INJURY Article Open access 02 June 2024 INTRODUCTION Hereditary spastic paraplegia (HSP) is a heterogeneous group
of neurodegenerative disorders clinically characterized by progressive weakness, spasticity and hyperreflexia of the lower limbs and pathologically characterized by retrograde axonal
degeneration of the corticospinal tracts and posterior columns.1, 2, 3 Pure HSP is limited to the lower limbs, whereas complex HSP includes other neurologic or systemic impairments that are
not attributed to coexisting disorders (cataracts, cognitive disorders, distal amyotrophy, and so on).4, 5 The genetics of HSP is complex and several modes of inheritance have been described
(autosomal dominant, autosomal recessive and X-linked recessive).2, 5 HSP is a rare condition; its prevalence in Europe ranges from 3 to 10 per 100 000 persons.6, 7 However, its onset might
occur at any time during childhood or adult life up to 70 years of age, which makes it a significant source of chronic neuro-disability.1 Neurogenic lower urinary tract impairments are well
recognized in patients with HSP. Indeed, the prevalence of low urinary tract symptoms in HSP population was reported at 77.6% in a recent Estonian study.3 However, in this population, the
urodynamics has been rarely studied or reported. To better characterize patients’ bladder and micturition function, the present retrospective study examined the urodynamics in a series of
HSP patients, together with demographic data and lower urinary tract symptoms. PATIENTS AND METHODS PATIENTS’ CHARACTERISTICS The study analyzed retrospectively the medical records of HSP
patients admitted to the neurological rehabilitation unit of a tertiary care institute, from January 1999 to December 2009, and who had undergone a urodynamic investigation (UDI). Patients
with any other documented neurological or urological disorder were excluded from the study. These patients were 16 men and 13 women whose ages ranged from 22 to 85 years (mean: 48.6±14.4)
and all of them had pure HSP. Age, sex, date of disease onset and UDI findings were specifically sought for. Urinary symptoms were collected using a standard form filled before the UDI. The
symptoms checked for were urgency (sensation of an imminent desire to urinate), frequency (voiding more than eight times per day), nocturia (voiding more than once per night), hesitancy
(difficulty to initiate voluntary voiding) and incontinence (involuntary loss of urine). A history of urinary tract infection was also noted. All the patients voided spontaneously and none
of them had any specific urological management before the UDI. THE URODYNAMIC STUDY The UDI included a free uroflowmetry, a measurement of post-void residual (PVR), a filling and voiding
cystometry, and a pressure profilometry using a multi-channel pressure recording technology. Whenever possible, the UDI started with the free uroflowmetry, then a 12-French-gauge (12 Fr)
urethral catheter was inserted to empty the bladder and measure the PVR volume at the same time. In filling cystometry, the 6-Fr dual-lumen urethral catheter was used for simultaneous
bladder infusion and intravesical pressure measurement. Another 4.5-Fr perforated rectal balloon catheter was inserted for abdominal pressure measurement. The bladder was infused with normal
saline (0.9%) at a medium filling rate (20–50 ml min−1) until the infusion reached the maximum cystometric capacity, then the patient was asked to void. Electromyography was recorded
through needle electrodes inserted into the urethral sphincter. Clinically relevant incomplete emptying was defined as a PVR >10% of the voided volume as measured immediately after
voiding. During pressure profilometry, sphincter hypertonia was defined as maximal urethral closure pressure >75 cm H2O in women and 90 cm H2O in men. During cystometry, four indicators
were considered: (i) detrusor activity, calculated as the difference between naive bladder pressure and abdominal pressure and classified as ‘normal activity’ or ‘detrusor overactivity (DO)’
(involuntary detrusor contractions during the filling phase); (ii) detrusor capacity, defined as the volume at which the patient reported feeling uncomfortable or full and classified as
‘normal capacity’ or ‘reduced capacity’ (maximum bladder volume ⩽300 ml); (iii) detrusor compliance, defined as the volume of bladder capacity divided by the corresponding change in detrusor
pressure, before any detrusor contractions, and classified as ‘normal compliance’ or ‘reduced compliance’ (<30 ml cm−1); (iv) detrusor sphincter dyssynergia (DSD, defined as an increase
in electromyographic activity during voiding and recorded as ‘present’ or ‘absent’). In each patient, only the first UDI before any urinary specific medication or intervention was considered
and, concomitantly to UDI, urinary tract ultrasounds were also examined. STATISTICAL ANALYSES All urinary symptoms (urgency, frequency, nocturia, hesitancy and incontinence) and urodynamic
