Play all audios:
ABSTRACT A retrospective single center study was performed to evaluate the safety and efficacy of valacyclovir for prevention of cytomegalovirus (CMV) infection (reactivation) after
allogeneic stem cell transplantation (SCT). We compared a group of 31 patients at risk for CMV reactivation (donor, recipient or both seropositive for CMV) who received valacyclovir at an
oral dose of 1 g three times a day for CMV prophylaxis with a matched cohort of 31 patients who did not receive the drug or any other form of CMV prophylaxis. Valacyclovir was used as
primary prophylaxis in 12 patients and as secondary prophylaxis (after a prior CMV reactivation was effectively treated with either ganciclovir or foscarnet and without CMV antigenemia at
the start of valacyclovir) in the remaining 19 patients. The two treatment groups were well matched for the donor–recipient CMV serological status and other pre-transplant characteristics.
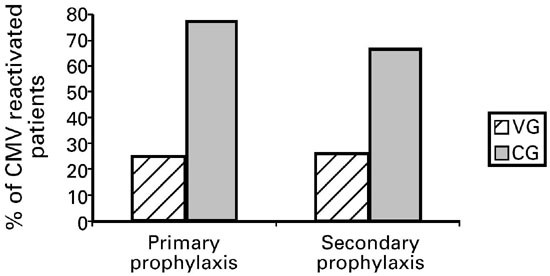
CMV reactivation was detected by blood antigenemia testing using a commercially available immunofluorescence assay for CMV lower matrix protein pp65 in circulating leukocytes. For primary
prophylaxis, 3/12 patients who received valacyclovir reactivated CMV compared to 24/31 patients in the control group (_P_ < 0.001). For secondary prophylaxis, 5/19 valacyclovir patients
reactivated compared to 16/24 control patients (_P_ < 0.05). Valacyclovir was well tolerated except for infrequent and mild gastrointestinal side-effects. There was no difference in the
incidence of CMV disease in the two groups. Prophylaxis with valacyclovir appears to be safe and efficacious in preventing both primary and secondary CMV reactivation in at-risk patients
after allogeneic SCT. Larger prospective randomized studies will be required to confirm these observations. _Bone Marrow Transplantation_ (2001) 28, 265–270. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and
online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS EFFICACY OF PROPHYLACTIC LETERMOVIR FOR CYTOMEGALOVIRUS REACTIVATION IN HEMATOPOIETIC CELL TRANSPLANTATION: A MULTICENTER REAL-WORLD DATA Article 02 November 2020 LETERMOVIR FOR
CYTOMEGALOVIRUS INFECTION IN PEDIATRIC PATIENTS UNDERGOING ALLOGENIC HEMATOPOIETIC STEM CELL TRANSPLANTATION: A REAL-LIFE STUDY BY THE INFECTIOUS DISEASES WORKING GROUP OF ITALIAN
ASSOCIATION OF PEDIATRIC HEMATOLOGY-ONCOLOGY (AIEOP) Article 25 January 2024 HIGH-DOSE ACICLOVIR IN CMV INFECTION PROPHYLAXIS AFTER ALLOGENEIC HSCT: A SINGLE-CENTER LONG-TERM EXPERIENCE
Article Open access 23 August 2023 REFERENCES * Wingard JR . Infections in allogeneic bone marrow transplant recipients _Semin Oncol_ 1993 20: 80–87 CAS PubMed Google Scholar * Winston
DJ, Ho WG, Champlin RE . Cytomegalovirus infections after allogeneic bone marrow transplantation _Rev Infect Dis_ 1990 12: S776–S792 Article PubMed Google Scholar * Osarogiagbon RU, Defor
TE, Weisdorf MA _et al_. CMV antigenemia following bone marrow transplantation: risk factors and outcomes _Biol Blood Marrow Transplant_ 2000 6: 280–288 Article CAS PubMed Google Scholar
* Humar A, Wood S, Lipton J _et al_. Effect of cytomegalovirus infection on 1-year mortality rates among recipients of allogeneic bone marrow transplants _Clin Infect Dis_ 1998 26: 606–610
Article CAS PubMed Google Scholar * Maltezou H, Whimbey E, Abi-Said D _et al_. Cytomegalovirus disease in adult marrow transplant recipients receiving ganciclovir prophylaxis: a
retrospective study _Bone Marrow Transplant_ 1999 24: 665–669 Article CAS PubMed Google Scholar * Goodrich JM, Bowden RA, Fisher L _et al_. Ganciclovir prophylaxis to prevent
cytomegalovirus disease after allogeneic marrow transplant _Ann Intern Med_ 1993 118: 173–178 Article CAS PubMed Google Scholar * Mori T, Okamoto S, Matsuoka S _et al_. Risk-adapted
pre-emptive therapy for cytomegalovirus disease in patients undergoing allogeneic bone marrow transplantation _Bone Marrow Transplant_ 2000 25: 765–769 Article CAS PubMed Google Scholar
* Winston DJ, Ho WG, Bartoni K _et al_. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled,
double-blind trial _Ann Intern Med_ 1993 118: 179–184 Article CAS PubMed Google Scholar * Bacigalupo A, Tedone E, Van Lint MT _et al_. CMV prophylaxis with foscarnet in allogeneic bone
marrow transplant recipients at high risk of developing CMV infections _Bone Marrow Transplant_ 1994 13: 783–788 CAS PubMed Google Scholar * Prentice HG, Gluckman E, Powles RL _et al_.