findings (detrusor overactivity, reduced detrusor capacity, reduced detrusor compliance, hypertonic sphincter, DSD and PVR) were considered in terms of presence or absence. Descriptive
statistics including size and frequencies for categorical variables, and means and s.d. for continuous variables were determined. The distributions of sex, age and disease duration were
compared for each urinary symptom. The possibility of any association between the presence of a PVR and each of the other urodynamic findings was examined. Considering the sample size,
non-parametric tests were used to assess associations; that is, Fisher exact test for categorical variables and Wilcoxon test for continuous variables. Epi-info software was used for all
statistical analyses. A _P_ value <0.05 was considered for statistical significance. ETHICS The study was carried out in agreement with French laws and the Helsinki declaration on
protecting human subjects. The local ethics committee waived the need for ethical approval because the study design did not involve changes of the usual course of patient management. RESULTS
LOW URINARY TRACT SYMPTOM Table 1 presents the distributions of the patients according to the different urinary symptom groups. All patients had at least one micturition complaint. Urgency
and frequency were the most common urinary symptoms and were present in 21 patients (72.4%) and 19 patients (65.5%), respectively. Nocturia and hesitancy were reported by 15 patients (51.7%)
and urinary incontinence by 16 patients (55.2%). The mean disease duration was of 22.23±11.61 years. Women had a higher risk of increased voiding frequency than men (_P_=0.028). They also
had a higher risk of increased incontinence (_P_=0.039). Otherwise, neither age, nor sex or disease duration was a significant risk factor for any low urinary tract symptoms in HSP patients
(Table 1). Thirteen patients (44.8%) had a history of symptomatic urinary tract infection but there were no significant associations between past infection and age, sex, or disease duration.
URODYNAMIC INVESTIGATION UDI results were unremarkable only in two women whose symptoms consisted of hesitancy and frequency. The UDI showed isolated sphincter hypertonia in three men. In
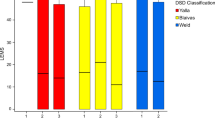
the remaining 24 patients, three major urodynamic profiles could be identified: obstructive bladder with DSD generally accompanied by sphincter hypertonia (37%), overactive bladder with DO
(21%) and an association of the two (42%). The main urodynamic findings in the patient series are summarized in Table 2. DSD, sphincter hypertonia and DO were the most common micturition
abnormalities. They were found in 65.5%, 58.6% and 51.7% of the patients, respectively. Sphincter hypertonia was significantly associated with the disease duration (_P_=0.043), but there
were no other significant relationships between disease duration and other urodynamic findings or between urodynamic findings and age or sex. Table 3 shows a comparison of urodynamic
findings in the presence and absence of significant PVR. DO and reduced detrusor compliance were significantly associated with the presence of PVR. A reduced detrusor capacity was more
frequent among patients with PVR than among the others, although this association did not reach statistical significance (_P_=0.074). RELATIONSHIP BETWEEN UROLOGICAL SYMPTOMS AND URODYNAMIC
FINDINGS As shown in Table 4, frequency and nocturia were significantly associated with DO, reduced detrusor capacity and reduced compliance. On the other hand, with borderline statistical
significance, sphincter hypertonia was less frequent among patients complaining from frequency than among the others (_P_=0.053). Otherwise, hesitancy, urgency and incontinence had no
significant relationships with either urodynamic finding. The urinary tract ultrasound examinations performed within 30 days with regard to UDI were unremarkable in 24 patients (82.7%). They
revealed discrete prostate hypertrophy in three men; all of them reported dysuria but only one had sphincter hypertonia as revealed by urethral profilometry. There were signs of bladder
outlet obstruction with thickening of the bladder wall and normal prostate size in one man having 49 years of disease duration. Bladder stones were found in one woman having 14 years of
disease duration. DISCUSSION Although neurogenic bladder dysfunction is a well-recognized and frequent problem in patients with HSP,8, 9, 10, 11 only few studies have attempted to document
such specific disturbances and evaluate their occurrence, type and severity. The paucity of urodynamic data in HSP patients is most probably due to the relative rarity of this
neurodegenerative disorder. In 1993, Bushman _et al._12 reported UDI findings in only three HSP patients and showed different combinations of abnormalities such as DO along with low detrusor
compliance or low detrusor capacity with or without DSD.12 However, a recent cross-sectional study carried out in 49 HSP patients by Braschinsky _et al._3 allows more confident comparisons
with the present series of 29 patients, one of the largest studies with complete urodynamic data. In the HSP population studied here, incontinence and hesitancy were the less common
complaints, whereas Braschinsky _et al._3 have put them on the top list of patient complaints (69.4% and 59.2%, respectively). This disagreement suggests that, in HSP patients, all potential
urinary symptoms should be thoroughly assessed. However, the increased voiding frequency and incontinence in women versus men is in line with Braschinsky's team findings. This
difference in voiding frequency is probably related to the larger bladder capacities in men;13 indeed, a reduced bladder capacity was significantly associated with an increased voiding
frequency. Besides, an intrinsic sphincter weakness in men may be masked by the obstructive anatomy of the urethra. It is commonly agreed that incomplete bladder emptying is a significant
risk factor for symptomatic urinary tract infections and upper urinary tract complications. This was well established in central nervous system disorders like multiple sclerosis or spinal
cord injuries.14 PVR was important in 12 subjects (41.4%) and was significantly associated with DO. Whereas Bradinsky _et al._ could associate PVR values over 100 ml with an increased risk
of symptomatic lower urinary tract infection in HSP patients, we did not find such an association with lower but relatively important PVR values (over 10% of the voided volume). In HSP
patients, a PVR >100 ml seems to correlate negatively with the walking speed and positively with the degree of leg spasticity.3 In the present study, all the patients had spasticity of
both lower limbs. However, because spasticity assessment was not concomitant to urodynamic evaluation, correlations could be inaccurate. In the present study population, the high prevalence
of DSD, a neurogenically-determined failure of coordination of detrusor and urethra, is worrisome because it entails adverse clinical consequences. The failure of the urethral sphincter to
relax when the detrusor contracts causes a functional urethral obstruction, which may not only hinder bladder emptying but may also develop a high detrusor intravesicular pressure that
endangers the upper urinary tract.15 DSD is caused by the interruption of autonomic spinal pathways connecting the pontine and sacral micturition centers.16, 17 These reticular spinal tracts
are close to the pyramidal tracts in the lateral columns of the spinal cord but are not yet known to be affected by the degenerative changes in HSP patients. Indeed, neuropathological
studies of patients with pure HSP have documented axonal degeneration of selected motor (corticospinal tracts) and sensory (dorsal column) fibers within the spinal cord.18, 19 As
hypothesized by Bushmann _et al._,12 this apparent lack of supporting histopathological evidence could mean a selective loss of neurotransmitter expression or release, or that a small
subgroup of neurons involved in micturition and urine storage might have been missed by the histopathological examination. On the other hand, DSD may be aggravated by pelvic floor spasticity
that generally accompanies lower limb spasticity in paraplegic patients. UDI is essential in differentiating various urological disturbances that lead to the same clinical symptoms. In the
present study, UDI was performed after a mean disease duration of 22.2 years, which may be explained by the typically slow progression of HSP. Besides, the presence of some complaints
suggested specific UDI abnormalities that were verified. Indeed, increased frequency of micturition and nocturia were significantly associated with DO, reduced capacity and reduced
compliance, which is in line with the results of studies of central nervous system disorders such as multiple sclerosis or spinal cord injury.20, 21 Otherwise, no association between the
remaining symptoms and UDI could be detected. Hence, HSP patients presenting a low urinary tract symptom should undergo a UDI to determine the exact micturition disturbances, initiate the
appropriate treatments and avoid complications. The presence of different urodynamic profiles might be related to different genetic forms of the disease and different sites of neurologic
lesions. However, this remains controversial because of the great variety of disorders having the same genetic basis, including intra-familial variations.22 Braschinsky _et al._3 reported
similar incidence of urinary dysfunction in both pure and complicated HSP clinical forms, with no difference between patients with or without SPG4 gene mutations in terms of prevalence,
character and severity of the neuro-urological complaints. Here, correlations between our findings and patients’ genetics could not be assessed for lack of genetic data in most patients.