Long-term survival in allogeneic bone marrow transplant recipients following acyclovir prophylaxis for CMV infection. The European Acyclovir for CMV Prophylaxis Study Group _Bone Marrow
Transplant_ 1997 19: 129–133 Article CAS PubMed Google Scholar * Meyers JD, Reed EC, Shepp DH _et al_. Acyclovir for prevention of cytomegalovirus infection and disease after allogeneic
marrow transplantation _New Engl J Med_ 1988 318: 70–75 Article CAS PubMed Google Scholar * Boeckh M, Gooley TA, Bowden RA . Effect of high-dose acyclovir on survival in allogeneic
marrow transplant recipients who received ganciclovir at engraftment or for cytomegalovirus pp65 antigenemia _J Infect Dis_ 1998 178: 1153–1157 Article CAS PubMed Google Scholar * Weller
S, Blum MR, Doucette M _et al_. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers _Clin Pharmacol Ther_
1993 54: 595–605 Article CAS PubMed Google Scholar * Purifoy DJ, Beauchamp LM, de Miranda P _et al_. Review of research leading to new anti-herpesvirus agents in clinical development:
valaciclovir hydrochloride (256U, the L-valyl ester of acyclovir) and 882C, a specific agent for varicella zoster virus _J Med Virol_ 1993 (Suppl. 1) 139–145 Article Google Scholar *
Soul-Lawton J, Seaber E, On N _et al_. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans _Antimicrob
Agents Chemother_ 1995 39: 2759–2764 Article CAS PubMed PubMed Central Google Scholar * Lowance D, Neumayer HH, Legendre CM _et al_. Valacyclovir for the prevention of cytomegalovirus
disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group _New Engl J Med_ 1999 340: 1462–1470 Article CAS PubMed Google
Scholar * Himmelmann-Jud B, Wuthrich RP, Weinreich T, Binswanger U . Successful use of oral valacyclovir in post-transplant cytomegalovirus infection in renal allograft recipients _Nephrol
Dial Transplant_ 1998 13: 1326–1327 Article CAS PubMed Google Scholar * Emery VC, Sabin C, Feinberg JE _et al_. Quantitative effects of valacyclovir on the replication of cytomegalovirus
(CMV) in persons with advanced human immunodeficiency virus disease: baseline CMV load dictates time to disease and survival. The AIDS Clinical Trials Group 204/Glaxo Wellcome 123–014
International CMV Prophylaxis Study Group _J Infect Dis_ 1999 180: 695–701 Article CAS PubMed Google Scholar * Feinberg JE, Hurwitz S, Cooper D _et al_. A randomized, double-blind trial
of valaciclovir prophylaxis for cytomegalovirus disease in patients with advanced human immunodeficiency virus infection. AIDS Clinical Trials Group Protocol 204/Glaxo Wellcome 123–014
International CMV Prophylaxis Study Group _J Infect Dis_ 1998 177: 48–56 Article CAS PubMed Google Scholar * Landry ML, Ferguson D, Stevens-Ayers T _et al_. Evaluation of CMV Brite kit
for detection of cytomegalovirus pp65 antigenemia in peripheral blood leukocytes by immunofluorescence _J Clin Microbiol_ 1996 34: 1337–1339 CAS PubMed PubMed Central Google Scholar *
Salzberger B, Bowden RA, Hackman RC _et al_. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome
_Blood_ 1997 90: 2502–2508 CAS PubMed Google Scholar * Boeckh M, Gooley TA, Myerson D _et al_. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovirversus ganciclovir
at engraftment after allogeneic marrow transplantation: a randomized double-blind study _Blood_ 1996 88: 4063–4071 CAS PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS
AND AFFILIATIONS * Division of Hematology and Oncology, Department of Medicine, Vanderbilt University School of Medicine and VA Medical Center, Nashville, TN, USA M Vusirikala, SN Wolff, RS
Stein, SJ Brandt, DS Morgan, JP Greer, FG Schuening & SA Goodman * Department of Medicine, and the Division of Infectious Diseases, Department of Medicine, Vanderbilt University School
of Medicine and VA Medical Center, Nashville, TN, USA JS Dummer Authors * M Vusirikala View author publications You can also search for this author inPubMed Google Scholar * SN Wolff View
author publications You can also search for this author inPubMed Google Scholar * RS Stein View author publications You can also search for this author inPubMed Google Scholar * SJ Brandt
View author publications You can also search for this author inPubMed Google Scholar * DS Morgan View author publications You can also search for this author inPubMed Google Scholar * JP
Greer View author publications You can also search for this author inPubMed Google Scholar * FG Schuening View author publications You can also search for this author inPubMed Google Scholar
* JS Dummer View author publications You can also search for this author inPubMed Google Scholar * SA Goodman View author publications You can also search for this author inPubMed Google
Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Vusirikala, M., Wolff, S., Stein, R. _et al._ Valacyclovir for the prevention of cytomegalovirus
infection after allogeneic stem cell transplantation: a single institution retrospective cohort analysis. _Bone Marrow Transplant_ 28, 265–270 (2001). https://doi.org/10.1038/sj.bmt.1703129
Download citation * Received: 18 December 2000 * Accepted: 16 May 2001 * Published: 04 September 2001 * Issue Date: 01 August 2001 * DOI: https://doi.org/10.1038/sj.bmt.1703129 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * cytomegalovirus * valacyclovir * allogeneic SCT