Finally, urinary tract ultrasound examination did not reveal upper urinary tract complications after 22 years of mean follow-up. Moreover, low urinary tract complications were found in only
two patients, one of whom had the highest disease duration (49 years). The absence of these complications in the remaining 27 patients may be probably explained by the slow progression of
the disease. These findings suggest that HSP patients do not seem to have the same risk of developing upper urinary tract complications, as spinal cord injury or multiple sclerosis
populations, even in the presence of risk factors such as DO and DSD. However, unless long-term urinary outcome data are available in HSP patients, a long-term imagery follow-up remains
necessary to detect potential complications. This retrospective study has some limitations. Despite a substantial number of participants, the study lacks power because some subgroups were
too small to allow definitive conclusions. On the other hand, the upper urinary tract outcome was assessed only based on ultrasonography examination. The biological outcome (creatinine
clearance) was not available for all of the patients and was thus not analyzed. Moreover, the technical limitations inherent to the urodynamic evaluation should be kept in mind while
interpreting the results. To conclude, despite the interesting trends shown, the present work will add to the previous and will benefit from future studies to clarify urodynamic
abnormalities in HSP patients. Future studies may focus on the etiopathology and long-term outcomes of the disease. DATA ARCHIVING There were no data to deposit. REFERENCES * Salinas S,
Proukakis C, Crosby A, Warner TT . Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. _Lancet Neurol_ 2008; 7: 1127. Article CAS Google Scholar * Harding AE .
Hereditary spastic paraplegias. _Semin Neurol_ 1993; 13: 333. Article CAS Google Scholar * Braschinsky M, Zopp I, Kals M, Haldre S, Gross-Paju K . Bladder dysfunction in hereditary
spastic paraplegia: what to expect? _J Neurol Neurosurg Psychiatry_ 2010; 81: 263. Article Google Scholar * Harding AE . Classification of the hereditary ataxia and paraplegias. _Lancet_
1983; 1: 1156. Google Scholar * Fink JK . Advances in the hereditary spastic paraplegias. _Exp Neurol_ 2003; 184 (Suppl 1): 106. Article Google Scholar * McMonagle P, Webb S, Hutchinson M
. The prevalence of ‘pure’ autosomal dominant hereditary spastic paraparesis in the island of Ireland. _J Neurol Neurosurg Psychiatry_ 2002; 72: 43. Article CAS Google Scholar * Silva
MC, Coutinho P, Pinheiro CD, Neves JM, Serrano P . Hereditary ataxias and spastic paraplegias: methodological aspects of a prevalence study in Portugal. _J Clin Epidemiol_ 1997; 50: 1377.
Article CAS Google Scholar * Harding AE . Hereditary ‘pure’ spastic paraplegia: a clinical and genetic study of 22 families. _J Neurol Neurosurg Psychiatry_ 1981; 44: 871. Article CAS
Google Scholar * Harding AE . _The Hereditary Ataxias and Related Disorders_. Edinburgh: Chirchill Livingstone, 1984 pp 174. Google Scholar * Opjordsmoen S, Nyberg-Hansen R . Hereditary
spastic paraplegia with neurogenic bladder disturbances and syndactylia. _Acta Neurol Scand_ 1980; 61: 35–41. Article CAS Google Scholar * Kolodny EH, Boustany RM, Rouleau GA, Growden JH,
Martin JB . Familial spastic paraplegia: clinical observations and genetic studies. _Prog Clin Biol Res_ 1989; 306: 205. CAS PubMed Google Scholar * Bushman W, Steers WD, Meythaler JM .
Voiding dysfunction in patients with spastic paraplegia: urodynamic evaluation and response to continuous intrathecal baclofen. _Neurourol Urodyn_ 1993; 12: 163. Article CAS Google Scholar
* Wyndaele JJ . Normality in urodynamics studied in healthy adults. _J Urol_ 1999; 161: 899–902. Article CAS Google Scholar * Foxman B . Epidemiology of urinary tract infections:
incidence, morbidity, and economic costs. _Am J Med_ 2002; 113 (Suppl 1A): 5. Article Google Scholar * Fowler CJ . Integrated control of lower urinary tract-clinical perspective. _Br J
Pharmacol_ 2006; 147 (Suppl 2): 14. Article Google Scholar * Blaivas JG . The neurophysiology of micturition: a clinical study of 550 patients. _J Urol_ 1982; 127: 958. Article CAS
Google Scholar * De Groat WC, Booth AM, Yoshimura N . Neurophysiology of mictirution and its modification in animal models of human disease. In: Maggi CA (ed). _The Autonomic Nervous
System_. Harwood Academic: London, 1993, pp 227. Google Scholar * Behan WM, Maia M . Strümpell's familial spastic paraplegia: genetics and neuropathology. _J Neurol Neurosurg
Psychiatry_ 1974; 37: 8. Article CAS Google Scholar * Boustany RM, Fleischnick E, Alper CA, Marazita ML, Spence MA, Martin JB _et al_. The autosomal dominant form of ‘pure’ familial
spastic paraplegia: clinical findings and linkage analysis of a large pedigree. _Neurology_ 1987; 37: 910. Article CAS Google Scholar * Litwiller SE, Frohman EM, Zimmern PE . Multiple
sclerosis and the urologist. _J Urol_ 1999; 161: 743. Article CAS Google Scholar * Van Brummen HJ, Heintz AP, Van der Vaart CH . The association between overactive bladder symptoms and
objective parameters from bladder diary and filling cystometry. _Neurourol Urodyn_ 2004; 23: 38–42. Article CAS Google Scholar * Orlacchio A, Kawarai T, Totaro A, Errico A, St
George-Hyslop PH, Rugarli EI _et al_. Hereditary spastic paraplegia: clinical genetic study of 15 families. _Arch Neurol_ 2004; 61: 849. Article Google Scholar Download references
ACKNOWLEDGEMENTS We wish to thank Jean Iwaz (PhD, Hospices Civils de Lyon) for his editorial assistance. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * INSERM, M Fourtassi, S
Jacquin-Courtois, J Luaute & G Rode * UMR-S 864, Bron, France M Fourtassi, S Jacquin-Courtois, J Luaute & G Rode * Université Lyon 1, Villeurbanne, France M Fourtassi, S
Jacquin-Courtois, M C Scheiber-Nogueira, A Hajjioui, J Luaute, K Charvier & G Rode * Hospices Civils de Lyon, Hôpital Henry Gabrielle, Service de Médecine Physique et de Réadaptation,
Saint-Genis-Laval, France M Fourtassi, S Jacquin-Courtois, J Luaute, K Charvier & G Rode * Hospices Civils de Lyon, Hôpital Henry Gabrielle, Service de Neuro-Urologie, Saint-Genis-Laval,
France M C Scheiber-Nogueira * Hospices Civils de Lyon, Service de Biostatistique, Lyon, France D Maucort-Boulch * CNRS, D Maucort-Boulch * UMR 5558, Laboratoire Biostatistique Santé,
Pierre-Bénite, France D Maucort-Boulch Authors * M Fourtassi View author publications You can also search for this author inPubMed Google Scholar * S Jacquin-Courtois View author
publications You can also search for this author inPubMed Google Scholar * M C Scheiber-Nogueira View author publications You can also search for this author inPubMed Google Scholar * A
Hajjioui View author publications You can also search for this author inPubMed Google Scholar * J Luaute View author publications You can also search for this author inPubMed Google Scholar
* K Charvier View author publications You can also search for this author inPubMed Google Scholar * D Maucort-Boulch View author publications You can also search for this author inPubMed
Google Scholar * G Rode View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to M Fourtassi. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Fourtassi, M., Jacquin-Courtois, S.,
Scheiber-Nogueira, M. _et al._ Bladder dysfunction in hereditary spastic paraplegia: a clinical and urodynamic evaluation. _Spinal Cord_ 50, 558–562 (2012).
https://doi.org/10.1038/sc.2011.193 Download citation * Received: 11 August 2011 * Revised: 29 December 2011 * Accepted: 29 December 2011 * Published: 31 January 2012 * Issue Date: July 2012
* DOI: https://doi.org/10.1038/sc.2011.193 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * bladder dysfunction * hereditary spastic paraplegia *
urodynamic study * detrusor-sphincter dyssynergia * detrusor overactivity
